What is gas chromatography?
Gas chromatography (GC) is a sophisticated technique that separates, identifies, and quantifies chemical components in complex mixtures. It operates on the principle of distributing the components between a mobile phase and a stationary phase.
In GC, the mobile phase is an inert gas like helium, and it acts as a carrier for the sample through a column. In both gases and liquids, the sample is first vaporized in the injection port, and then it flows through the column with the help of carrier gas.
The stationary phase lines the interior of the column. It may be liquid or solid. Along its way down through the column, parts of the sample will interact differently with the stationary phase and separate according to whether and how it mingled with the stationary phase.
This method especially works well when studying small molecular weight compounds. The use of gas chromatography extends to all fields such as medicine, checking the environment, crime investigation, and beauty products. Its use separates as well as detects gases. This is very different from other chromatography methods.

Definition of Gas chromatography
Gas chromatography (GC) is an analytical technique used to separate, identify, and quantify volatile compounds in a mixture by passing them through a column with a stationary phase and a carrier gas.
Principle of Gas chromatography
Gas chromatography is an analytical technique primarily based on a partitioning mechanism, whereby different components of the analyte distribute between two distinct phases. These consist of the mobile phase, formed by an inert gas, moving through the sample column, while the stationary phase consists of an ultrathin layer of liquid or solid coat covering the walls within which the column itself is formed.
These will be the compounds that have a higher affinity to the stationary phase, will be retained longer within the column, and thus it takes longer to elute. Conversely, those compounds that have a higher affinity to the mobile phase, move more rapidly through the column and elute early. Essentially, it depends on intermolecular interactions as well as on polarity that can be controlled to achieve even better separations.
In a heated injection port, the sample is first vaporized. This vaporized sample is carried by the carrier gas into the column. This sample continuously experiences adsorption and desorption by the stationary phase throughout the column.
Thus, the intricate dance of these adsorptions and desorptions separates the solutes into individual zones according to their partition coefficients. Subsequently, each eluting solute emerges at different times, yielding sharp peaks resembling the Gaussian curve in an ideal scenario.
The solutes, which are collected and exit the column, will be detected and recorded as a series of signals on a detector. These peaks, in regard to time, height, width, and area, are analyzed to yield quantifiable data on components within the sample. Such detailed partitioning and separation allow for the appropriate identification and measurement of volatile compounds in complex mixtures.
How does gas chromatography work? (Mechanism of Separation in Gas Chromatography)
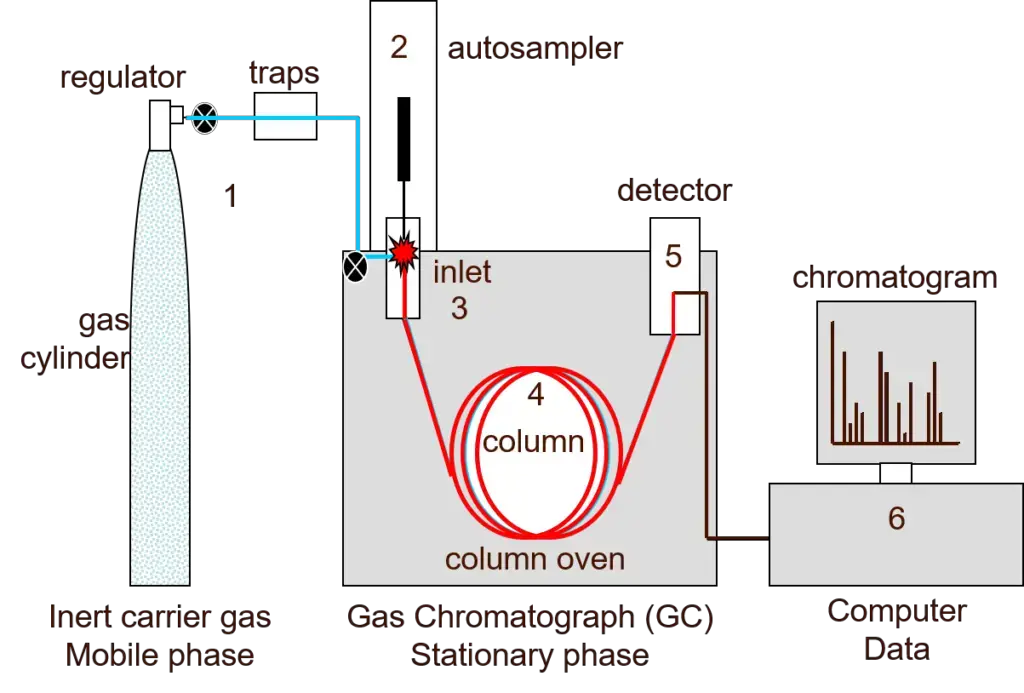
GC is a powerful technique of separating and analyzing volatile compounds within a sample. The process of separation has critical steps, and each one needs to be carried out carefully to achieve accurate separation and detection.
- Preparation of Sample- The sample is introduced into the gas chromatograph through the injection port. This can either be done through a syringe or an autosampler. The injection port contains a septum which allows for injection of the sample without the carrier gas escaping. After injection, the sample rapidly vaporizes if it has not already in its gaseous state.
- Mobile Phase or Carrier Gas – The carrier gas, typically an inert gas such as helium or nitrogen, is used as the mobile phase. The mobile phase carries the vaporized sample molecules throughout the GC instrument with no chemical interaction with either the sample or other parts of the instrument. The flow rate and type of carrier gas can be changed based on what the analysis requires.
- Analytical Column (Separation Process)- The vaporized sample enters the analytical column, which is a long and thin tube made of fused silica or metal. The inside walls of the column are covered with a stationary phase, usually liquid; and they can consist of polyethylene glycol (PEG) or polydimethylsiloxane (PDMS). The sample elements are separated along the column, depending on how they interact with the stationary phase.
- Stationary Phase Interactions: Nonpolar stationary phases, such as dimethylpolysiloxane, allow for the separation of nonpolar compounds. Polar stationary phases, such as polyethylene glycol, are usually used for compounds that are polar and, therefore, exhibit hydrogen bonding or acids and alcohol. The selected stationary phase mainly depends on volatility and functional groups of the compounds to be analyzed.
- Column Oven- The analytical column is in a column oven that heats to facilitate elution of the less volatile compounds. It is possible to program the temperature of the oven to vary over time, hence enhancing the separation of different components in the sample.
