Enteropathogenic Escherichia coli (EPEC)
Enteropathogenic Escherichia coli (EPEC) are a group of strains of Escherichia coli that have been associated with diarrheal illnesses. The understanding of EPEC has evolved over time with the discovery of different mechanisms of pathogenesis. Here is a summary of the key points about EPEC:
- The term EPEC was introduced in 1955 to describe certain strains of E. coli that were implicated in infant diarrhea cases in the 1940s and 1950s. The definition of EPEC has been modified as additional serotypes were associated with infantile diarrhea.
- EPEC strains have been recognized as significant causes of infant diarrhea in both developed and developing countries.
- The pathogenic nature of EPEC strains was confirmed through human volunteer studies.
- The original definition of EPEC was based on serotyping and excluded strains associated with enterotoxins or Shigella-like invasiveness.
- Adherence to HEp-2 cells in cell culture, known as localized adherence (LA), was identified as a characteristic of EPEC strains. The presence of a 60-MDa plasmid, known as the EPEC adherence factor (EAF) plasmid, was associated with the ability to adhere in a localized pattern.
- Differences in adherence patterns led to the recognition of other categories of diarrheagenic E. coli, such as diffusely adherent E. coli (DAEC) and enteroaggregative E. coli (EAEC).
- EPEC strains were found to cause attaching and effacing (A/E) lesions, characterized by the loss of microvilli, intimate attachment of bacteria to the host, and the formation of pedestals at the attachment sites.
- EPEC strains are now defined based on their ability to cause diarrhea, produce A/E lesions, and the absence of Shiga toxins. They are classified into “typical” and “atypical” subtypes based on the presence or absence of the pEAF plasmid.
Advancements in techniques and a better understanding of EPEC strains’ genomes and virulence mechanisms have contributed to the current classification and characterization of EPEC.
Disease Caused by Enteropathogenic Escherichia coli (EPEC)
Enteropathogenic Escherichia coli (EPEC) is known to cause a specific type of diarrhea, particularly in infants and young children. Here are some important points regarding the diseases caused by EPEC:
- Infantile diarrhea outbreaks: EPEC is a frequent cause of outbreaks of diarrhea in infants. These outbreaks can occur in various settings, such as hospitals, daycare centers, or communities. The transmission of EPEC is often through contaminated food or water, person-to-person contact, or contact with contaminated surfaces.
- Sporadic diarrhea in adults: While EPEC primarily affects infants and young children, it can occasionally cause sporadic diarrhea in adults. In adults, EPEC infections may be associated with travel to regions where the disease is endemic or exposure to contaminated food or water sources.
- Common cause in children: EPEC is a significant cause of diarrhea in children under 5 years old, particularly in developing countries. The disease burden is higher in resource-limited settings, where factors such as poor sanitation, inadequate hygiene practices, and limited access to clean water contribute to the transmission and persistence of EPEC infections.
- Impact on child mortality: In developing countries, EPEC infections, along with other diarrheal diseases, contribute to high child mortality rates. After rotavirus infections, EPEC is considered one of the leading causes of death in children under 5 years old in these regions. The severity of EPEC-associated diarrhea can lead to dehydration and other complications if left untreated.
Mode of Transmission of Enteropathogenic Escherichia coli (EPEC)
The mode of transmission for Enteropathogenic Escherichia coli (EPEC) is primarily through the fecal-oral route. Here are the key points regarding the transmission of EPEC:
- Fecal-oral transmission: EPEC is transmitted when fecal matter containing the bacteria comes into contact with the mouth of another person. This can occur through various means, including contaminated hands, contaminated weaning foods or formula, or contact with contaminated fomites (objects or surfaces).
- Contaminated hands: Poor hand hygiene practices, such as inadequate handwashing after using the toilet or handling contaminated materials, can contribute to the transmission of EPEC. Infected individuals can contaminate their hands with EPEC present in their feces, and if proper handwashing is not followed, they can spread the bacteria to others through direct contact or by touching objects and surfaces.
