Grocott-Gomori’s Methenamine Silver (GMS) stain is the histological staining method that is used mainly for the detection of fungal microorganisms in tissue sections and smears. It is the process that was first developed by Gomori and later modified by Grocott, and it is used because of its high sensitivity to demonstrate carbohydrates present in the fungal cell wall.
It has been used earlier for missing tissues and diseases of the liver and rectum and later it became important for the identification of Pneumocystis jirovecii, a fungus that causes pneumocytosis in immunocompromised and immunosuppressed patients. It is the stain that shows better sensitivity compared to Periodic Acid-Schiff stain and Gridley stain, and it is used in paraffin prepared sections to detect fungi and other polysaccharide-rich organisms. The major reagents used are Gomori’s methenamine-silver nitrate and chromic acid, and its application in histology makes it suitable for demonstrating fungi in aspirates, tissues, and smears.
Grocott-Gomori’s Methenamine Silver Staining is based on the argentaffin reaction in which the fungal cell wall has the ability to reduce the silver ions into visible metallic silver. In this step chromic acid is used as a strong oxidizing agent. It is the process that oxidizes the mucopolysaccharides of the fungal cell wall and produces aldehyde groups, while other carbohydrates in the tissue are over-oxidized into non-reactive carboxylic acids.
These are important because they prevent background staining and only the fungal cell wall remains reactive. After oxidation the section is treated with methenamine silver solution which is usually heated, and the aldehyde groups now reduce the silver ions to form black metallic silver on the fungal structures. The slide is then toned with gold chloride which helps in giving a clear black color and removes background discoloration.
Sodium thiosulfate is used to fix the reaction and remove excess unreacted silver so that the slide does not become dark later. Finally, a counterstain like Light Green is applied. The stained fungi appear as black structures against a pale green background which helps in observing the size, shape, and internal details of fungal organisms.
Objectives
- To prove that fungi are present in a sample.
- Pneumocystis jirovecii and Histoplasma spp. must be shown to exist.
Grocott-Gomori’s Methenamine Silver Staining Principle
Grocott-Gomori’s Methenamine Silver Staining works on the principle of the argentaffin reaction, in which the fungal cell wall has the capacity to reduce a silver solution into visible metallic silver. It is the process in which the polysaccharides present in fungal cell walls, especially the 1,2-glycol groups, are first oxidized by using chromic acid. This oxidation converts these groups into aldehydes, and at the same time the chromic acid over-oxidizes other surrounding carbohydrates into carboxylic acids which are non-reactive. It is important because this over-oxidation suppresses the background staining and only the fungal structures remain capable of reacting.
After oxidation, the section is treated with the methenamine-silver working solution. In this alkaline solution the aldehyde groups formed on the fungal wall now act as the reducing centers and they reduce the silver ions into metallic silver. The metallic silver is precipitated as a black deposit on the fungal cell wall which makes the fungi clearly visible.
The slide is then toned with gold chloride, and this step helps in converting the brownish silver deposits into a sharp black color and also improves background clarity. Finally sodium thiosulfate is used to remove any unreacted silver so the section does not become dark with time. It is the overall reaction that gives a clear black fungal outline against a pale background and is used for demonstrating fungi in tissues.
Reagents Requireds
The reagents used in Grocott-Gomori’s Methenamine Silver Staining are selected to oxidize the fungal wall, reduce silver ions, and finally visualize the organisms. These are prepared freshly because the silver solution is easily altered by impurities, and the reaction is highly sensitive.
1. Oxidizing Reagents
Chromic acid (4–5%) is the major oxidizing reagent. It is used because it converts the 1,2-glycol groups of fungal cell wall into aldehydes, and at the same time it over-oxidizes other tissue carbohydrates into non-reactive acids. Sodium bisulfite or sodium metabisulfite (1%) is used afterward for removing the residual chromic acid.
2. Silver Impregnation Reagents
Silver nitrate solution (around 5%) and methenamine solution (3%) are used together with borax solution (5%). These are prepared in distilled water because impurities may cause unwanted silver deposition. This mixture forms the alkaline methenamine-silver complex which is reduced by the fungal aldehydes.