- Component Detection– When the eluted components of the sample get into the detector, it produces a response by the chemical nature of each one of them. Types of Detectors:
- Flame Ionization Detector (FID): Responds to species containing C-H bonds.
- Electron Capture Detector (ECD): Very sensitive to electron-capturing species, particularly halogenated species.
- Element-Specific Detectors: Sensitive species, which are associated with particular elements like sulfur, nitrogen, or phosphorus.
- The detector produces a signal for each compound eluting from the column, thus producing a chromatogram—a plot of signal intensity versus time.
- Data Acquisition and Analysis – The detector signals are recorded by software in a computer, which creates a chromatogram. The chromatogram provides numerical data, such as retention time, peak height, width, and area for each part. This data helps in identifying and measuring the parts in the sample.
- Modes of Operation –
- Split Mode: This mode is used for samples with high concentration, where only some of the sample goes into the column to avoid overload.
- Splitless Mode: This is used for low concentration samples where the entire vaporized sample is transferred into the column, thus ensuring maximum sensitivity.
Instruments of Gas Chromatography
| Part | Function |
|---|---|
| Carrier Gas System | Delivers an inert carrier gas (e.g., Helium, Nitrogen) at a consistent pressure and flow rate to transport the sample through the column. |
| High-Pressure Cylinder | Stores the carrier gas under high pressure for use in the gas chromatograph. |
| Pressure Regulators & Flow Meters | Ensures the carrier gas is delivered at the correct pressure and flow rate, crucial for consistent chromatographic performance. |
| Sample Injection System | Introduces the sample into the carrier gas stream. Liquid samples are vaporized before entering the column. |
| Heated Metal Block | Vaporizes liquid samples by heating, ensuring they are in the gas phase before entering the separation column. |
| Separation Column | The heart of the gas chromatograph, where the components of the sample are separated based on their interactions with the stationary phase and carrier gas. |
| Stationary Phase | Coats the inside of the column, interacting with the sample components to separate them based on their chemical properties. |
| Liquid Phases | Various liquid coatings within the column used to achieve separation; choice depends on the nature of the analytes (non-polar, intermediate, polar, etc.). |
| Support Materials | Provide a surface for the stationary phase, ensuring efficient interaction with the sample while maintaining column integrity. |
| Detector System | Senses and measures the quantity of separated components as they exit the column, generating a signal proportional to the concentration or mass of the analytes. |
| Temperature Control System | Maintains a consistent temperature within the column, essential for reproducible separation and analysis of components. |
| Flow Rate Control System | Regulates the flow rate of the carrier gas through the column, ensuring consistent operation and accurate separations. |
| Recorder & Data Processing System | Captures and processes the detector’s signal, producing a chromatogram that visually represents the separation and quantification of the sample components. |
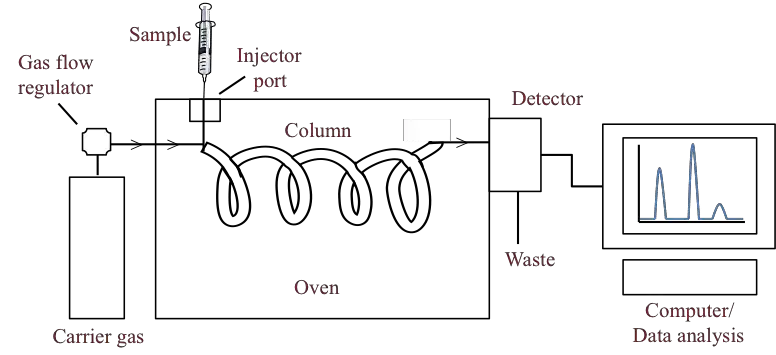
Below is a detailed overview of the key parts of a gas chromatograph, emphasizing their roles in the chromatographic process.
1. Carrier Gas System
In gas chromatography (GC), the carrier gas plays a pivotal role as the mobile phase. Its primary function is to transport the sample through the chromatographic column, facilitating the separation and analysis of the sample components. The selection of carrier gas influences the efficiency, resolution, and overall performance of the chromatographic system. Below are the commonly used carrier gases, their characteristics, and considerations for their use:
Common Carrier Gases
- Helium (He):
- Characteristics:
- Inert: Helium does not react with the sample or the column material, ensuring stable operation.
- Low Viscosity: This property contributes to high column efficiency and resolution.
- Compatibility: It works well with most types of detectors.
- Applications:
- General Use: Suitable for a wide range of applications, including both routine and specialized analyses.
- Efficiency: Provides excellent separation efficiency and resolution.
- Characteristics:
- Hydrogen (H2):
- Characteristics:
- Low Viscosity: Allows for faster analysis times compared to other gases.
- High Diffusion Coefficient: Facilitates rapid movement through the column.
- Flammable: Requires stringent safety measures due to its flammability.
- Applications:
- Low Molecular Weight Compounds: Effective for analyzing hydrocarbons and volatile organic compounds (VOCs).
- Enhanced Speed: Provides quicker analysis but necessitates careful handling and safety precautions.
- Characteristics:
- Nitrogen (N2):
- Characteristics:
- Inert: Suitable for analyzing non-reactive or thermally stable compounds.
- Cost-Effective: Less expensive than helium and hydrogen, making it economical for routine analyses.
- Versatility: Usable with most detectors, though not ideal for detectors requiring more easily ionizable gases.
- Applications:
- Routine Analyses: Commonly used in everyday applications due to its affordability.
- Detector Compatibility: Can be used with most GC detectors except flame ionization detectors (FID).
- Characteristics:
- Argon (Ar):
- Characteristics:
- Higher Viscosity and Density: Results in longer retention times compared to helium.
- Inert: Similar to helium and nitrogen in its non-reactivity.
- Applications:
- Specialized Uses: Often used in specific applications, including in gas chromatography-mass spectrometry (GC-MS) systems.
- Retention Time: May influence the separation profile due to its higher viscosity.
- Characteristics:
Factors Influencing Carrier Gas Selection
- Column Compatibility:
- The carrier gas should be compatible with the column’s material and the stationary phase to ensure optimal performance and prevent damage.
- Detector Type:
- The choice of carrier gas must align with the requirements of the detector. For instance, detectors like flame ionization detectors (FID) may not work well with nitrogen.
- Analyte Properties:
- The physical and chemical properties of the analytes, such as their volatility and reactivity, can affect the choice of carrier gas.
