The most common cause for human disease is viral infections. Millions are still dying from viral infections, including hepatitis and human immunodeficiency viruses (HIV).
Emerging viruses are also causing severe problems for the human population. There have been several outbreaks of emerging viruses in various countries, including avian influenza A (H5N1), severe acute respiratory syndrome-coronavirus SARS-CoV (SARS-CoV), pandemic swine flu A (H1N1) virus 2009, Ebola virus (ZIKV 2015), and pandemic SARS-CoV-2 in 2015.
Recent coronavirus disease 2019 outbreak caused by SARS/CoV-2 is an excellent example of how viral infection can pose a serious threat to the global economy and public health. The first step to combating viral infections is to receive a prompt and accurate diagnosis. It is essential to detect the virus in a patient’s sample early and accurately for proper treatment, control and prevention of epidemics.
Introduction Diagnostic Techniques of Viruses
- Traditional laboratory diagnosis of medical viruses is done by isolating the virus in embryonated chicken eggs, tissue culture or animal models. Then, electron microscopy is used to visually examine any viral particles in samples.
- Conventional diagnostic tools are often difficult to use, costly, slow, inefficient, and not reproducible. Molecular diagnostics have revolutionized virology, detecting the presence of virus nucleic acid in patient samples.
- Despite the fact that many traditional methods have been replaced by nucleic-acid-based ones, immuno-based techniques continue to play an important role in the detection and surveillance of viral infections.
- The detection of viral infections using immunological methods involves the identification of antiviral antibodies and viral antigens in clinical samples. We will be discussing some molecular diagnostic and immunological methods for diagnosing medical viruses.
Molecular Diagnostic Techniques of Viruses
Diagnostic virology has been revolutionized by nucleic acid-based molecular diagnostic techniques. They are faster, more sensitive and more specific. These methods can detect specific sequences of nucleic acids and can be used to diagnose virtually all viruses that are known to affect humans.
A. Nucleic Acid-Based Amplification Techniques
- The amplification of viral genome material by molecular techniques is extremely sensitive and precise. It allows for rapid diagnosis and can detect multiple viruses simultaneously.
- For the detection of viruses that are difficult or impossible to cultivate, slow-growing viruses in culture, or viruses with antigenic variations, nucleic acid amplifying techniques can be very helpful.
- In diagnosing viral infections caused from several viruses, the nucleic acid amplifying tests are extremely popular. They include hepatitis C virus, dengue virus and Epstein-Barr virus(EBV), influenza viruses as well as Zika virus (ZIKV), Ebolavirus, and coronavirus.
Examples of Nucleic Acid-Based Amplification Techniques
There are a variety of nucleic acids amplification methods that can be used to diagnose viral infections in the laboratory. These are all discussed below.
1. Polymerase Chain Reaction (PCR)
- PCR is a common example of a nucleic acid amplification test.
- Since its creation by Mullis and Faloona, it has revolutionized molecular diagnostics.
- PCR involves the extraction and purification DNA molecules and exponential amplification using an exponential DNA polymerase.
- The amplified product can then be detected using a variety of techniques after the PCR reaction. These include gel electrophoresis and colorimetric methods as well as sequencing.
- Since its inception, PCR is used to detect human viral infections. The overall clinical sensitivity ranges from 77.8% up to 100% and the clinical specificity ranges from 89% up to 100%.
- The versatility of PCR makes it a versatile tool. There are many variations of PCR, but the two most important ones are reverse transcription-PCR (RTPCR) and real-timePCR. The first was used to amplify ribonucleic acids (RNA) targets. The second was used to measure deoxyribonucleic acids (DNA) in real-time during PCR reactions.
Advantages
- Sensitive, specific
- A widely used nucleic acid-based method of detection
- Multiplex detection potential
Limitations
- High contamination risk
- Inhibitors are not recommended
- It is labor-intensive and time-consuming
- Qualitative
- Gel documentation apparatus and thermal cycler are required
2. Reverse Transcription-PCR (RT-PCR)
- RT-PCR is designed to amplify RNA targets.
- This technique uses reverse transcriptase to convert viral RNA targets to complementary DNA (cDNA). The resulting cDNA can then be amplified using conventional PCR.
