What is Ligase?
In biochemistry, ligases are a class of enzymes that incorporate joining of two large molecules by forming new bond. This process which is referred to as ligation, commonly entails a hydrolysis of a indivisible chemical group of one of the substrates which allows formation of new linkages that include C-O, C-S or C-N. For instance, DNA ligase is a certain enzyme that is included in the group of ligases It connects the ends of the prerequisites of two nucleic acids or, in other words, it performs joining of complementary fragments of nucleic acids, and prepares single-strand breaks for double helix DNA during replication.
The basic reaction catalyzed by ligases can be represented by the following dehydration reaction:
A-OH+B-H→A-B+H2O
Equation at B shows how the hydrogen atom of the hydroxyl group of molecule A –OH interacts with the lone hydrogen atom of molecule B-H to form a covalent bond with the simultaneous release of water. Consequently, the function of ligases is to catalyse different biochemical synthesis pathways involving coupled formation of molecule linkages.
Numbering and nomenclature of ligases can be confusing since these enzymes are assigned a number of synonyms based on their function. As with DNA ligase, the term ‘ligase’ is used in the name of the enzyme but there might be other terms such as ‘ synthetase’ or ‘ synthase’ where the enzyme appears to be synthesizing tal or in specified molecules. In addition, some of the ligases have a specific name that shows their involvement in catalyzing carboxylation reactions and end with the suffix -ase, called carboxylases. There appears to be a certain irregularity in naming these enzymes, but notably, synthetase and synthase are used synonymously to refer to ligases.
Ligases according to the Enzyme Commission (EC) number classification system belong to the category EC 6. Each of this classification has been subdivided into a number of subclasses depending on the type of bond they in form. Specifically, these subclasses include:
EC 6.1: Ligases that catalyze the formation of carbon-oxygen bond.
EC 6.2: Carbon—Sulfur ligases that are involved in the formation of carbon-sulfur bonds.
EC 6.3: Enzymes that function in synthesizing carbon –nitrogen bonds, including argininosuccinate synthetase.
EC 6.4: Carbon-carbon bond synthesizing ligases, as represented by acetyl-CoA carboxylase.
EC 6.5: Ligases that synthesize phosphoric ester bond involve DNA ligase.
EC 6.6: Specific ligases that catalyse the formation of N – metal bonds as shown in chelatases.
What is DNA Ligase?
- The DNA ligase is an important enzyme that is needed for the maintenance and integrity of DNA in life. It is mainly involved in catalyst of joining of DNA strands of phosphodiester bond formation and is a key step DNA repair, replication and recombination.
- DNA ligase in the context of DNA repair ligates single stranded breaks in duplex DNA with a complementary strand as a template. DNA ligase finishes the repair process by creating the final phosphodiester bond when a single strand break occurs. If the DNA is to be accurately and fully used, it must retain its proper genetic information, thus this mechanism. Even in specialized forms of DNA ligase, one that is particularly adept at repairing double strand breaks (where both strands of the DNA helix are broken), it is not entirely clear how.
- Furthermore, DNA ligase is important for DNA replication. DNA polymerases synthesize newly formed DNA strands, which are subsequently joined by it to ensure that the newly formed strands are continuous. DNA ligase is involved in the last phosphodiester linkage between 5′ phosphate group from DNA polymerase III and 3′ hydroxy group of DNA polymerase I, specifically. The importance of this action is that it maintains structural integrity of the replicated DNA.
- DNA ligase is an energy dependent mechanism of action. The enzyme of DNA joining in most organisms requires ATP or NAD+ for the joining of DNA fragments. Eukaryotic cells contain ATP dependent ligases, whereas bacterial, archaeal, and viral ligases can utilise ATP or NAD+. This molecule must ‘liberate energy’ in order to break the phosphate bonds required to join DNA nicks — places in the DNA where the phosphodiester linkage between two adjacent nucleotides is severed.
- Apart from its natural biological function, DNA ligase has been widely applied in molecular biology. In laboratory uses, such as gene cloning, it is widely used. The researchers purify DNA ligase because by doing so, they are able to join DNA molecules joined together, resulting into recombinant DNA, which can be used in biotechnological and therapeutic purposes.