- Safety Considerations:
- Safety is a critical factor, particularly for flammable gases like hydrogen. Appropriate safety measures must be in place when handling and using these gases.
- Cost and Availability:
- Economic considerations and the availability of gases can influence the choice, with nitrogen often being a cost-effective option for routine analyses.
2. Separation Column
- Column Structure: The separation column is the core of gas chromatography. It is often made of metal, coiled into various shapes (e.g., U-shaped, spiral), and can withstand high temperatures.
- Column Packing: The column is packed with a stationary phase, which interacts with the components of the sample to separate them based on their different affinities to the stationary and mobile phases.
- Swagelok Fittings: These fittings simplify the insertion and removal of the column, ensuring a secure and leak-proof connection.
3. Sample Injection System
Sample injection is a fundamental step in gas chromatography (GC), pivotal for ensuring accurate and reproducible results. This process involves introducing a prepared sample into the chromatographic system, where it is then analyzed. The choice of injection method impacts the quality of the data obtained, influencing factors such as sensitivity, precision, and the ability to handle various sample types.
Common Sample Injection Methods
- Split Injection:
- Overview: In split injection, a fraction of the sample is diverted away from the column via a split vent.
- Application: This method is used when analyzing samples with relatively high concentrations.
- Function:
- Fractional Introduction: Only a portion of the sample enters the column, reducing the risk of overloading.
- Column Protection: Minimizes potential damage to the column caused by high sample concentrations.
- Details:
- Split Ratio: The ratio of the split vent flow to the column flow determines how much of the sample is diverted.
- Suitability: Ideal for situations where the sample concentration is high, requiring only a small amount for effective analysis.
- Splitless Injection:
- Overview: In splitless injection, the entire sample is introduced into the column without any diversion.
- Application: This technique is suitable for trace analysis where high sensitivity is essential.
- Function:
- Maximum Sample Transfer: Ensures that all of the sample is transferred to the column, which improves detection limits.
- Enhanced Sensitivity: Useful for detecting low concentrations of analytes.
- Details:
- Injection Port Design: Often involves a heated port that vaporizes the entire sample before introduction.
- Use Case: Preferred for analyzing trace-level compounds where every molecule is critical.
- On-Column Injection:
- Overview: On-column injection, or direct injection, involves introducing the sample directly onto the column without prior vaporization.
- Application: This method is ideal for compounds that are thermally labile or have high boiling points.
- Function:
- Direct Application: Reduces the risk of decomposition or adsorption that can occur during vaporization.
- Sample Integrity: Preserves the sample’s original state for accurate analysis.
- Details:
- Sample Compatibility: Suitable for compounds that might degrade or adsorb during the heating process.
- Injection Technique: Requires precise control to avoid sample loss or column contamination.
- Programmable Temperature Vaporization (PTV) Injection:
- Overview: PTV injection provides controlled vaporization of large sample volumes by initially trapping the sample in a cooled liner and then heating it.
- Application: This technique is employed for samples that need enhanced sensitivity and when dealing with large sample volumes.
- Function:
- Controlled Vaporization: Allows for the introduction of large volumes by first trapping the sample and then rapidly heating it.
- Improved Sensitivity: Enhances the detection of trace components by ensuring complete vaporization.
- Details:
- Liner Design: The sample is initially held in a cooled liner, which is then heated to vaporize the analytes.
- Volume Handling: Effective for samples with higher concentrations or when large sample sizes are required.
Optimization of Injection Parameters
- Injection Volume: The amount of sample introduced affects the sensitivity and resolution of the analysis. Careful calibration is necessary to avoid overloading the column.
- Injection Speed: The rate at which the sample is introduced can influence peak shapes and resolution.
- Liner Choice: The selection of liners impacts the sample’s interaction with the injection port and can affect the overall results.
4. Liquid Phases
- Non-Polar Phases: These include paraffin, squalane, and silicone greases. Non-polar phases separate components primarily by boiling point.
- Intermediate Polarity Phases: These phases, like diethylhexyl phthalate, contain a polar group on a non-polar skeleton, allowing them to separate both polar and non-polar solutes.
- Polar Phases: Materials such as carbowaxes are used for separating highly polar compounds.
- Hydrogen Bonding Phases: Phases like glycol with high hydrogen bonding capabilities are used for specific separations, such as unsaturated hydrocarbons.
- Specific Purpose Phases: These phases involve chemical reactions with solutes to achieve separation, for example, using AgNO3 in glycol for unsaturated hydrocarbons.
5. Detector System
In gas chromatography (GC), detectors are crucial components that identify and quantify the separated analytes as they exit the chromatographic column. Each detector type has unique attributes and is selected based on the nature of the analytes, sensitivity needs, and specific analytical goals. Below is a detailed overview of commonly used detectors in GC:
a. Flame Ionization Detector (FID)
- Principle of Operation:
- Ionization Process: FID detects organic compounds by ionizing them in a hydrogen/air flame. The ionized molecules generate a current that is proportional to their concentration.
- Measurement: The detector measures the resulting ion current, providing a quantitative signal.
- Characteristics:
- Sensitivity: Highly sensitive to organic compounds, making it effective for trace analysis.
- Universality: Provides broad detection capability for various organic molecules.
- Applications: Commonly used in environmental, petrochemical, and pharmaceutical analyses.
- Advantages:
- High Sensitivity: Capable of detecting very low concentrations of organic compounds.
- Stable Performance: Offers reliable and consistent results.
b. Thermal Conductivity Detector (TCD)
- Principle of Operation:
- Thermal Conductivity Measurement: TCD detects changes in thermal conductivity as analytes elute from the column. The detector measures the difference in thermal conductivity between the carrier gas and the analyte.
- Characteristics:
- Nondestructive: Does not alter the analytes, preserving their integrity.
- Versatility: Can detect both inorganic and organic compounds.
- Applications: Useful for applications where non-destructive analysis is required, such as in the detection of gases and volatile compounds.
- Advantages:
- Wide Range: Capable of detecting a broad spectrum of compounds.
- Stability: Maintains consistent performance across a variety of conditions.
c. Electron Capture Detector (ECD)
- Principle of Operation:
- Electron Capture: ECD detects compounds that capture electrons, such as halogenated compounds and some pesticides. The detector measures a decrease in current caused by the capture of electrons by analyte molecules in the presence of a radioactive beta-emitting source.
- Characteristics:
- High Sensitivity: Extremely sensitive to compounds with electron-capturing properties.