- Contaminated weaning foods or formula: EPEC can contaminate weaning foods or formula, especially when prepared or stored under unhygienic conditions. Consumption of contaminated foods or drinks can lead to infection and subsequent transmission to others.
- Contaminated fomites: Fomites are objects or surfaces that can become contaminated with EPEC and serve as vehicles for transmission. Examples include toys, utensils, countertops, and bathroom fixtures. If these items are not properly cleaned and sanitized, they can contribute to the spread of the bacteria.
- Person-to-person spread: EPEC can be transmitted directly from one person to another through close contact. This can occur through activities such as caring for an infected individual, sharing utensils or food, or engaging in practices that involve oral-oral contact, particularly in settings such as households, childcare centers, or healthcare facilities.
- Reservoirs: Humans are the primary source of typical EPEC strains, meaning these strains primarily circulate among human populations. However, atypical strains of EPEC can also be found in a variety of animal hosts, making them potential reservoirs for transmission to humans.
Epidemiology of EPEC
The epidemiology of Enteropathogenic Escherichia coli (EPEC) is influenced by various factors such as incidence, age distribution, geographic region, and transmission patterns. Here are the key points regarding the epidemiology of EPEC:
- Incidence: The prevalence of EPEC infection can vary between different epidemiological studies due to variations in study populations, age groups, detection methods, and geographic regions. EPEC-induced diarrhea is primarily reported in children under 1 year of age, particularly in urban areas and among socioeconomically disadvantaged populations. The association between typical EPEC serotypes and infantile diarrhea was previously strong, but recent studies have shown a shift in the epidemiology of EPEC.
- Age distribution: Typical EPEC infections are predominantly observed in infants less than 6 months of age. The prevalence of EPEC decreases with age, possibly due to the loss of specific receptors or development of immunity in older children and adults.
- Changing epidemiology: In recent years, the proportion of atypical EPEC strains has increased and surpassed typical EPEC strains as a cause of childhood diarrhea. Atypical EPEC strains have been associated with diarrhea in both developing and developed countries. The prevalence of atypical EPEC strains can vary across regions and studies.
- Transmission and reservoirs: Typical EPEC is transmitted through the fecal-oral route, primarily through contaminated surfaces, contaminated weaning fluids, and human carriers. EPEC outbreaks among adults are rare but may occur through the ingestion of contaminated food and water. Humans, including symptomatic and asymptomatic children and adults, are the known reservoir for typical EPEC. In the case of atypical EPEC, these strains have been found in diarrheic as well as healthy animals and environmental sources. While some animal-associated serogroups have been identified, direct transmission from animals to humans has not been confirmed. Foods such as raw meats, pasteurized milk, meat samples, vegetables, and water have also been implicated as potential vehicles for atypical EPEC transmission to humans.
Pathogenesis of Enteropathogenic Escherichia coli (EPEC)
The pathogenesis of Enteropathogenic Escherichia coli (EPEC) involves a series of steps that result in the characteristic attaching and effacing (A/E) histopathology. Here is an overview of the key points regarding the pathogenesis of EPEC:
- Plasmid-mediated A/E histopathology: EPEC induces A/E lesions, which involve the disruption of the normal structure of microvilli on the surface of intestinal epithelial cells. This disruption leads to malabsorption and diarrhea.
- Non-toxigenic and non-invasive: Unlike other diarrheagenic E. coli strains, EPEC is non-toxigenic and non-invasive. It does not produce toxins that cause diarrhea, and it does not invade deeper layers of the intestinal tissue.
- Bacterial attachment and effacement: The infection begins with the attachment of EPEC to the epithelial cells of the small intestine. This initial attachment leads to the effacement (destruction) of the microvilli on the surface of the epithelial cells.