3. Toning and Fixing Reagents
Gold chloride solution (0.1–0.5%) is used for toning. It is the step that gives the black color to the fungal structures and clears the background. Sodium thiosulfate (2–5%) is used after toning to remove any unreacted silver so that the section does not darken later.
4. Counterstaining Reagents
Light Green SF yellowish is commonly used as the counterstain. Some procedures may also use Neutral Red or other routine stains, but Light Green is preferred because it gives a clear contrast to the black fungal structures.
5. General Laboratory Reagents
Xylene is required for deparaffinization and clearing. Ethanol of different strengths is used for hydration and dehydration steps. A suitable mounting medium is used for preparing permanent slides.
Other Requirements
1. Glassware and Handling Tools
Acid-cleaned glassware is required for preparing silver solutions because any contamination can cause background precipitates. Coplin jars or plastic staining jars are used for processing. Non-metallic forceps are important since metal contact may reduce silver ions prematurely.
2. Heating Apparatus
A water bath maintained around 45–60°C is commonly required for the silver impregnation step. Some procedures also use microwave heating, but specially designed laboratory microwave ovens are used.
3. Slides
Adhesive or charged slides are preferred because the chromic acid step and high-temperature silver impregnation may detach the tissue from ordinary slides.
4. Control Tissue
A known positive control, such as tissue containing Pneumocystis, Aspergillus, or Histoplasma, is processed along with the test sample. These are used to ensure that the staining reaction is valid.
5. Safety Requirements
Chromic acid and silver solutions require handling inside a fume hood. Lab coat, gloves, and safety goggles are used during reagent preparation, especially with oxidizing agents.
These reagents and tools together allow proper oxidation, silver reduction, and visualization of fungal organisms, and they are used freshly to maintain the sensitivity of the Grocott-Gomori’s Methenamine Silver staining process.
Solution Preparation
The solutions used in Grocott-Gomori’s Methenamine Silver staining are prepared carefully because the reagents like chromic acid and silver nitrate are corrosive and sensitive to impurities. All the glassware used for preparing the silver solutions is acid-cleaned and washed with distilled water so that no contaminants cause unwanted silver deposition. The preparation is done with protective gloves, lab coat, and goggles because these reagents may damage the skin and eyes.
1. Preparation of Oxidizing Solution
The oxidizing reagent is usually chromic acid. It is prepared by dissolving 4–5 g of chromium trioxide in 100 ml of distilled water to make a 4–5% solution. This solution is stable for some months but must be discarded if it becomes brown. In some procedures a weaker solution, around 2%, is made by dissolving 10 g in 500 ml of water especially when microwave heating is used. After the oxidation step a sodium bisulfite or sodium metabisulfite solution (1%) is used to remove any remaining chromic acid. It is prepared by dissolving 1 g of the salt in 100 ml of distilled water.
2. Preparation of Methenamine Silver Solutions
These are prepared in two steps. The stock methenamine-silver solution is prepared by mixing 100 ml of 3% methenamine solution with 5 ml of 5% silver nitrate. A white precipitate forms initially which dissolves on shaking. This stock can be stored in the refrigerator. The working solution is prepared freshly before each staining. Around 25 ml of the stock solution is mixed with 25 ml of distilled water. To this, 2–3 ml of 5% borax solution is added. Borax creates the alkaline condition needed for the silver reduction to occur.
3. Preparation of Toning and Fixing Solutions
Gold chloride solution is used for toning. It is usually made as 0.1–0.5% by dissolving the proper amount of gold chloride in distilled water. A common method is diluting 10 ml of 1% gold chloride stock with 90 ml of water. The fixing reagent is sodium thiosulfate, prepared by dissolving 2–5 g in 100 ml of distilled water to make a 2–5% solution. This reagent removes unreduced silver and prevents darkening of the slide.
4. Preparation of Counterstain
Light Green SF yellowish is used commonly. A 0.2% solution is made by dissolving 0.2 g of the dye in 100 ml of distilled water. Around 0.2 ml of glacial acetic acid is added to adjust the pH and give a clear staining result.
These solutions together form the complete set required for oxidation, silver impregnation, toning, fixing, and counterstaining in Grocott-Gomori’s Methenamine Silver staining, and most of them are prepared fresh to maintain the accuracy and sensitivity of the staining reaction.