- Since its inception, RTPCR has been used to diagnose human infection by RNA virus viruses.
- Conventional RTPCR showed overall sensitivity of 73% to 100%, and specificity of 99% to 100% for the detection or viral infection.
Advantages
- Sensitive, specific
- Multiplex detection potential
Limitations
- Handling RNA might prove difficult
- Multiplex detection potential
- It is time-consuming and cumbersome
- Relatively costly
- Inhibitors are not recommended
- Some RNA viruses may have high mutation rates and mutant regions within PCR, which can lead to decreased sensitivity.
3. Real-Time PCR
- Real-time PCR systems allow for simultaneous viral nucleic acids amplification and detection.
- The amount of fluorescence from the specimen is what will determine the presence of an amplification product.
- Special thermal cyclers monitor fluorescence emission from specimens.
- SYBR green, TaqMan and molecular beacon chemicals can be used to detect and quantify amplification products.
- SYBR Green dye binds to the minor loop of double-stranded dsDNA (dsDNA), and when excited by appropriate light, it displays improved fluorescence. This is directly proportional with the amount of accumulated dsDNA.
- TaqMan probes are DNA oligonucleotides that have a fluorescent dye called reporter at one end and a quencher at the other. TaqMan probes can be used to hybridize with an internal region of a product PCR.
Advantages
- Highly sensitive and precise
- Closed tube operation reduces cross-contamination risk.
- Rapid and labor-intensive
- Multiplex detection Genotyping
- Quantitative determination of viral load
Limitations
- Expensive laboratory equipment and fluorescent probe required
- TaqMan probe design requires complete information about the target nucleic acids sequence
- SYBR green method: Primer dimer artifact can be a problem
- Inhibitors are not recommended
4. Transcription-Based Amplification Methods
- Transcription-based amplification method includes nucleic acid sequence-based amplification (NASBA) and transcription-mediated amplification (TMA).
- TMA and NASBA are very similar. They are both isothermal amplification techniques.
- The temperature at which the entire process of amplifying takes place is 41°C.
- Both cases involve the conversion of the viral RNA target into cDNA using RT, and then RNA Polymerase synthesizes multiple copies.
- TMA and NASBA have two enzymes (RT) and RNApolmerase (NASBA has three enzymes: Avian myeloblastosisvirus reverse transcriptase, RNase H and T7 RNA Polymerase).
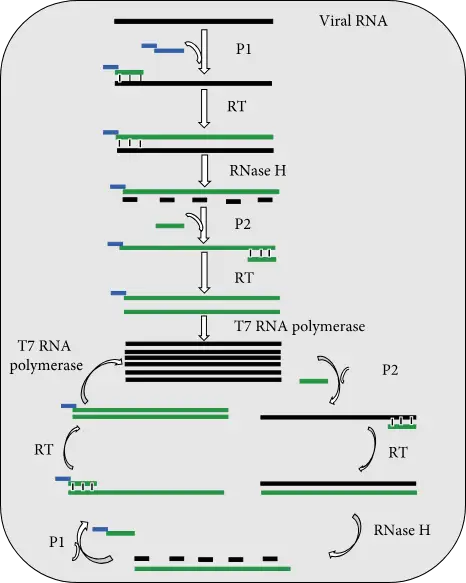
(i) NASBA process
- Three enzymes and two primers are used in the NASBA process to multiply the target viral RNA.
- Primer 1 (P1) has at its 5′ End T7 RNA Polymerase Promotor Region, and at its 3’ End P1 carries sequence which is complementary to a target virus RNA sequence.
- Primer 2 (P2) contains a sequence that is complementary to the cDNA strand.
- The amplification reaction starts with the production by RT of cDNA copies of the viral DNA using P1.
- RNase H is responsible for removing viral RNA from RNA DNA hybrid molecules.
- The released DNA strand is then used to synthesize dsDNA molecules by RT.
- T7 RNA Polymerase also uses dsDNA molecules to create templates for transcribe many copies of viral RNA.
- This cycle can be repeated many times and results in accumulations of many viral DNA copies and dsDNA molecules.