- Selectivity: Ideal for detecting specific classes of compounds.
- Applications: Frequently used in environmental monitoring and food safety testing.
- Advantages:
- High Detection Sensitivity: Effective for detecting trace levels of specific compounds.
- Specificity: Provides selective detection for compounds with particular chemical properties.
d. Mass Spectrometer (MS)
- Principle of Operation:
- Ionization and Fragmentation: MS ionizes the separated analytes and fragments them. The resulting ions are analyzed based on their mass-to-charge ratios.
- Analysis: Provides detailed information on the molecular structure and composition of the compounds.
- Characteristics:
- Versatility: Combines separation with identification and quantification capabilities.
- Resolution: Offers high-resolution analysis and specific identification of compounds.
- Applications: Widely used in research and complex mixtures where detailed compound identification is required.
- Advantages:
- Detailed Analysis: Provides comprehensive data on the molecular composition of the analytes.
- High Specificity: Enables precise identification and quantification.
e. Flame Photometric Detector (FPD)
- Principle of Operation:
- Photometric Emission: FPD detects specific elements, such as sulfur and phosphorus, by ionizing analytes in a hydrogen/air flame and measuring the resulting photometric emission.
- Characteristics:
- Element-Specific: Selective for elements with distinct photometric emission characteristics.
- Applications: Useful for analyzing compounds containing sulfur or phosphorus, such as in petrochemical analyses.
- Advantages:
- Selective Detection: Targets specific elements, providing focused analytical capabilities.
- Sensitivity: Effective for detecting trace amounts of elements.
Additional Detectors
- Nitrogen-Phosphorus Detector (NPD): Sensitive to nitrogen and phosphorus-containing compounds, similar to FPD but specifically targeting these elements.
- Photoionization Detector (PID): Detects compounds by ionizing them with ultraviolet light, useful for detecting volatile organic compounds.
- Electron Impact (EI) and Chemical Ionization (CI) Detectors: Often combined with mass spectrometry to enhance analytical capabilities.
6. Support Materials
- Inert Supports: The support material must be inert to prevent reactions with the stationary phase or analytes. It must immobilize a large volume of liquid phase as a thin film over its surface.
- Surface Area: A high surface area is critical for rapid equilibrium between the stationary and mobile phases.
- Diatomaceous Earth and Glass Beads: These are common supports, with diatomaceous earth being treated to create larger particle aggregates and glass beads providing low surface area and porosity.
- Porous Polymer Beads: These differ in the degree of cross-linking of styrene with alkyl-vinyl benzene and are stable up to 250°C.
7. Recorder and Data Processing System
- Recorder: The recorder is typically a 10 mV full-scale device equipped with a fast-response pen to capture the signal from the detector in real-time. It may be coupled with an integrator for more detailed data analysis.
- Data Processing System: Modern gas chromatographs are equipped with computer systems that process the data from the detector and display a chromatogram, which is a graphical representation of the detector’s response over time. This chromatogram is essential for analyzing the separated components.
8. Temperature Control System
- Column Oven: The column oven maintains a constant temperature throughout the column to ensure consistent separation of components. The temperature can be adjusted to optimize the separation process for different types of samples.
9. Flow Rate Control System
- Flow Controllers: The flow rate of the carrier gas is precisely controlled to maintain consistent conditions within the column. This is crucial for reproducibility and accuracy in the chromatographic process.
Stationary Phase in Gas Chromatography System
The stationary phase is a fundamental component in gas chromatography (GC) that plays a crucial role in the separation of sample components. It interacts with the sample molecules as they traverse the chromatographic column, facilitating their differential separation based on their interactions with the stationary phase. The stationary phase can be either a solid material or a liquid film adhered to a solid support. Understanding the nature and function of the stationary phase is essential for optimizing separation and achieving accurate analysis.
Types of Stationary Phases
- Gas-Solid Chromatography (GSC):
- Overview: In GSC, the stationary phase consists of a solid material packed into the column.
- Function:
- Adsorption: The sample molecules adhere to the surface of the solid stationary phase.
- Separation Mechanism: Differences in the affinity of molecules for the solid phase result in separation based on adsorption properties.
- Common Materials:
- Porous Polymers: These materials provide a high surface area for adsorption.
- Silica Gel: Known for its high surface area and polarity, often used for a wide range of compounds.
- Molecular Sieves: Used for separating molecules based on size.
- Activated Charcoal: Effective for adsorbing a variety of organic compounds.
- Considerations:
- Sample Affinity: The choice of solid material depends on the specific interactions required for the analysis.
- Gas-Liquid Chromatography (GLC):
- Overview: In GLC, the stationary phase is a liquid film coated onto a solid support material.
- Function:
- Partitioning: Sample molecules partition between the liquid film and the carrier gas.
- Combination of Adsorption and Partitioning: The separation occurs due to both adsorption onto the liquid film and partitioning between the liquid and the mobile phase.
- Common Liquid Phases:
- Polyethylene Glycol (PEG): A high-boiling liquid often used for polar analytes.
- Polydimethylsiloxane (PDMS): A nonpolar liquid suitable for a range of nonpolar compounds.
- Cyanopropylphenyl: Used for compounds that can interact via π-π interactions.
- Considerations:
- Chemical Bonding: The liquid phase must be chemically bonded to the solid support to remain stable during analysis.
Selection of Stationary Phase
The choice of stationary phase is guided by several factors:
- Analyte Properties:
- Polarity: Polar stationary phases are chosen for polar analytes, while nonpolar phases are suited for nonpolar compounds.
- Volatility: The boiling point of the stationary phase should be high enough to avoid volatilization during the analysis.
- Molecular Size: The stationary phase should be selected to accommodate the size of the analytes, particularly in GSC.
- Separation Efficiency:
- Selectivity: Different stationary phases offer varying degrees of selectivity, impacting the resolution of the separation.
- Efficiency: The stationary phase must provide efficient separation to achieve clear and distinct peaks in the chromatogram.
The procedure of Gas Chromatography
Gas chromatography (GC) is a powerful analytical technique used to separate and analyze compounds within a mixture. The process involves several key steps, each critical to achieving accurate results. Here is a detailed, step-by-step procedure for gas chromatography:
Step 1: Sample Injection and Vaporization
- Sample Preparation:
- Injection: A small volume of the liquid sample is drawn into a syringe. This step is crucial for accurate analysis, as the volume and consistency of the sample can impact results.