Procedure of Grocott-Gomori’s Methenamine Silver Staining
General Method
1. Preparation of Sections
It is done by taking thin paraffin sections (4–5 µm) which is hydrated to distilled water. The glassware used for the staining is kept acid-cleaned because metallic contamination may produce unwanted background.
2. Oxidation
In this step the sections is oxidized with 4% aqueous chromic acid at room temperature and it is kept for about 1 hour. It is the process where fungal cell wall polysaccharides is converted into aldehyde groups. After oxidation sections is washed in water for a few seconds.
3. Removal of Excess Chromic Acid
The sections is treated with 1% sodium metabisulphite for 1 minute. This is referred to as the step which remove the remaining chromic acid. Then it is washed in smoothly running tap water for about 3 minutes and again rinsed in distilled water.
4. Silver Impregnation
The slides is now placed in the pre-heated working methenamine silver solution in a water bath at about 60°C. It is kept for 15–20 minutes until the section turns yellowish-brown. The reaction is as follows– the aldehydes reduce the silver ions to metallic silver. It is checked microscopically to see fungi turned dark brown.
5. Rinsing
The sections is rinsed well in distilled water so that excess solution is removed.
6. Toning
It is done by adding 0.2% gold chloride to the section and it is left for 2 minutes. This helps in converting the brown deposits into black.
7. Fixing of Silver
The sections is treated with 2% sodium thiosulphate for 2 minutes. It is the process which remove unreduced silver so that the stain does not darken later.
8. Washing
The slides is washed in smoothly running tap water for about 5 minutes.
9. Counterstaining
The counterstain is done with Light Green for about 15 seconds. This provide a pale green background. Excess Light Green is removed with alcohol.
10. Dehydration and Mounting
Finally the sections is dehydrated, cleared and mounted.
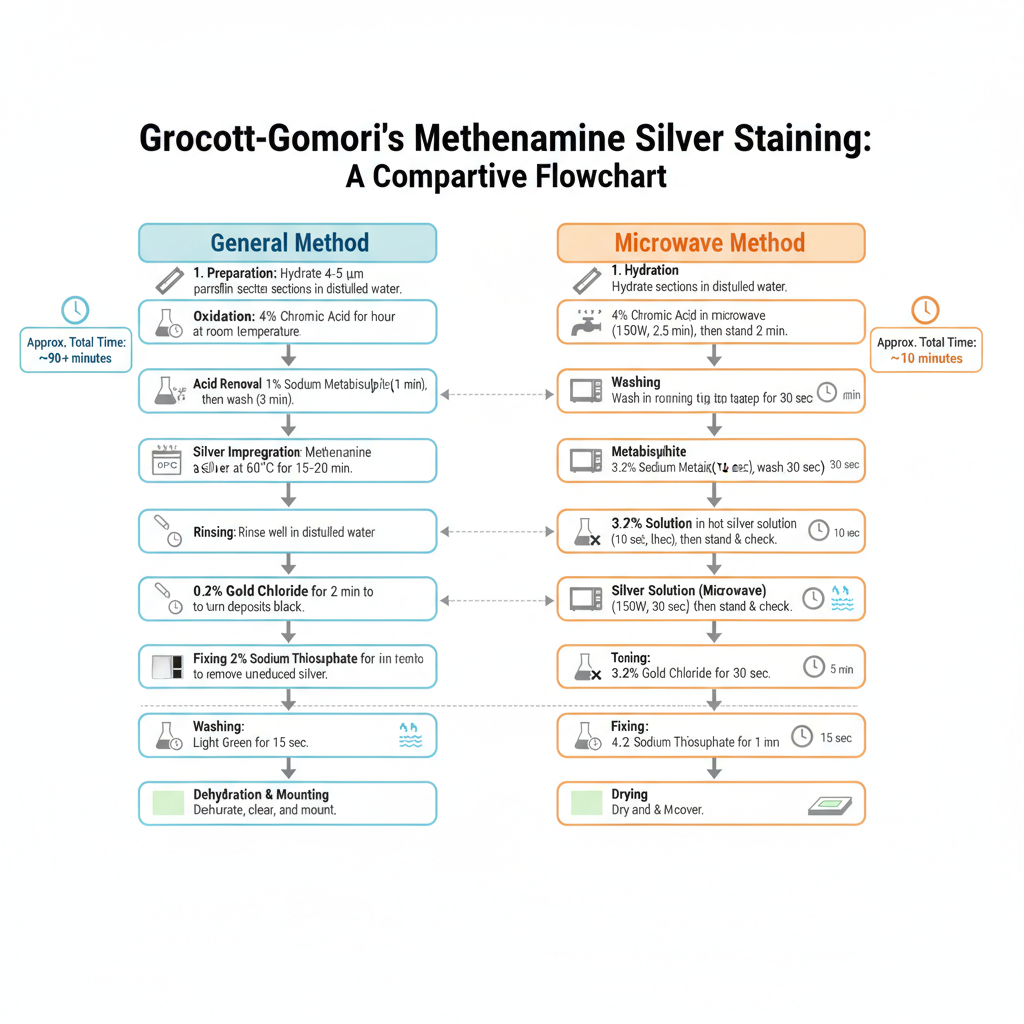
Microwave Method
1. Hydration
The sections is hydrated to distilled water.
