Gel Staining Procedure for PAGE
A. Coomassie Blue staining
Staining protein gels using Coomassie Brilliant Blue R-250 is common to see proteins that are resolved using SDS-PAGE. It is extremely sensitive and suitable for long-term storage of gels.
Reagents Required
- Gel Fix solution (500 mL) [Methanol (M3641) 250 mL, Glacial acetic acid (695092) 50 mL, Water 200 mL]
- Coomassie solution [CBB R-250 (B6529) 0.1%, Methanol (M3641) 40%, Glacial acetic acid (695092) 10%, Filter the stain solution using Whatmann No. 1 filter paper.]
- Destain solution [Methanol (M3641) 50 mL, Glacial acetic acid (#695092) 35 mL]
- Gel storage solution [Glacial acetic acid (695092) 25 mL, Water 475 mL]
Procedure
- After electrophoresis is completed, place the gel into the tray made of plastic that contains Gel Fix Solution. Set the tray on a rolling table and set the proteins for two hours.
- Take the gel fix solution off and then add Coomassie solution. Set the table on a rocking surface and leave the gel staining for 2 to 4 hours.
- After staining Wash your gel several times using distillate water to eliminate any stain.
- Add the destaining solution to the gel. Place it on the table to wait for approximately 4 hours, until blue bands with a clean background are evident.
- After destaining, gels can be stored in gel storage solutions and photographed when needed.
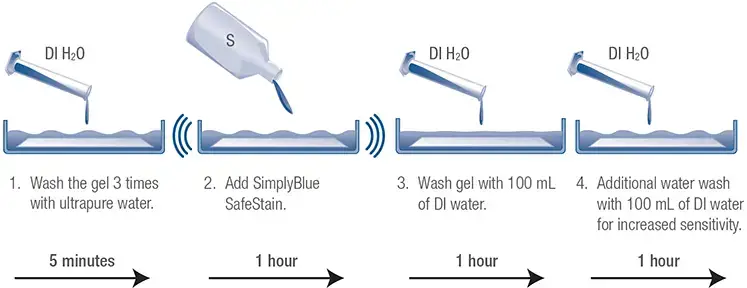
B. Silver Staining
We have a very sensitive silver stain detection kit for gels using SDS-PAGE.
Reagents Required
The following reagents are additionally needed:
- Fixing solution [Ethanol (E7023) 50 mL, Acetic acid 10 mL, Water 40 mL]
- 30% ethanol (E7023) solution
Procedure
- After the electrophoretic run, immerse the gel in the fixer for 40 minutes. An overnight soak in fixer will create a more clear background.
- Remove the fixing solution , and clean it off for 10 minutes with a 30% solution of ethanol and then rinse with ultrapure water for 10 minutes.
- Then, decant the water and soak the gel in the sensitizer solution for 10 minutes.
- Clean the sensitizer solution off and wash the gel 2 times with water, each time lasting 10 minutes.
- Discard the water and soak in the gel 10 minutes in the silver solution.
- Dissolve silver in a solution and rinse the gel with water for 1.5 minutes.
- Then, discard the water and then immerse the gel in the solution of developer for 3-7 minutes.
- Add 5 mL stopping solution in the solution for the developer, and allow to incubate for 5 minutes. Remove the stop solution/developer and rinse the gel with ultrapure water for about 15 minutes.
- The gel is able to be photographed and then kept in clean, ultrapure water.
To double stain you can stain the gel with CBB R-250 and then silver stain using the methods in the previous paragraphs.
The Fluorescent Stains are: We provide the fluorescent SYPRO and Lucy staining for electrophoresis of proteins. The gels can be submerged in the stain’s fluorescent light in dark , or the gel could mix with buffer cathode in the electrophoresis.
Reversible Gel Staining
Reversible gel staining allows the user to go on to the western-blotting process of protein immediately following SDS-PAGE.
A. R-PROB staining
R-PROB is an exclusive stain that detects proteins on gels as well as western blots.
Reagents Required
- Reversible Protein Detection Kit for Membranes and Polyacrylamide Gels (RPROB)
- Fixing solution (F7264)
- 10% acetic acid
- EDTA 50 mM
Procedure
- Submerge the gels following electrophoresis in the fixing solution 20 minutes. Repeat this process two times.
- Rinse the gel in the water two times, each time lasting 30 minutes.
- Incubate the gel in the staining solution for 20-40 minutes with gentle stirring.
- Get rid of any stain using 10 percent Acetic acid.
- To remove staining remove the gel, clean it with EDTA then follow by two washes lasting 15 minutes each in either water or a fixing solution.
B. Copper staining
To verify the movement as well as separation of protein the gel can be stained using the use of a reversible stain like CuCl2.
Reagents Required
- 0.3 M CuCl2 solution
- 0.25 M Tris and 0.25 M EDTA solution
Procedure
- Rinse the gels following electrophoresis in distillate water for a maximum of 30 minutes.
- The gel should be immersed within a 0.3 M CuCl2 solution for 10 min.
- Rinse the area with de-ionized, water.
- Proteins can be visualized as clear areas on blue backgrounds.
- To remove the stain completely to make a western blot clean the stained gels with 0.25 M Tris solution and 0.25 M EDTA solution, pH 9, repeatedly.
- Transfer the gel with the stained area to the transfer buffer prior to starting the transfer set-up.