- Injection Port: The syringe needle is inserted into the heated injection port of the gas chromatograph. Rapid injection is essential to ensure that the entire sample is introduced into the system at once, preventing variations in sample introduction time.
- Vaporization:
- Temperature Setting: The injection port temperature is set above the boiling points of the sample components. This ensures that the sample components vaporize completely, forming a gas phase suitable for analysis.
- Mixing with Mobile Phase: The vaporized sample components are then mixed with the inert carrier gas (mobile phase). This mixture is carried through the chromatographic column.
Step 2: Separation in the Column
- Separation Mechanism:
- Interaction with Stationary Phase: As the sample mixture moves through the column, components interact differently with the stationary phase, which may be a solid or a liquid coated on a solid support.
- Retention Time: Components that strongly adsorb or bind to the stationary phase will have longer retention times (Rt), spending more time in the column. Conversely, components that interact weakly will have shorter retention times and exit the column earlier.
- Effects of Polarity:
- Polar vs. Non-Polar Columns: In a polar column, more polar components (e.g., component A) will have longer retention times compared to less polar components (e.g., component B). In a non-polar column, this relationship is reversed, with more polar components eluting faster than less polar components.
Step 3: Detecting and Recording Results
- Detection:
- Timing of Detection: Components exit the column and reach the detector at different times based on their retention times. The detector identifies each component as it elutes.
- Signal Generation: The detector generates a signal proportional to the amount of each component present. This signal is then sent to a data recording system.
- Data Recording:
- Chart Recorder: The detector’s signal is recorded by a chart recorder, producing a chromatogram. This chromatogram displays peaks corresponding to the different components.
- Peak Identification: Components that exit the column first are represented by the initial peaks on the chromatogram, while those eluting later produce peaks further along the chart.
How do you read a chromatogram and what does it tell you?
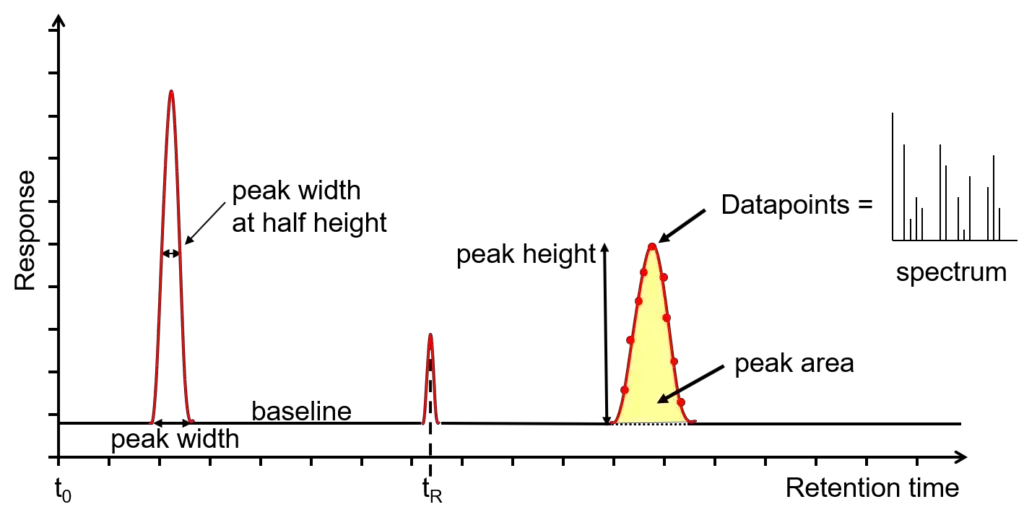
A chromatogram is a graphical representation of the separation process in gas chromatography (GC) or gas chromatography-mass spectrometry (GC-MS). Understanding how to interpret this data is crucial for analyzing both the performance of the chromatographic system and the qualitative or quantitative characteristics of the sample. Here is a detailed guide to reading a chromatogram and what it tells you:
Components of a Chromatogram
- X-Axis: Retention Time
- Definition: The x-axis represents retention time, which is the time elapsed from the injection of the sample into the GC until the analyte exits the column. Each analyte’s retention time is measured from the sample injection time to the apex of the peak.
- Significance: Retention time is used to identify the components of the sample. Different compounds have distinct retention times based on their interaction with the stationary phase and the mobile phase.
- Y-Axis: Detector Response
- Definition: The y-axis shows the response from the detector, which is proportional to the concentration of the analyte. This response is usually measured in terms of peak height or area.
- Significance: The detector response provides information about the amount of each analyte present in the sample. Higher peaks indicate higher concentrations.
- Baseline
- Definition: The baseline is the flat line representing the detector’s response when no analytes are being eluted. It is a combination of electrical noise and chemical noise from the system.
- Significance: A stable and low baseline indicates good system performance. An elevated or fluctuating baseline may suggest issues such as contamination, equipment malfunction, or inadequate maintenance.
Peak Analysis
- Peak Height and Area
- Height: Measured from the baseline to the peak’s apex. While useful, peak height is less reliable for quantitative analysis due to its susceptibility to variations.
- Area: Calculated by integrating the peak’s area under the curve. Peak area is preferred for quantitation as it provides a more accurate measure of analyte concentration, less affected by peak broadening.
- Peak Width
- Width at Baseline: The width of the peak at the baseline level, indicating the total spread of the analyte.
- Width at Half Height: The width of the peak at half its maximum height, providing insight into peak shape and resolution.
- Significance: Narrow, sharp peaks generally indicate better resolution and sensitivity. Wider peaks suggest band broadening, which can affect both the accuracy and resolution of the analysis.
- Peak Shape
- Gaussian Peaks: Ideally, peaks should be symmetrical and Gaussian in shape, with a sharp peak and symmetrical tails.
- Peak Tailing: When the peak tail is wider than the front, it may indicate issues such as column activity or dead volume in the system.
- Peak Fronting: When the peak front is wider, it may suggest column overload or issues with sample injection.
- Number of Data Points
- Ideal Range: A peak should ideally have 15-25 data points. This range ensures accurate peak shape representation without compromising signal-to-noise ratio.
- Too Few Data Points: Fewer data points can lead to inaccurate peak integration and poor resolution.
- Too Many Data Points: Excessive data points can increase noise and reduce sensitivity.
For GC-MS Data
- Mass Spectra
- Additional Dimension: In GC-MS, each data point on the chromatogram corresponds to a mass spectrum, providing a third dimension of data.