2. Oxidation in Microwave
Slides is placed in 40 ml of 4% chromic acid in a loosely covered plastic Coplin jar.
It is microwaved at 150 Watt for 2 min 30 sec.
After microwaving, slides is dipped up and down and kept for an extra 2 minutes.
3. Washing
Sections is washed in smoothly running tap water for 30 sec.
4. Metabisulphite Treatment
3.2% sodium metabisulphite is added for 10 sec and washed again with tap water for 30 sec.
5. Silver Solution (Microwave)
The working silver solution is preheated at 450 Watt for 60 sec.
Slides is rinsed thoroughly with distilled water and then placed in the hot silver solution.
It is microwaved at 150 Watt for 30 sec.
Slides is dipped up and down and allowed to stand for 1 minute.
Sections is checked microscopically. If fungi is not sufficiently stained, slides is dipped back into silver solution and checked every 1 minute until the fungi turns dark brown.
6. Toning
Toned with 3.2% gold chloride for 30 sec and rinsed in distilled water.
7. Fixing
4.2% sodium thiosulphate is added for 1 minute and washed with tap water for 15 sec.
8. Counterstaining
Counterstain is done with Light Green for 15 sec and excess stain is removed with alcohol.
9. Drying and Mounting
The slides is dried and covered.
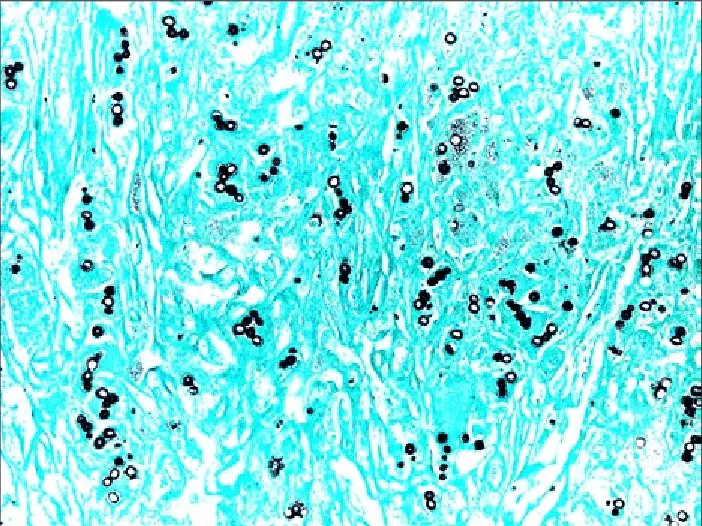
Results of Grocott-Gomori’s Methenamine Silver Staining
It is observed that fungal organisms appears as crisp black structures. The silver gets deposited on the fungal cell wall so the outline becomes very clear. Internal parts of the fungi sometimes shows gray or an old-rose appearance. The background is generally pale green because Light Green counterstain is used. This colour contrast helps in identifying the fungal elements easily in the section.
Yeasts, hyphae, and cyst forms is clearly visualized because the stain produces sharp margins. The tissue elements in the background appear faint and does not interfere with the black fungal outlines. In some sections mucin or melanin may show dark gray colour.