- DNA ligase first emerged in 1967 where it was first isolated from the bacterium Escherichia coli. In the late 1960s, subsequent research identified a number of other forms of this enzyme. DNA ligases are nucleotide transferases in the nucleotide transferase superfamily and include RNA ligases and mRNA capping enzymes. The evolutionary importance of these enzymes in nucleic acid metabolism is emphasized by this classification.
Structure of DNA Ligase
Refinement of DNA ligase structure is critical to understanding how it catalyzes the joining of DNA strands. Differences in size and composition exist among different organisms, as occurred in adaptations to their respective biological contexts. The structural components of DNA ligase enable us to understand its mechanisms of action and its overall roles in DNA repair and replication.
- Size Variability: Size of DNA ligases vary with organism. As a result, viral ATP dependent DNA ligases are commonly smaller than their counterparts in other organisms. The correlation with the particular requirements and efficiencies that DNA processing may need in different biological systems may be related to this size difference.
- Catalytic Core: The conserved catalytic core of both ATP dependent and NAD+ dependent DNA ligases is essential for their enzymatic activity. Six conserved motifs (I, II, III, IV, V, and VI) known as this core make for special binding cofactors such as ATP or NAD+, coordinating with metal ions, and fitting the ligation chemistry.
- Non-Catalytic Domain: Like other DNA ligases, many others also possess a conserved non catalytic domain (NCD) whose function is still uncharacterized. Finally, the potential role of this domain in stability or regulation of the ligase was assessed, but more extensive research is needed to determine specific contributions.
- Structure of ATP-Dependent Ligases:
- Domain Composition: Typically ATP dependent ligases, including those derived from bacteriophage T7, are organized into two distinct domains. The adenylation or nucleotide binding domain includes a pocket below one β sheet, which is the binding site for ATP, the first.
- Domain 1 Structure: The stable framework for ATP interaction includes three antiparallel β sheets flanked by six α helices contained within this domain.
- Oligonucleotide Binding Fold Domain: The second domain is an oligonucleotide binding fold containing one highly twisted β-sheet antiparallel close to the plane, one α-helix along one edge, and two residues inserted into the β-sheet.
- Linker Region: These two domains are joined by a flexible linker which interacts dynamically during ligation.
- Structure of NAD+-Dependent Ligases:
- Catalytic Core Composition: E. coli LigA is a ligase, NAD+-dependent, and the ligase catalytic core is made of a central core comprising the oligonucleotide binding fold and the nucleotidyltransferase domain.
- Domain Organization: The domains in these domains are at the edge of the N-terminal domain (domain a), which are associated with three C-terminal domains that contain a helix-hairpin-helix (HhH) domain, zinc finger domain and a BRTC domain.
- Role of Domain la: For this reaction of the ligase with NAD+, this domain is necessary, allowing formation of the ligase 5′-AMP intermediate, the key step in the ligation process.
Types of DNA Ligase
DNA ligases are enzymes critical for DNA replication and repair, classified primarily based on their cofactor requirements: ATP-dependent and NAD+-dependent DNA ligases. Understanding these types is essential for appreciating their diverse roles in different organisms, including bacteria, archaea, and eukaryotes.
- ATP-Dependent DNA Ligases: Most eukaryotic DNA ligases utilize ATP as a cofactor. These ligases exhibit variability in size and structure across different organisms. For example:
- Size Variation: The DNA ligase from Haemophilus influenzae comprises 268 amino acids, whereas larger cellular ligases, such as human DNA ligase I, consist of 912 amino acids, and ligase IV contains 844 amino acids.
- Bacteriophage T7: This ligase is the most widely used variant, characterized by its monomeric structure with a molecular weight of 41 kDa. It features two distinct domains. Domain 1 is responsible for ATP binding, composed of six α-helices surrounding three antiparallel β-sheets. Domain 2 consists of antiparallel β-sheets with an accompanying α-helix.
- Bacteriophage T4: This ligase can ligate cohesive ends or blunt ends of DNA, RNA-DNA hybrids, and RNA. It exists as a monomeric form with a molecular weight of 68 kDa.
- Mammalian DNA Ligases:
- DNA Ligase I: Functions primarily to ligate Okazaki fragments during lagging strand replication and assists in joining some recombinant fragments.
- DNA Ligase II: Involved in the purification or excision repair of alternatively spliced forms of DNA ligase III, typically found in non-dividing cells.