- Bundle-forming pili (BFP): Typical EPEC strains utilize bundle-forming pili, which are encoded by plasmids, for the initial aggregation and formation of microcolonies on the epithelial cell surface. However, these plasmids are not present in atypical EPEC strains.
- LEE pathogenicity island: The subsequent stages of attachment and destruction are regulated by genes encoded on the LEE (locus of enterocyte effacement) pathogenicity island. This island contains more than 40 genes that are responsible for the attachment of EPEC to host cells and the destruction of the host cell surface.
- Type III secretion system: EPEC employs a type III secretion system to actively secrete bacterial proteins into the host epithelial cells. These proteins play a role in manipulating the host cell machinery and establishing a favorable environment for the bacterium.
- Translocated intimin receptor (Tir): One of the secreted proteins, Tir, is inserted into the epithelial cell membrane and serves as a receptor for an outer membrane bacterial adhesin called intimin. The binding of intimin to Tir triggers a series of events, including the polymerization of actin, accumulation of cytoskeletal elements beneath the attached bacteria, loss of cell surface integrity, and eventual cell death.
- Formation of A/E lesions: The culmination of the pathogenic process is the formation of A/E lesions on the intestinal epithelium. These lesions disrupt the brush border epithelium, leading to increased secretion and watery diarrhea.
Clinical Presentation and Complications of Enteropathogenic Escherichia coli (EPEC)
Enteropathogenic Escherichia coli (EPEC) infections primarily affect children younger than 2 years old. The clinical presentation of EPEC infection is characterized by the following:
- Watery diarrhea: EPEC infection leads to the onset of watery diarrhea, which is the hallmark symptom. The diarrhea is typically non-bloody in nature.
- Vomiting: Vomiting is commonly associated with EPEC infection and is often seen alongside watery diarrhea.
- Fever: Patients with EPEC infection may experience fever as a systemic response to the infection.
The severity and duration of the symptoms can vary among individuals, but in some cases, the watery diarrhea can be severe and protracted, leading to dehydration and requiring hospitalization. The onset of symptoms can occur rapidly, sometimes within a few hours after ingesting EPEC-contaminated food or water.
While most EPEC infections resolve within a few days, some cases may experience persistent diarrhea that requires medical attention and hospitalization. Persistent diarrhea is characterized by the prolonged duration of watery stools and can lead to dehydration and nutritional deficiencies if not properly managed.
It is important to note that complications can arise from EPEC infections, especially in vulnerable populations such as infants and young children. Dehydration, electrolyte imbalances, and malnutrition are potential complications that can occur due to prolonged and severe diarrhea. Prompt medical intervention, including rehydration therapy and supportive care, is crucial in managing EPEC infections and preventing complications.
Overall, recognizing the clinical presentation of EPEC, especially in young children, is vital for early detection, appropriate treatment, and prevention of further complications associated with the infection.
EPEC virulence factors and genetics
Enteropathogenic Escherichia coli (EPEC) possesses various virulence factors and exhibits distinct genetic characteristics. Here are the key points regarding EPEC virulence factors and genetics:
- Localized adherence (LA): Typical EPEC strains display a characteristic pattern of adherence known as localized adherence (LA). This pattern is mediated by bundle-forming pili (BFP) associated with the EPEC adherence factor (EAF) plasmid. LA results in the formation of compact microcolonies on cell surfaces.
- Atypical adherence patterns: Atypical EPEC strains may exhibit variant adherence patterns such as the LA-like (LAL) pattern. The LAL pattern is characterized by the presence of loose compact microcolonies or clusters of bacteria. Some atypical EPEC strains also display other adherence phenotypes such as diffuse adherence (DA) and aggregative adherence (AA).
- Attaching and effacing (A/E) lesion: EPEC infection is characterized by the ability of the bacteria to intimately attach to epithelial cells and efface microvilli. This attaching and effacing histopathology is observed in tissue biopsies of infected individuals and is mediated by various factors, including the type III secretion system (T3SS) and the intimin protein.