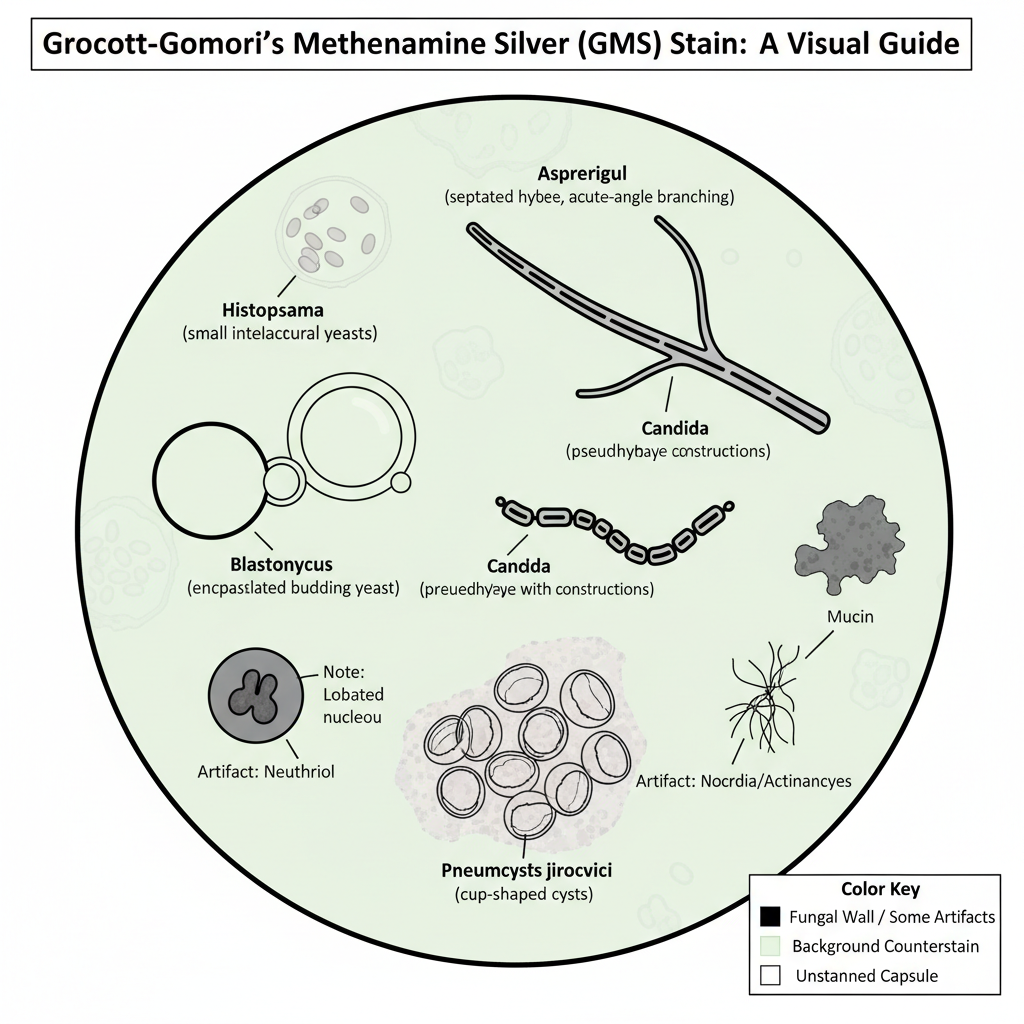
Interpretation
1. Yeasts
These are seen as round or oval black-outlined bodies. The budding pattern is important in interpretation. Cryptococcus may shows encapsulated budding yeasts. Blastomyces shows broad-based budding. Histoplasma appears as small clustered yeasts inside macrophages.
2. Hyphae
The hyphal elements appears as long filamentous black structures. It is checked whether the hyphae is septated or not. Aspergillus is identified by regularly septated hyphae with acute-angle branching. Candida may show pseudohyphae which is irregular and constricted at intervals.
3. Pneumocystis
Pneumocystis jirovecii is seen as cysts which shows a cup-like or crescent shape. The cyst wall becomes black while the center appears pale.