- DNA Ligase III: The only mammalian ligase located in mitochondria, it plays a role in nucleotide excision repair and the ligation of recombinant fragments.
- DNA Ligase IV: Crucial for homologous recombination repair, this ligase is important for immune cell development and other vital cellular activities.
- NAD+-Dependent DNA Ligases: These ligases are predominantly found in bacterial species but also exist in some mimivirus and entomovirus. Additionally, some bacteria, archaea, and viruses may utilize both NAD+ and ATP for their ligases. For example, Mycobacterium tuberculosis codes for NAD+-dependent ligases and has at least three types of ATP-dependent ligases. The size of these ligases tends to be uniform, ranging from 656 to 837 amino acids. Notable examples include:
- E. coli DNA Ligases: This monomeric enzyme has a molecular weight of 74 kDa and requires NAD+ as a cofactor. It catalyzes the formation of phosphodiester bonds in double-stranded DNA containing cohesive ends. The ligases LigA and LigB, products of the lig gene, are predominantly utilized in E. coli.
- Taq DNA Ligases: Derived from thermophilic bacteria, Taq ligases are thermostable and require NAD+ as a cofactor. They demonstrate higher stability and activity at extreme temperatures compared to conventional DNA ligases, making them valuable in DNA amplification reactions for detecting mutations in mammalian DNA.
Mechanism of DNA ligase
DNA ligase is a critical DNA methabolism process, it joins DNA fragments by forming phosphodiester bonds. It is an enzyme that catalyses DNA single strand breaks repair and is critical for DNA replication. The ligation usually proceeds generally in three main steps, all of which need to be properly completed to allow the ligation of DNA.
- Adenylation of DNA Ligase/Activation of DNA Ligase: The first step, in turn, is to activate DNA ligase by ATP. In this case, ATP is hydrolyzed to adenosine monophosphate (AMP) releasing energy. The AMP is subsequently the attached to an amino group of a lysine residue that resides at the conserved active site of the enzyme. This attachment is by a phosphoramidite bond such that an enzyme-AMP intermediate is formed, an essential step in the ligation reaction.
- Activation of 5’ Phosphate in Nicks: After formation of the enzyme-AMP complex, the activated ligase migrates to the DNA break site where the 5’ phosphate of AMP is substituted for as a so-called ‘nick.’ Upon encountering the ligase enzyme in this interaction, the AMP moiety sequesters the phosphate group to release the ligase enzyme from the complex. This interaction turns out to be effective for ligation at the 5’ end of the single-stranded DNA.
- DNA Ligation: Finally, the 5’ phosphate group is activated and this newly activated, oxidised phosphate group is then attacked nucleophilically by its opposite number at the 3’ hydroxyl group of an adjacent DNA strand. The attacked site leads to the formation of a covalent phosphodiester bond between the two nucleotides, making the DNA strand impermeable. This ligation reaction releases free AMP and hydrogen ions completing the ligation.
Interesting is that in bacterial systems the ligase may be adenylated with NADH2, the process which yields nicotinamide mononucleotide. Here, we focus on one variation which demonstrates the range of ligation mechanisms employed across different organisms, while the major aspects remain the same.