- Invasiveness: While EPEC is generally considered a noninvasive pathogen, intracellular EPEC strains have been observed in tissue culture and small intestinal biopsies. Some EPEC strains contain plasmid sequences that confer invasiveness.
- Biofilm formation: EPEC has the ability to form biofilms on abiotic surfaces, which requires various adhesive structures such as type 1 pili, antigen 43, BFP, and the EspA filament. Atypical EPEC strains have also been shown to adhere to abiotic surfaces using non-fimbrial adhesins.
- EAF plasmid: Typical EPEC strains carry a large virulence plasmid called the EAF plasmid. This plasmid varies in sequence among different EPEC strains but is conserved to some extent. The EAF plasmid contains genes encoding BFP and a transcriptional activator called plasmid-encoded regulator (Per).
- Bundle-forming pili (BFP): BFP is a type IV pilus produced by typical EPEC strains. It interconnects bacteria within microcolonies, contributing to the LA phenotype. The major structural subunit of BFP is encoded by the bfpA gene.
- Locus of enterocyte effacement (LEE) and type III secretion system (TTSS): The LEE is a pathogenicity island in EPEC that contains genes essential for A/E lesion formation. It encodes a T3SS, which enables the secretion of effector proteins into host cells. The LEE also contains genes encoding intimin (eae) and its translocated receptor, Tir.
- Intimin and Tir: Intimin is an outer membrane adhesin encoded by the eae gene. It is required for the intimate adherence of EPEC to epithelial cells. Intimin binds to its receptor Tir, leading to the formation of pedestals beneath adherent bacteria.
- Other potential adhesins: EPEC strains may possess additional adhesins such as rodlike fimbriae, fibrillae, lymphocyte inhibitory factor (LifA), and the E. coli common pilus (ECP). These adhesins contribute to bacterial adherence to host cells and interactions between bacteria.
- Flagella: Flagella have been suggested to play a role in EPEC adherence to epithelial cells, although their involvement is not fully confirmed.
Toxin Produced by Enteropathogenic Escherichia coli (EPEC)
EspC Toxin
EspC is a secreted protein produced by enteropathogenic Escherichia coli (EPEC) that plays a role in the pathogenesis of the bacterium. Here are the key points about EspC:
- Function: EspC induces cytopathic effects on epithelial cells, causing damage to the cytoskeleton. It is a member of the serine protease autotransporters of the Enterobacteriaceae (SPATE) family of autotransporter proteins.
- Transport mechanism: EspC possesses its own transport mechanism, known as autotransport, which allows it to be secreted from the bacterial cell and interact with host cells.
- Protein hydrolysis: EspC has the ability to degrade various proteins. It has been shown to interact with and degrade hemoglobin, as well as hydrolyze proteins such as pepsin, factor V, and spectrin. This proteolytic activity contributes to the pathogenic effects of EPEC.
- Lysozyme resistance: EspC confers enhanced resistance to the antimicrobial enzyme lysozyme. This resistance allows EPEC to evade the immune response mediated by lysozyme, enhancing its survival in the host.
- Adherence and biofilm formation: EspC serves as a substratum for adherence, enabling EPEC to attach to host cells. It also plays a role in biofilm formation, a process by which bacteria form a protective community on surfaces.
- Protection against antimicrobial compounds: EspC helps protect EPEC from antimicrobial compounds. Its presence contributes to the bacterium’s ability to resist the action of antimicrobial agents, promoting its survival and persistence in the host.
- Chromosomal location: The gene encoding EspC is located within a specific 15-kilobase chromosomal island that is specific to EPEC1 strains. This chromosomal island is unique to certain EPEC strains and contributes to their virulence.
Overall, EspC is a multifunctional protein produced by EPEC that contributes to the pathogenicity of the bacterium. Its proteolytic activity, adherence properties, resistance to antimicrobial compounds, and role in biofilm formation are important factors in EPEC’s ability to cause disease and establish infection. Further research is needed to fully understand the precise mechanisms and interactions of EspC in EPEC pathogenesis.