Non-Fungal Structures and Artifacts
1. Bacteria
Some bacteria like Nocardia and Actinomyces may also reduce silver and appear black. These shows filamentous branching rods, so the appearance may overlap with fungi.
2. Tissue Components
Mucin, glycogen and melanin may appear dark gray to black. But due to chromic acid oxidation the background staining is usually less as compared to PAS staining.
3. Neutrophils
Sometimes neutrophils take silver and appear black. These cells can be mistaken as yeast. The distinguishing feature is that neutrophils shows a lobated nucleus inside the black cytoplasm while fungi shows a distinct wall with clear or gray center. Presence of stained neutrophils also indicates that the silver reaction has worked properly even when fungi is absent.
Precautions
- Lab workers must wear gloves, coat and eye protection during the staining process.
- The reagents is handled inside a fume hood because toxic vapors may be produced.
- Chromic acid is corrosive and carcinogenic, so it must be used with great care.
- Silver nitrate is an irritant and can cause burning of skin and eyes.
- All chemical waste containing silver or chromium must be discarded by proper method.
- All glassware used in silver solution must be acid-cleaned and rinsed well with distilled water.
- Only distilled or deionized water is used because tap water contains minerals that precipitate silver.
- Metal forceps is not used during silver impregnation.
- Plastic forceps or paraffin-coated forceps is used to avoid unwanted silver reduction.
- The temperature of the silver solution is kept within the required range.
- Overheating may cause the silver to break down and precipitate.
- During microwave method the Coplin jars must be loosely covered to avoid pressure build up.
- Chromic acid solution must be fresh. If the colour turns brown it is replaced.
- Silver solution that becomes cloudy or shows mirror-like deposits is discarded.
- Control slide is checked under microscope at intervals to stop the reaction at correct stage.
- Under-oxidation gives high background staining while over-oxidation prevents fungal staining.
- A known positive control slide is always run to confirm the reagents are working properly.
Applications of Grocott-Gomori’s Methenamine Silver Staining
- It is used for detecting fungal infections in tissue sections.
- It shows a wide range of fungal organisms like Aspergillus, Candida, Histoplasma, Cryptococcus, Blastomyces, Sporothrix and Coccidioides.
- It is the method used for identifying Pneumocystis jirovecii because the cysts appear as cup-shaped or crescent forms.
- It helps in studying fungal morphology such as the wall thickness, branching angle, budding pattern and size of yeast cells.
- It can detect filamentous bacteria like Actinomyces and Nocardia which may appear similar to fungi.
- It is used for differentiating these bacteria from true fungal hyphae based on staining pattern and filament structure.
- It may demonstrate internal organs of Strongyloides larvae after staining.
- It can show intranuclear inclusions in cells infected with Cytomegalovirus.
- It stains mucin and glycogen which appears dark gray to black in the section.
- It may also detect endospores of Bacillus species.
- It is applied in immunocompromised patients for detection of low fungal burden.
- It is used on cytology samples like BAL fluid for screening respiratory fungal pathogens.
- It is used in research work to assess fungal load in animal models.
Advantages of Grocott-Gomori’s Methenamine Silver Staining
- It is considered a highly sensitive method for detecting fungi in tissue sections.
- It gives a very clear contrast because fungal cell walls appear black on a pale background.
- It can detect even very small or scattered fungal elements that may be missed in routine stains.
- It helps in studying fungal morphology like septation, width of hyphae, and branching pattern.
- It is useful for identifying Pneumocystis jirovecii cysts which become sharply outlined.
- It suppresses background staining because chromic acid over-oxidizes most tissue carbohydrates.
- It produces clean sections with minimum background reaction which helps in easy interpretation.
- It distinguishes fungi better than PAS because PAS may show excess background.
- It can be used in immunocompromised patients where fungal load is very low.
- It helps in rapid screening of cytology smears where fungi is difficult to visualize with H&E.
Limitations of Grocott-Gomori’s Methenamine Silver Staining
- It is not fully specific because many non-fungal structures can also take silver.
- It may stain neutrophil cytoplasm black and this can look similar to small yeasts or cyst forms.
- It can stain mucin, glycogen, melanin and keratin which creates background that makes interpretation difficult.
- It may also show bacteria like Nocardia and Actinomyces which appear filamentous and can confuse diagnosis.