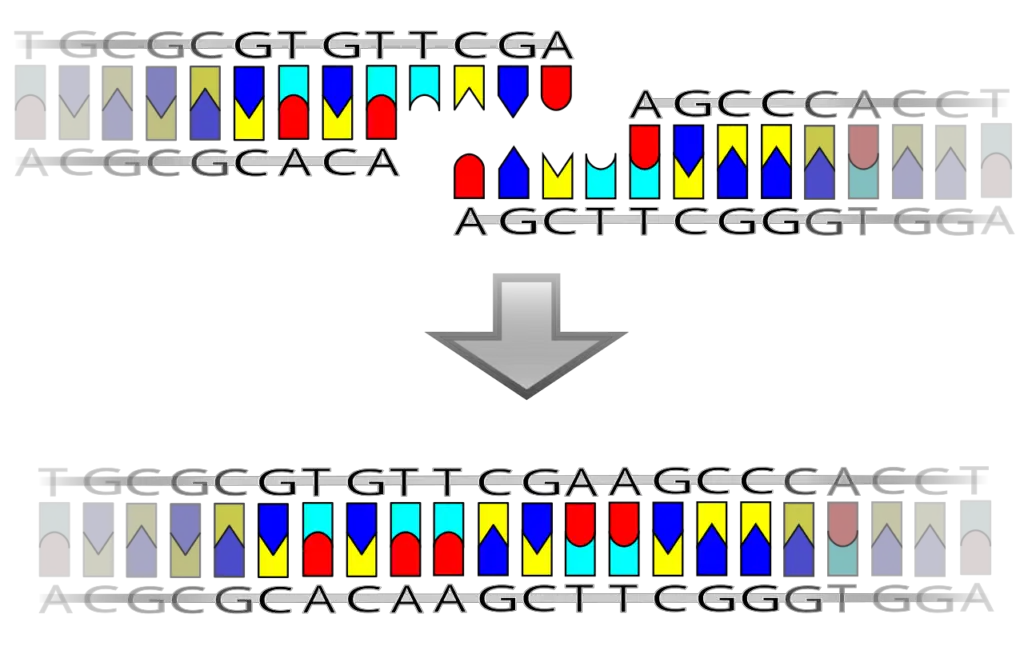
DNA Ligation Reactions
DNA ligation reactions are fundamental processes in molecular biology, enabling the joining of DNA fragments to form recombinant DNA molecules. These reactions utilize specific ends created by restriction enzymes, leading to two primary types of ligation: blunt-end ligation and sticky-end ligation. Each method has distinct mechanisms and efficiencies, which are crucial for various genetic engineering applications.
- Blunt-End Ligation:
In this reaction, both the plasmid vector and the DNA insert are cut by restriction enzymes to create blunt ends. Blunt ends are flat, with no overhanging nucleotides, allowing for the direct joining of the fragments. DNA ligase facilitates the formation of a phosphodiester bond between these blunt ends. Although this method can successfully ligate fragments, it is generally less efficient than sticky-end ligation due to the lack of complementary overhangs that help stabilize the binding of the fragments prior to ligation. - Sticky-End Ligation:
This method involves a more complex interaction. Restriction enzymes cleave the plasmid vector and the DNA insert at two different positions, resulting in short, single-stranded overhangs at the 3’ and 5’ ends of the nucleic acid fragments. These overhangs are known as sticky ends and must be complementary for successful ligation. The presence of complementary sticky ends enhances the efficiency of the reaction because the overhangs can base-pair with one another, providing a stable initial interaction before the DNA ligase catalyzes the formation of the phosphodiester bond. This increased stability contributes to a higher likelihood of successful ligation, making sticky-end ligation a preferred choice in many cloning procedures.
Functions of DNA Ligase
Below I present detailed functions of DNA ligase to illustrate its usefulness in cellular processes and biotechnology.
- DNA Replication: DNA ligase is necessary for DNA replication, but particularly for synthesis of the lagging strand of DNA. It can join Okazaki fragments through formation of phosphodiester bonds between the nicks produced by DNA polymerase. This ensures that the DNA strand remains continuous for accurate and complete duplication of the genetic material.
- DNA Repair: Repair of single strand breaks in DNA requires the enzyme. DNA ligase is an enzyme which helps the DNA strands become reconnected after the DNA is damaged in an environment, due to errors in replication or other stresses in the cellular environment. The ligase seals these nicks and maintains genome stability by preventing accumulation of mutations.
- Recombinant DNA Technology: In the construction of recombinant DNA molecules DNA ligases are indispensable in the field of genetic engineering. Ways they are also used are to insert foreign DNA fragments, such as genes, into plasmids, together with restriction endonucleases. This whole process is very important for gene cloning; you can make genetically modified organisms or make specific proteins for research or therapeutic purposes.
- High-Efficiency Cloning: For high efficiency full length complementary DNA (cDNA) cloning in molecular biology labs, E. coli DNA ligases are commonly used. Thanks to their ability to ligate DNA fragments, they are useful tools for constructing cDNA libraries and for the expression of eukaryotic genes in prokaryotic systems.
- Thermostable Applications: The ability of thermostable Taq ligases to withstand high temperatures means they are utilised commercially in polymerase chain reactions (PCR). As a result of their stability, they are good candidates for amplification reactions which improve the efficacy and specificity of DNA amplification processes.