- Significance: The mass spectra allow for detailed identification and quantification of compounds based on their mass-to-charge ratios, enhancing the analytical capabilities of GC-MS.
What informations chromatogram tells you ?
A chromatogram is a crucial tool in gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) that conveys several important types of information about the sample being analyzed. Understanding how to interpret this data is essential for drawing meaningful conclusions from the chromatographic analysis. The information provided by a chromatogram can be categorized into three main areas:
1. The Nature of the Sample
- Peak Count
- Definition: The number of distinct peaks observed on a chromatogram corresponds to the number of different compounds present in the sample.
- Interpretation: For example, if a chromatogram displays three distinct peaks, it indicates that the sample contains at least three separate compounds. Conversely, a single peak suggests a more homogeneous sample, ideally confirming the purity of the substance under analysis.
- Peak Characteristics
- Retention Time (tR): Each peak on the chromatogram corresponds to a specific retention time, which is the time taken for a compound to travel through the chromatographic column to the detector.
- Significance: By evaluating the retention times, one can assess the complexity of the sample. A more complex sample will have more peaks, whereas a simpler or purer sample will have fewer peaks.
2. The Identity of the Sample
- Retention Time Matching
- Definition: The retention time of a peak can be compared to known retention times of reference compounds to identify the substances in the sample.
- Considerations: Accurate identification requires that the conditions of the chromatographic run (e.g., carrier gas flow rate, temperature, and column length) be consistent with those used for the reference compounds. Variations in these conditions can affect the retention time.
- Reference Materials and Literature Values
- Procedure: Injecting known reference materials under the same conditions as the sample allows for direct comparison of retention times.
- Significance: Matching the retention times of peaks to those of known compounds helps in identifying the substances present in the sample. GC-MS further enhances identification by providing mass spectral data for each compound, offering more precise identification.
3. The Amount of Sample
- Peak Area
- Definition: The area under each peak on the chromatogram is proportional to the concentration of the corresponding compound in the sample.
- Measurement: Modern GC systems use software to integrate the area under each peak, providing quantitative data on the concentration of the compounds.
- Quantitative Analysis
- Relative Quantification: By comparing the peak areas of different compounds, the relative amounts of these compounds can be determined.
- Absolute Quantification: To calculate actual concentrations, a standard calibration curve is used. This curve is derived from known standards and allows for the conversion of peak areas into concentration values.
Types of Gas Chromatography
Gas chromatography (GC) is a powerful analytical technique used to separate and analyze compounds in a mixture. It operates based on the interaction between the stationary and mobile phases. The primary types of gas chromatography are Gas-Solid Chromatography (GSC) and Gas-Liquid Chromatography (GLC). Each type has distinct applications and functionalities, which are detailed below.
1. Gas-Solid Chromatography (GSC)
Gas-solid chromatography involves a stationary phase that is in a solid state, while the mobile phase is a gaseous substance. The solid stationary phase is often coated onto the inner walls of the chromatographic column.
Key Points:
- Stationary Phase: The stationary phase is a solid material that interacts with the sample components.
- Mobile Phase: The mobile phase is a gas that moves through the stationary phase.
- Applications: GSC is used primarily for separating volatile components. Due to the low volatility and stability of the solid stationary phase, GSC can operate effectively at high temperatures.
Detailed Components:
- Column Construction: The stationary phase is applied to the interior surface of the column, which is typically a long, narrow tube.
- Separation Mechanism: Separation occurs based on the adsorption of sample components onto the solid phase. Components that adsorb strongly will elute later.
- Temperature Range: High-temperature capabilities make GSC suitable for compounds that require elevated temperatures for effective separation.
2. Gas-Liquid Chromatography (GLC)
Gas-liquid chromatography is a more commonly used form of GC where the stationary phase is a liquid that is either coated onto a solid support or immobilized on the inner surface of a capillary column. The mobile phase remains an inert gas, such as helium or nitrogen.
Key Points:
- Stationary Phase: The liquid stationary phase is adsorbed onto a solid support or coated onto the inner wall of a column.
- Mobile Phase: An inert gas, such as helium or nitrogen, acts as the mobile phase.
- Separation Principle: Separation is based on the different affinities of sample components for the liquid stationary phase and their volatility.
Detailed Components:
- Column Types: GLC columns can be packed with a solid support material or consist of a capillary tube with the stationary phase coated on the inner surface.
- Affinity and Volatility: Components with a higher affinity for the liquid stationary phase will interact more strongly and take longer to elute, leading to a longer retention time.
- Application Scope: GLC is widely used for separating a broad range of compounds, including those with varying volatilities and affinities.
Detection Methods
GC can also be categorized based on the type of detector employed, which is crucial for identifying and quantifying the separated compounds. Common detectors include:
- Flame Ionization Detector (FID): Measures ions formed during the combustion of organic compounds in a flame. It is highly sensitive and suitable for detecting hydrocarbons.
- Thermal Conductivity Detector (TCD): Detects changes in the thermal conductivity of the gas stream compared to the carrier gas. It is used for detecting a wide range of compounds.
- Electron Capture Detector (ECD): Sensitive to compounds that capture electrons, such as halogenated compounds, and is used in environmental analysis.
- Mass Spectrometry (MS): Provides detailed molecular information about the compounds by measuring their mass-to-charge ratio.
Applications of Gas Chromatography
- Chemical Analysis in Pharmaceuticals
- Analysis of Drug Compound: GC evaluates the chemical composition of drugs to assure the presence of the active constituents in the required concentrations.
- Purity Determination : This method provides a measure for pharmaceutical purity via the detection of impurities-the key to successful product quality and safety.
- Environmental Analysis
- Pollutant Detection: GC analyzes air, water, soil, and sediments for organic pollutants, an important tool for environmental contamination surveys and regulatory compliance monitoring.
- Contaminant Analysis: This technology determines and quantifies environmental pollutants with the aim of prevention and remediation of pollution.
- Food and Beverage Analysis
- Quality Control: GC analyzes foods and beverages for flavorings, additives, preservatives, adulterants or contaminants.
- Authenticity Testing: This technique authenticates food and beverages, including tracing the source of essential oils or detecting fraud.
- Forensic Analysis: GC is used in forensic science for the analysis of biological specimens such as blood, urine, and saliva and trace evidence such as hair and fibers to link substances to crimes.
- Toxicology: GC detects and measures the amount of compounds in forensic samples to help investigate poisoning and drug-related activities.