- It sometimes stains elastin and reticulin fibers which increases background noise.
- It is a technically difficult staining method and needs careful temperature control.
- If the silver solution is too cool the fungi appears faint, and if too hot the silver may precipitate.
- The chromic acid oxidation step must be balanced because under-oxidation leaves background and over-oxidation prevents fungal staining.
- The silver solution is highly sensitive to contamination and unclean glassware can produce unwanted black deposits.
- Overstaining may hide important fungal details like septation and budding pattern.
- Larger fungi stain faster while very small organisms like Pneumocystis stain slowly, so imbalance may occur in mixed infections.
- Excess Light Green counterstain may cover small fungal elements and produce false negative results.
- Chromic acid used in the procedure is toxic and carcinogenic and needs careful handling and disposal.
FAQ
Q1. What is Grocott-Gomori’s Methenamine Silver (GMS) stain?
A. It is a special histological stain used for detecting fungi in tissue sections. It is the method where fungal cell walls is stained black by silver deposition.
Q2. What is Grocott-Gomori’s Methenamine Silver Staining used for?
A. It is used for identifying fungal organisms in tissues and cytology smears. It is also used for detecting Pneumocystis, some filamentous bacteria and certain argentaffin structures.
Q3. What is the principle of Grocott-Gomori’s Methenamine Silver Staining?
A. It is the process where chromic acid oxidizes tissue components to form aldehydes, and these aldehydes reduce silver ions into metallic silver. The fungi resist over-oxidation, so the cell walls become black.
Q4. What is the procedure for Grocott-Gomori’s Methenamine Silver Staining?
A. The section is oxidized with chromic acid, treated with sodium metabisulphite, rinsed well, then kept in pre-heated methenamine silver solution until it turns brown. It is toned with gold chloride, fixed in sodium thiosulphate, counterstained with Light Green, and then dehydrated and mounted.
Q5. What organisms can Grocott-Gomori’s Methenamine Silver Staining identify?
A. It identifies fungi like Aspergillus, Candida, Cryptococcus, Histoplasma, Blastomyces, Sporothrix and Pneumocystis. It may also stain Nocardia and Actinomyces.
Q6. How do you interpret the results of Grocott-Gomori’s Methenamine Silver Staining?
A. Fungi appears as black outlined structures with gray or pale centers. Yeasts show budding pattern, hyphae show septation and branching angle, and Pneumocystis shows cup-shaped cysts.
Q7. What color do fungi appear with GMS stain?
A. Fungi appear black.
Q8. What reagents are used in Grocott-Gomori’s Methenamine Silver Staining?
A. Chromic acid, sodium metabisulphite, methenamine silver working solution, gold chloride, sodium thiosulphate, and Light Green counterstain.
Q9. What are the applications of Grocott-Gomori’s Methenamine Silver Staining?
A. It is used for fungal detection, Pneumocystis identification, studying fungal morphology, detecting filamentous bacteria, and screening cytology fluids and research samples.
Q10. What are the pitfalls of Grocott stain?
A. It may stain neutrophils, mucin, melanin, glycogen and bacteria, causing confusion. Over-oxidation or under-oxidation may hide fungi. Temperature variation can cause silver precipitation.
Q11. How does GMS stain compare to other fungal stains?
A. It is more sensitive than PAS and H&E because it gives higher contrast. Background staining is less due to chromic acid oxidation.
Q12. Who developed Grocott-Gomori’s methenamine silver stain?
A. It was developed by Grocott based on the earlier silver techniques of Gomori.
Q13. Can neutrophils stain with Grocott methenamine silver?
A. Yes. Neutrophils sometimes take the stain and appear black.
Q14. How can black stained neutrophils be differentiated from fungi in GMS stain?
A. Neutrophils show a lobated nucleus inside the black cytoplasm, while fungi show a clear outer wall outline with pale or gray center.
Q15. What fixative is recommended for GMS staining?