Other toxins
In addition to its primary virulence factors, Enteropathogenic Escherichia coli (EPEC) strains have been found to produce other toxins that may contribute to their pathogenesis. Here are two notable toxins associated with EPEC:
- Cytolethal distending toxin (CDT): Scott and Kaper discovered a cytolethal distending toxin in an EPEC strain, which has been shown to induce chromatin disruption in target cells [89]. This disruption leads to cell cycle arrest in the G2/M phase, eventually resulting in cell death [90]. The CDT gene is believed to be present in most EPEC strains associated with diarrhea [91]. However, the exact role of CDT in EPEC pathogenesis is still being investigated.
- Enteroaggregative E. coli heat-stable enterotoxin 1 (EAST1): EAST1 is another toxin found in EPEC strains. Originally identified in enteroaggregative E. coli (EAEC), EAST1 has also been detected in certain EPEC isolates [92]. Studies using EAST1 DNA probes have suggested that this toxin is expressed by a number of clinical EPEC isolates [18, 93]. The precise role of EAST1 in EPEC pathogenesis is not yet fully understood.
Both CDT and EAST1 represent additional toxin factors that may contribute to the virulence of EPEC strains. Further research is needed to elucidate the mechanisms by which these toxins function and their significance in the overall pathogenicity of EPEC.
Model of EPEC pathogenesis
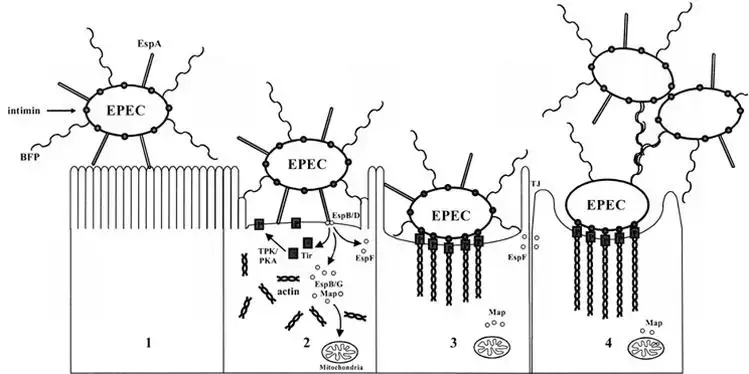
The pathogenesis of Enteropathogenic Escherichia coli (EPEC) can be described using a three-stage model. This model was initially proposed in the early 1990s and has since been expanded upon by researchers. The stages of EPEC pathogenesis are as follows:
- Stage 1: Localized adherence – The first stage involves the attachment of typical EPEC strains to the surface of the host intestinal epithelium. This attachment is primarily mediated by bundle-forming pili (BFP), which are filamentous structures produced by the bacteria. Additionally, a filament called EspA can also contribute to attachment, albeit to a lesser extent. Atypical EPEC strains may rely on EspA for adherence. This initial attachment allows the bacteria to establish contact with the host cells.
- Stage 2: Signal transduction – During the second stage, various proteins are translocated into the host cells by the type III secretion system, a specialized apparatus used by EPEC to inject virulence factors into host cells. One of the key translocated proteins is translocated intimin receptor (Tir), which is inserted into the host cell membrane. This interaction triggers a cascade of cell-signaling events, involving other translocated effector proteins such as EspB, EspD, EspF, EspG, and Map. These effectors activate signaling pathways within the host cells, leading to alterations in the host cell cytoskeleton. This results in actin accumulation and loss of microvilli, the finger-like projections on the surface of intestinal cells.