- V(D)J Recombination: DNA ligase IV is essential for V(D)J recombination, a means to generate diversity at immunoglobulin and T cell receptor loci during immunoglobulin and T cell receptor development in the immune system. A robust adaptive immune response requires this function for the production of a large panoply of antibodies and T cell receptors.
How does DNA ligase work?
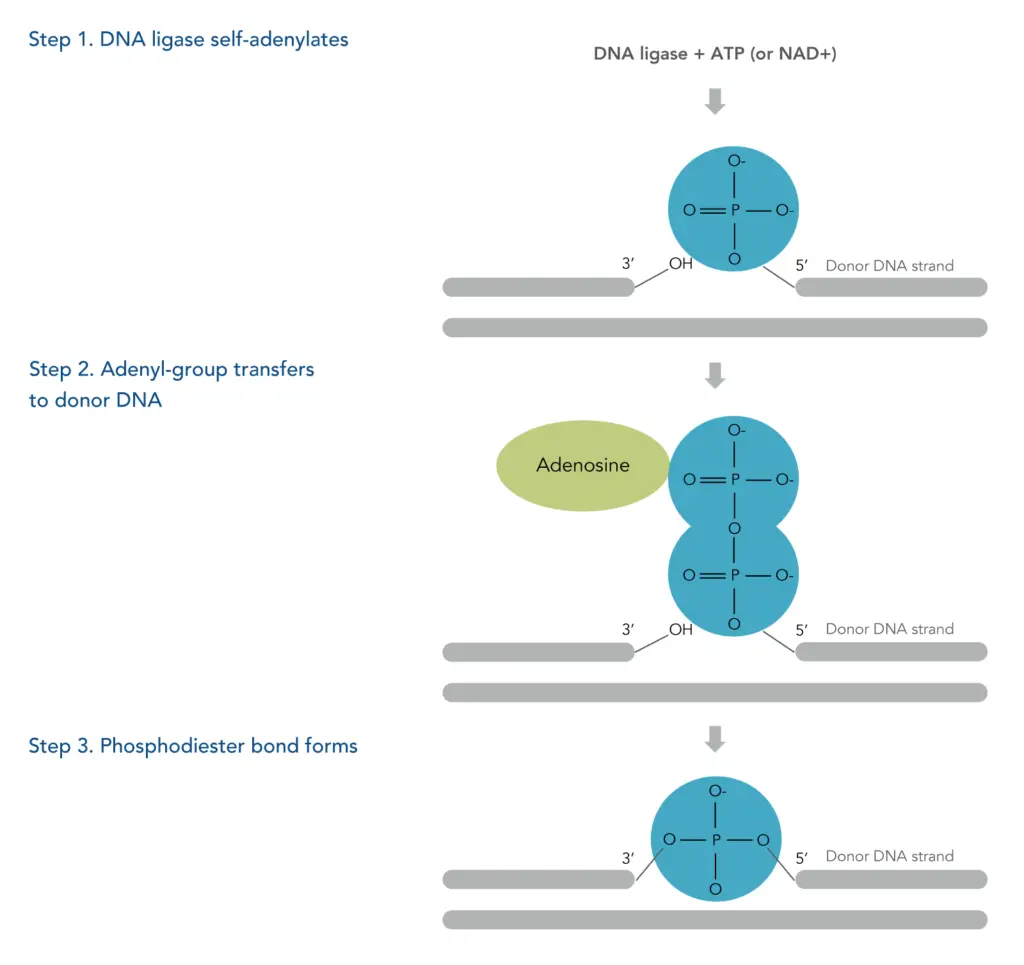
Understanding how DNA ligase functions is crucial for grasping the mechanisms of DNA replication, repair, and recombinant DNA technology. DNA ligase facilitates the joining of DNA fragments by forming covalent phosphodiester bonds. The process can be divided into three distinct steps, illustrating the enzyme’s action in catalyzing the ligation reaction.