- Petrochemical Analysis: GC is used in analyzing petroleum products, crude oil, natural gas, and refinery gases, and hence in determining their composition and qualities to aid the refining and manufacture of new products.
- Specific Analyses
- Airborne Pollutants: GC identifies airborne pollutants such as VOCs and dangerous substances.
- Performance-Enhancing Drugs: In sports, GC detects performance-enhancing drugs in urine to ensure fair competition.
- Oil Spills: The technique analyzes oil spills; it determines contamination levels and helps in environmental responses.
- Essential Oils in Perfume: GC analyzes and formulates the essential oils of perfumes. This ensures the proper composition and quality of a perfume.
Advantages of Gas Chromatography
The significance of gas chromatography (GC) in analytical chemistry is derived from the following advantages:
- High Sensitivity
- Trace Analysis: GC can easily detect trace levels of compounds that are suitable for trace analysis at parts-per-billion or picomole levels.
- Detection of Minor Components: It is sensitive and useful in identifying minor components of complex mixtures.
- High Resolution
- Separation of Mixtures: GC is able to separate the most complex mixtures into single components, giving detailed information on compounds.
- Detailed Analysis: With high resolution, it is possible to identify and quantify with much accuracy.
- Rapid Analysis
- Speed: GC analyzes samples fast, usually within minutes, hence useful for applications that require rapid analysis.
- Efficiency in High-Throughput Settings: GC’s quick turnaround is ideal for processing many samples efficiently.
- Small Sample Usage
- Cost-Effective and Eco-Friendly: GC uses minimal sample amounts, saving costs and reducing environmental impact.
- Less Sample Preparation: The small volume decreases extensive prep needs.
- Good Selectivity
- Separation of Similar Compounds: GC successfully separates compounds with similar characteristics, which can be helpful in the identification of unknowns.
- Identification of Complex Mixtures: Selectivity is an important characteristic of distinguishing compounds with close resemblance.
- Wide Application
- Versatility: GC finds application in pharmaceuticals, environmental analysis, forensics, and clinical research.
- Versatility: It analyzes all kinds of samples and is therefore versatile across disciplines.
- Sophisticated Applications
- Long columns and high gas velocities speed up separation of compounds and thereby increase the efficiency of analysis. GC can function up to 500°C that enables it to analyze thermally stable materials. It can be relied on for continuous operations.
- Consistent Performance: GC is reliable and ideal for routine monitoring and industrial use because of its near-continuous operation.
- Environmental Monitoring: Its dependable operation makes it favored for environmental monitoring where consistent data is important.
- Suitability for Specific Molecules: GC effectively detects small, volatile molecules and non-aqueous solutions.
Limitations of Gas Chromatography
- Volatility Limits
- Volatile Compound Analysis: GC excels at volatile compound analysis. This method may fail to evaluate compounds with low vapor pressures or high boiling points.
- Exclude Non-Volatile Compounds: High-boiling-point or non-volatile compounds may require derivatization or other procedures to evaluate.
- Derivatization Needs
- Derivatization increases volatility and stability for GC analysis in certain chemicals.
- Error: Derivatization takes time and may introduce errors, lowering analysis accuracy.
- Degrading Column
- GC columns can be damaged by inappropriate handling or operation. Column deterioration might result from oven operation without carrier gas flow or improper syringes.
- Loss of Efficiency: Damaged columns impair efficiency and resolution, decreasing GC system performance.
- Peak Tailing
- Accuracy Impact of Peak Tailing: Certain compounds may experience peak tailing, where the peak extends beyond its intended form. This can distort quantitative measurements and lower analytical accuracy.
- Tailing peaks can make compound quantification inaccurate, leading to data misinterpretation.
- Instrument Care
- GC findings must be accurate and reliable, thus regular instrument maintenance is necessary. Parts are calibrated, cleaned, and replaced as needed.
- Proper maintenance prevents failures and extends GC equipment life.
- Thermal Degradation
- High Temperature Effects: The high temperatures necessary for GC operation might contribute to thermal deterioration of certain substances. This degradation alters analyte chemical composition, affecting results accuracy.
- Stability Under Operating Conditions: GC-analyzed compounds must be stable at high temperatures.
- Compound Specific Constraints
- Compounds examined must be stable under GC working conditions and have a vapor pressure higher than zero.
- Size Limitations: GC works best for compounds under 1,000 Da. Larger compounds may be difficult to vaporize and analyze.
- Salts can interfere with GC analysis, hence samples should be salt-free and ion-free.
- Reference Standard Comparison
- Reference Standards: To accurately quantify a sample, it is frequently required to compare it to a pure, suspected drug reference standard. Compare findings to guarantee dependability.
Chromatography-Mass Spectrometry (GC-MS)
Chromatography-Mass Spectrometry (GC-MS) is an advanced analytical technique that combines the separation capabilities of gas chromatography (GC) with the identification and quantification power of mass spectrometry (MS). This integrated method provides a comprehensive analysis of complex mixtures, offering detailed insights into the composition of samples.
Overview of GC-MS
- Gas Chromatography (GC):
- Function: Separates volatile components in a mixture based on their interactions with a stationary phase and their volatility.
- Mechanism: Compounds are vaporized and carried by an inert gas through a column coated with a stationary phase. As they travel through the column, they are separated based on their affinity for the stationary phase.
- Mass Spectrometry (MS):
- Function: Identifies and quantifies compounds by measuring the mass-to-charge ratio (m/z) of ions.
- Mechanism: Ions are generated, analyzed, and detected to produce a mass spectrum, which displays the relative abundances of ions against their m/z ratios.
Components and Functions of GC-MS
- Ionization:
- Purpose: Converts sample molecules into ions, enabling their analysis by the mass spectrometer.
- Methods:
- Electron Ionization (EI): Molecules lose an electron to form positive ions. It is suitable for compounds with moderate volatility.
- Chemical Ionization (CI): Uses a reagent gas to ionize the sample, often producing less fragmentation and more intact molecular ions.
- Mass Analysis:
- Purpose: Separates ions based on their mass-to-charge ratios.
- Types of Mass Analyzers:
- Quadrupole: Uses oscillating electric fields to filter ions by their m/z ratio.
- Time-of-Flight (TOF): Measures the time ions take to travel a fixed distance, with lighter ions reaching the detector faster.
- Orbitrap: Measures the frequency of oscillating ions trapped in an electrostatic field.