- Stage 3: Intimate attachment with pedestal formation – In the third stage, the bacteria establish intimate adherence to the host cells through interactions between intimin, an outer membrane adhesin produced by EPEC, and Tir on the host cell surface. This intimate attachment amplifies the accumulation of actin filaments and other cytoskeletal proteins, leading to the formation of pedestal-like structures beneath the attached bacteria. These structures provide stability to the bacteria-host cell attachment and play a role in the colonization of the intestine.
Throughout the stages of EPEC pathogenesis, the translocated effector proteins disrupt various host cell processes. They interfere with tight-junction integrity, leading to increased permeability of the intestinal epithelium and electrolyte loss. Furthermore, they affect mitochondrial function and contribute to cell death.
The model of EPEC pathogenesis helps to understand the stepwise process by which the bacteria attach to host cells, manipulate cellular signaling pathways, and cause structural and functional changes in the intestinal epithelium, ultimately leading to the characteristic symptoms and manifestations of EPEC-induced diarrhea.
Diagnosis and Testing for Enteropathogenic Escherichia coli (EPEC)
The diagnosis and testing of Enteropathogenic Escherichia coli (EPEC) involve both phenotypic and genotypic approaches. Here are the key points regarding the methods used for the detection of EPEC:
- Phenotypic approach: The phenotypic approach involves the use of cell cultures and fluorescence microscopy to observe the attaching-and-effacing (A/E) histopathology, which is the hallmark of EPEC infections. This histopathology can be observed in intestinal biopsy specimens from patients or infected animals, as well as in cell culture models. The presence of A/E lesions indicates the presence of EPEC.
- Genotypic approach: The genotypic approach involves the use of DNA hybridization or polymerase chain reaction (PCR) techniques to detect specific genetic markers associated with EPEC. Probes and amplification assays have been developed for various targets, including the plasmid-encoded bundle-forming pili (BFP) and gene targets on the “locus of enterocyte effacement” (LEE) pathogenicity island. These genetic markers help identify the presence of EPEC strains.
- Fecal leukocytes and inflammatory diarrhea: EPEC infections may not always result in the presence of fecal leukocytes, which are white blood cells in the stool. However, more sensitive tests for inflammatory diarrhea, such as the anti-lactoferrin latex bead agglutination test, can frequently yield positive results in cases of EPEC infection. These tests help identify the presence of inflammation associated with EPEC-related diarrhea.
In summary, the laboratory diagnosis of EPEC involves both phenotypic and genotypic methods. Phenotypic approaches focus on the observation of A/E histopathology using cell cultures and fluorescence microscopy. Genotypic approaches involve the use of DNA-based techniques, such as DNA hybridization and PCR, to detect specific genetic markers associated with EPEC. Additionally, tests for inflammatory diarrhea can provide further evidence of EPEC infection. Accurate diagnosis is crucial for appropriate management and control of EPEC-related illnesses.
Management and Therapeutic Approaches for Enteropathogenic Escherichia coli (EPEC)
The management and therapeutic approaches for Enteropathogenic Escherichia coli (EPEC) infections primarily focus on supportive care and addressing the complications associated with the infection. Here are the key points regarding the management and treatment of EPEC:
- Supportive care: The cornerstone of management for EPEC infections is supportive care, which aims to maintain hydration and electrolyte balance. Oral rehydration therapy or, in severe cases, intravenous fluids may be administered to prevent or treat dehydration. Replenishing fluids and electrolytes helps to restore the body’s balance and prevent complications associated with fluid loss.
- Nutritional support: In cases where EPEC infections cause prolonged or severe diarrhea, nutritional support may be necessary. This can involve providing adequate nutrition through oral feeds, breastfeeding, or formula feeding. In severe cases where oral intake is not possible, enteral or parenteral nutrition may be required.
- Antibiotic therapy: Antibiotics are generally not recommended for routine treatment of EPEC infections, especially in non-severe cases. EPEC strains are typically resistant to common antibiotics, and the use of antibiotics may increase the risk of complications and antibiotic resistance. However, in certain situations where EPEC infections are severe, prolonged, or associated with systemic complications, antibiotics may be considered under the guidance of a healthcare professional.