- Self-Adenylation of DNA Ligase:
The process begins with the activation of DNA ligase. The enzyme reacts with either ATP or NAD+, resulting in the formation of a phosphamide bond. This bond is established between a lysine residue within the enzyme and an adenosine monophosphate (AMP) molecule derived from the cofactor. This step is crucial as it prepares the ligase for subsequent interactions with the DNA strands. - Transfer of the Adenyl Group:
Once the DNA ligase is activated and the AMP is attached, the next step involves the transfer of the adenyl group to the 5’ phosphorylated end of the donor DNA strand. This modification is essential because it activates the 5’ end, making it more reactive and primed for ligation. - Formation of the Phosphodiester Bond:
The final step in the mechanism occurs when the hydroxyl group of the acceptor DNA strand attacks the adenylated 5’ end of the donor DNA. This reaction results in the formation of a covalent phosphodiester bond, effectively sealing the nick in the DNA strand. The completion of this reaction releases AMP, allowing the ligase to continue functioning and participate in further ligation events.
Therefore, the action of DNA ligase is integral to maintaining the integrity of the DNA molecule by enabling the repair of nicks and gaps. The enzyme’s ability to facilitate the formation of phosphodiester bonds is essential for various biological processes, including DNA replication and repair, making it a vital component in molecular biology and genetic engineering.
Disorders of DNA ligase
The following points outline some notable disorders linked to dysfunctional DNA ligases:
- LIG4 Syndrome: LIG4 syndrome (Ligase IV syndrome) is generated by mutations of the DNA ligase 4 (LIG4) gene. This defect hinders the repair of double-stranded breaks in DNA, leading to severe immunodeficiency in people carrying this condition. In this case patients presented with developmental anomalies like microcephaly and bone marrow hypoplasia found in half of those.
- Xeroderma Pigmentosum (XP): Xeroderma pigmentosum is a disease in which people are highly sensitive to ultraviolet (UV) radiation. Because XP patients are particularly sensitive to skin damage and skin cancers following UV exposure, these impaired DNA repair mechanisms are particularly significant to their skin susceptibility. As a rule, this is a skin and eye condition, and some patients have neurological complications as well.
- Ataxia-Telangiectasia: Mutations in the ATM gene lead to ataxia-telangiectasia, a condition characterised by severely weakened or destroyed immune cells which damage the cells surrounding them. It is a disorder of progressive movement, balance and neurological symptoms including slurred speech and oculomotor apraxia. With the severity of motor difficulties, so many people need wheelchair assistance by adolescence.
- Fanconi Anemia (FA): Fanconi anaemia is a rare genetic disorder of the blood that results in bone marrow failure. Because of this, there’s not enough healthy blood cells being produced, leading to severe health problems that can include an increased risk of leukaemia. The disorder represents a failure of the DNA repair pathways critical for maintaining genetic stability in hematopoietic cells.
- Bloom Syndrome: Bloom syndrome is characterised by ataxia telangiectasia, a heightened sensitivity to sunlight that leads to a butterfly shaped rash across the nose and cheeks, plus a variety of other skin manifestations. Patients may also develop telangiectases and pigment changes to nonexposed skin areas. Defects in DNA repair mechanisms can increase the risk of cancer, and the disorder is thought to be related.
- Eun, H.-M. (1996). Ligases. Enzymology Primer for Recombinant DNA Technology, 109–144. doi:10.1016/b978-012243740-3/50005-3
- Shuman S. DNA ligases: progress and prospects. J Biol Chem. 2009 Jun 26;284(26):17365-9. doi: 10.1074/jbc.R900017200. Epub 2009 Mar 27. PMID: 19329793; PMCID: PMC2719376.
- Doherty AJ, Suh SW. Structural and mechanistic conservation in DNA ligases. Nucleic Acids Res. 2000 Nov 1;28(21):4051-8. doi: 10.1093/nar/28.21.4051. PMID: 11058099; PMCID: PMC113121.
- https://www.khanacademy.org/science/biology/biotech-dna-technology/dna-cloning-tutorial/a/restriction-enzymes-dna-ligase
- https://byjus.com/neet/dna-ligase/
- https://www.cell.com/molecular-cell/fulltext/S1097-2765(07)00144-X
- https://www.eurekaselect.com/article/9441
- https://lifesciences.danaher.com/us/en/library/unveiling-dna-ligation.html
- https://study.com/academy/lesson/dna-ligase-definition-role-quiz.html
- https://www.idtdna.com/pages/education/decoded/article/how-dna-ligation-works
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/dna-ligase