- Detection:
- Purpose: Measures the abundance of ions and records their m/z ratios.
- Techniques:
- Electron Multiplier: Detects and amplifies the signal of ions by converting them into an electrical current.
- Detector Array: Measures multiple ions simultaneously, providing a detailed mass spectrum.
Applications of GC-MS
- Qualitative Analysis:
- Identification: Provides structural information about compounds by analyzing their fragmentation patterns and m/z ratios.
- Characterization: Determines the molecular weight and structure of unknown compounds.
- Quantitative Analysis:
- Concentration Measurement: Quantifies the amount of each compound in a sample by comparing the area under the peaks in the mass spectrum to calibration standards.
- Sensitivity: Capable of detecting trace amounts of compounds due to the high sensitivity of the mass spectrometer.
- Environmental and Forensic Analysis:
- Detection of Pollutants: Identifies and quantifies environmental pollutants such as pesticides and hydrocarbons.
- Forensic Investigations: Analyzes substances in biological samples for forensic purposes, including drug testing and toxicology.
Common problems with gas chromatography
Several typical difficulties with gas chromatography (GC) can influence the instrument’s performance and reliability. Here are some of the most typical GC issues:
- Leaks: Leaks in the GC system can disturb the flow of the mobile phase (gas) and jeopardize the analysis’s accuracy and repeatability. Proper component installation and regular leak testing are critical for identifying and addressing any leaks that may develop.
- Tailing Peaks and Activity: Activity refers to the interaction of polar analytes with the column and liners, which results in tailing peaks in the chromatogram. Silanol-based groups on the glass column and liners can produce this problem, especially at trace amounts of more polar compounds. A buildup of dirt or pollutants within the system might also lead to activity-related issues. Regular system maintenance and cleaning, including the use of deactivated inlet liners, can assist reduce activity-related concerns.
- Catalytic Breakdown or Adsorption: The presence of catalytic materials or adsorptive surfaces inside the GC system might generate unwanted reactions or adsorption of analytes, resulting in irreproducible findings or loss of analyte peaks in some situations. To avoid these concerns, care should be made to reduce the presence of catalytic materials and to maintain a clean system.
- Issues with the Inlet: The inlet is an important location in GC since it is where the sample is injected, evaporated, and transported to the GC column. Problems with the intake might result in poor sample vaporization, insufficient transfer, or contamination, reducing the analysis’s accuracy and repeatability. Regular inlet maintenance, such as cleaning and changing consumables such as inlet liners, is required to keep the instrument in good operating order.
FAQ
What is gas chromatography?
Gas chromatography (GC) is an analytical technique used to separate and analyze the components of a mixture based on their interactions with a stationary phase and a mobile phase (carrier gas). It is widely used in various industries and research fields for qualitative and quantitative analysis.
How does gas chromatography work?
In gas chromatography, the sample is vaporized and injected into a column containing a stationary phase. The sample components interact differently with the stationary phase, causing them to separate as they travel through the column. The separated components are then detected and recorded.
What are the advantages of gas chromatography?
Gas chromatography offers fast separation, high temperature capabilities, and versatility in analyzing a wide range of compounds. It provides accurate results, even in trace-level analysis, and is particularly useful for analyzing volatile and non-polar substances.
What are the limitations of gas chromatography?
Some limitations of gas chromatography include the requirement for sample stability under GC conditions, limited applicability to volatile and low molecular weight compounds, the need for non-aqueous solutions, and the necessity of reference standards for accurate quantification.
What types of detectors are used in gas chromatography?
There are various detectors used in gas chromatography, including flame ionization detector (FID), thermal conductivity detector (TCD), electron capture detector (ECD), mass spectrometry (MS), and others. The choice of detector depends on the specific analytical needs and the nature of the analytes.
How is gas chromatography used in environmental analysis?
Gas chromatography plays a crucial role in environmental analysis, allowing the detection and quantification of pollutants in air, water, and soil samples. It is used to analyze volatile organic compounds (VOCs), pesticides, persistent organic pollutants (POPs), and other environmental contaminants.
Can gas chromatography be coupled with other analytical techniques?
Yes, gas chromatography can be coupled with other analytical techniques, such as mass spectrometry (GC-MS) or infrared spectroscopy (GC-IR). These hyphenated techniques provide additional information about the composition, structure, and properties of the analytes.
What is the importance of column selection in gas chromatography?
The selection of the appropriate column is crucial in gas chromatography. Different columns have different stationary phases, such as polar or non-polar, and varying lengths and internal diameters. The column choice depends on the analytes of interest and their interactions with the stationary phase.
How can I interpret a gas chromatogram?
Gas chromatograms consist of peaks representing separated analytes. The retention time indicates the time it takes for each analyte to elute from the column. The peak area or height provides information about the quantity of the analyte. Comparing peak patterns and retention times can aid in qualitative and quantitative analysis.
What are the applications of gas chromatography?
Gas chromatography finds applications in various fields, including environmental monitoring, pharmaceutical analysis, food and beverage industry, forensics, petrochemical analysis, quality control in manufacturing, and research and development. It is a versatile technique used for analyzing and identifying a wide range of compounds.
References
- Ardrey, R. E. (2017). Understanding and Troubleshooting Gas Chromatography. Elsevier.
- https://bitesizebio.com/28687/carrying-gas-chromatography/
- https://gentechscientific.com/overview-of-gas-chromatography-components/
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Instrumental_Analysis_(LibreTexts)/27%3A_Gas_Chromatography/27.02%3A_Instruments_for_Gas-Liquid_Chromatography
- https://www.creative-proteomics.com/blog/index.php/introduction-to-gas-chromatography-principles-characteristics-and-process/
- https://mvpsvktcollege.ac.in/wp-content/uploads/2022/11/3-TYGC.pdf
- https://www.drawellanalytical.com/what-are-4-key-components-of-a-gas-chromatography-system/
- https://bitesizebio.com/28687/carrying-gas-chromatography/
- https://www.geeksforgeeks.org/gas-chromatography/#instruments-of-gas-chromatography
- https://lab-training.com/gas-chromatography/
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Instrumentation_and_Analysis/Chromatography/Gas_Chromatography
- https://www.technologynetworks.com/analysis/articles/gas-chromatography-how-a-gas-chromatography-machine-works-how-to-read-a-chromatograph-and-gcxgc-335168
- https://www.jove.com/science-education/10187/gas-chromatography-gc-with-flame-ionization-detection