- Infection control measures: Preventing the spread of EPEC infections is important, especially in settings such as healthcare facilities or childcare centers. Strict adherence to hand hygiene practices, proper sanitation of surfaces and objects, and appropriate food handling and preparation techniques can help prevent the transmission of EPEC to others.
- Monitoring and follow-up: Close monitoring of the patient’s symptoms, hydration status, and overall well-being is essential during the management of EPEC infections. Follow-up visits with healthcare providers are important to assess the progress of the infection, ensure proper hydration and nutrition, and address any complications or concerns.
FAQ
What is Enteropathogenic Escherichia coli (EPEC)?
Enteropathogenic Escherichia coli (EPEC) is a specific strain of Escherichia coli bacteria that causes gastrointestinal infections, particularly in infants and young children. It is known for its ability to cause diarrhea, often in outbreaks.
How is EPEC diagnosed?
The diagnosis of EPEC can be done through both phenotypic and genotypic methods. Phenotypic approaches involve cell cultures and fluorescence microscopy, while genotypic methods utilize DNA hybridization or PCR techniques. The presence of characteristic histopathological changes, such as attaching and effacing (A/E) lesions, can also aid in the diagnosis.
What are the complications of EPEC infection?
While most EPEC infections resolve after a few days, complications can arise. Persistent diarrhea requiring hospitalization can occur, especially in young children. Additionally, EPEC is one of the leading causes of death in children in developing countries, particularly in combination with other factors like malnutrition.
Who is most at risk of EPEC infection?
EPEC infections primarily affect children younger than 2 years old, especially those living in developing countries. However, EPEC can cause sporadic diarrhea in adults as well, though it is less common.
What are the symptoms of EPEC infection?
EPEC infection typically presents with watery diarrhea, non-bloody stools, vomiting, and fever. The diarrhea can be severe and protracted, and in some cases, hospitalization may be required due to persistent diarrhea.
How is EPEC transmitted?
EPEC is primarily transmitted through the fecal-oral route. Contaminated hands, contaminated weaning foods or formula, and contaminated fomites (inanimate objects) can serve as vehicles for transmission. Person-to-person spread is also possible.
How is EPEC managed and treated?
Management of EPEC infection primarily involves supportive care to maintain hydration and electrolyte balance. Rehydration therapy, such as oral rehydration solution or intravenous fluids, may be necessary in severe cases. Antibiotics are generally not recommended unless there are specific indications.
What are the virulence factors of EPEC?
EPEC possesses various virulence factors that contribute to its pathogenicity. These include bundle-forming pili (BFP), intimin, type III secretion system (TTSS), and other effector proteins. These factors aid in the attachment and colonization of host cells, disruption of cell signaling, and induction of cytopathic effects.
Can EPEC produce toxins?
Yes, EPEC strains can produce toxins in addition to their other virulence factors. Cytolethal distending toxin (CDT) and enteroaggregative E. coli heat-stable enterotoxin 1 (EAST1) have been identified in some EPEC strains. The role of these toxins in EPEC pathogenesis is still being studied.
How can EPEC infections be prevented?
Prevention of EPEC infections involves practicing good hygiene, such as regular handwashing with soap and water, especially before handling food. Proper sanitation and safe food handling practices are also crucial in reducing the transmission of EPEC. In some cases, vaccination against specific EPEC serotypes may be considered for high-risk populations.
References
- Sastry A.S. & Bhat S.K. (2016). Essentials of Medical Microbiology. New Delhi : Jaypee Brothers Medical Publishers.
- Murray, P. R., Rosenthal, K. S., & Pfaller, M. A. (2013). Medical microbiology. Philadelphia: Elsevier/Saunders
- Parija S.C. (2012). Textbook of Microbiology & Immunology.(2 ed.). India: Elsevier India.
- https://www.intechopen.com/chapters/68887