A. Neutral buffered formalin is commonly recommended for routine GMS staining.
- Carson, F. L., & Richmond, R. (2010, August). Periodic acid cannot replace chromic acid in Grocott’s method for fungi. Biotechnic and Histochemistry. ResearchGate. https://www.researchgate.net/publication/45280037
- CK-12 Foundation. (n.d.). Flexi answers – Is Jones reagent the same as chromic acid? https://www.ck12.org/flexi/chemistry/lewis-acids-and-bases/is-jones-reagent-the-same-as-chromic-acid/
- Doan, C. (n.d.). Special stains – Which one, why and how? Part III: Microorganisms – Bacteria and fungi. Leica Biosystems. https://www.leicabiosystems.com/us/knowledge-pathway/special-stains-which-one-why-and-how-part-iii-microorganisms-bacteria-and-fungi/
- Ellis, R. (2024, January 26). Grocott’s methenamine silver staining protocol. IHC World. https://ihcworld.com/2024/01/26/grocotts-methenamine-silver-staining-protocol/
- iHisto. (n.d.). GMS stain service: High-sensitivity detection of fungi and microorganisms. https://www.ihisto.io/routine-histology/gms-stain
- Lees, G. E., Berridge, B. R., & Cianciolo, R. E. (n.d.). Renal biopsy stains. Clinician’s Brief. https://assets.ctfassets.net/4dmg3l1sxd6g/1MMOZtVchp2esCi3nvJ9DS/8eaba9851e4f98ebe2cc66b9208d1d85/renalbiopsystains-3402-article.pdf
- Life Worldwide. (n.d.). Histopathology of fungi. https://en.fungaleducation.org/histopathology/
- National Cancer Institute. (n.d.). 5.3 Grocott’s methenamine silver (GMS) staining procedure. https://ccrod.cancer.gov/confluence/download/attachments/119178148/Procedure%20Manual%205.3%20Special%20Stains%20-%20GMS%20Staining%20Procedure.pdf
- NephSIM. (n.d.). Case 43: Pathology. https://nephsim.com/case-43-pathology/
- Newcomer Supply. (n.d.). Grocott methenamine silver set, GMS [Product Catalog]. https://www.newcomersupply.com/product/grocott-methenamine-silver-set-gms/
- Newcomer Supply. (2025, March). GMS silver set staining procedure (Part 1142) [Technical Memo]. https://www.newcomersupply.com/documents/staining/procedures/GMS_Silver_Set.1142.pdf
- Siena, D., & Grizzle, W. (2020, October 15). The performance and uses of silver staining: The views of the histotechnologist and pathologist. National Society for Histotechnology. https://elearn.nsh.org/products/the-performance-and-uses-of-silver-staining-the-views-of-the-histotechnologist-and-pathologist
- Sigma-Aldrich. (n.d.). Methenamine silver plating kit acc. to Gomori for the detection of argent-affine structures in histological tissue. https://www.sigmaaldrich.com/US/en/product/mm/100820
- StainsFile. (n.d.). Gomori’s methenamine silver for glycogen and fungi. STEMCELL Technologies. https://www.stainsfile.com/protocols/gomoris-methenamine-silver-for-glycogen-and-fungi/
- The Grocott-Gomori’s Methenamine Silver (GMS) stain: A definitive review of principles, procedures, and diagnostic applications. (n.d.). [Source text provided].
- VitroVivo Biotech. (n.d.). VitroView™ Grocott-Gomori’s methenamine silver (GMS) stain kit. https://vitrovivo.com/product/vitroview-grocott-gomoris-methenamine-silver-stain-kit/
- WebPath. (n.d.-a). Basement membranes – Jone’s methenamine silver. University of Utah. https://webpath.med.utah.edu/HISTHTML/MANUALS/JONES.PDF
- WebPath. (n.d.-b). GMS – Methenamine silver – Grocott’s, modified. University of Utah. https://webpath.med.utah.edu/HISTHTML/MANUALS/GMS.PDF
- Wenk, P. A. (2010, Fall). Troubleshooting the GMS stain. On Stage, 29(4), 1, 4-5, 11-12. New York State Histotechnological Society. http://www.nyhisto.com/wp-content/uploads/2011/08/OnStage_V29_I4_1010.pdf
- Wikipedia. (n.d.-a). Grocott’s methenamine silver stain. https://en.wikipedia.org/wiki/Grocott%27s_methenamine_silver_stain
- Wikipedia. (n.d.-b). Hexamethylenetetramine. https://en.wikipedia.org/wiki/Hexamethylenetetramine
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.