- You can detect the amplified product either by gel electrophoresis at end of assay, or in real-time using molecular beacon.
- One of the advantages of transcription-based amplification is that they don’t require a thermal cycler. This means that developing countries and laboratories with limited budgets can perform the assays. They also have rapid kinetics (requires less cycles) and produce single-stranded DNA products that are suitable for detection using various techniques.
- For diagnosing human viral infections caused RNA viruses, Transcription-based Amplification Methods are appropriate.
- They can amplify viral messenger RNA, viral genomic RNA and ribosomal DNA.
Application
- Ayele et al. Ayele et al. This assay was able to detect both C and C subtypes with high sensitivity and specificity (90.5% sensitivity, 100% specificity and 95.2% specificity respectively for C beacons) and sequencing is considered the gold standard for genotyping.
- Moore et al. NSABA was also used to detect influenza A H5N1 virus infection in clinical specimens. It has a LoD at 10 RNA copies/ml, the same sensitivity and turnaround time as RTPCR.
- The NASBA test was used to detect dengue viral RNA. It had a LoD of 1 PFU/ml and no cross-reactions with JEV. Turnaround time was 3 hours.
- Ender et al. TMA was used to screen blood donors for HIV-1 and HCVRNA. TMA had LoDs 16.2 IU/ml HIV-1 and 3.5IU/ml HCV.
- Multiplex NASBA was used to simultaneously detect HIV-1 and HCV from plasma samples. The HIV-1 and HCV LoD was 1000 copies/ml. There were no cross-reactions.
- Swenson and his coworkers used real-time TMA to detect HSV-1 in lesion swab samples. They had overall sensitivities at 98.2%, 99.4%, and specificity at 97.8%, respectively, as compared with culture.
(ii) TMA-based assays
- TMA-based tests for HIV-1 and HCV detection are available commercially. They were developed by Hologic (San Diego CA, USA).
- Aptima HCVRNA qualitative assay can be used to detect HCVRNA in serum or plasma.
- TMA is used to amplify conserved areas within the 5′-UTR HCV genome.
- The assay has a LoD of 7.5IU/ml and a specificity 99.6%.
- BioMerieux Clinical Diagnostics also offers commercially available NASBA-based kits to detect HIV-1, CMV and enterovirus.
- BioMerieux, Marcy l’Etoile in France developed the NucliSens Easy Q RSV B assay. It is used to detect RSV in different types of respiratory samples.
- This assay uses real-time NASBA and targets the F gene of RSV.
Advantages of TMA and NASBA
- Sensitive, specific
- It is simple and quick (fewer cycles are needed).
- Potential for multiplexing
- Quantification
- Genotyping
- It doesn’t require a thermal cycler, as the reaction occurs isothermally at 41°C
Limitations of TMA and NASBA
- Handling RNA might prove difficult
- Three enzymes are required for NASBA
- Use of enzymes which are not thermostable
- As the temperature at which the amplification takes place is lower (41degC), non-specific interactions may rise.
5. Loop-Mediated Isothermal Amplification (LAMP)
- LAMP is an alternative isothermal nucleic acids amplification method. It is widely used for the sensitive, specific and rapid detection of DNA and RNA viruses within human specimens.
- Notomi and colleagues were the first to develop this method. It quickly gained popularity in diagnostic viralology.
- To amplify target DNA, the method uses four to six different primers and DNA polymerase with DNA strand-displacement activity.
- The LAMP assay can be used to quickly detect a variety of DNA viruses in human samples. This includes HSV-1 with LoD 10 copies of HSV-1DNA/ml with no cross-reactivity and no cross reactions with other select viruses. hAdV40/hAdV41 with LoD between 50 and 100 DNA/reaction with no cross-reactivity and turnaround time time of 60 mins. EBV with sensitivity and specificity of 86.4% and CMV with LoD 10 DNA copies/ml with no cross-reactivity and 1 hour after RNA extraction.
Advantages
- Highly sensitive and precise.
- It is easy to do.
- It does not require a costly thermal cycler.
- Rapid (results within 1 hour).
- Quantitative.
- Genotyping.
- Simple detection systems (using naked eye).
- The sample is relatively resistant to any inhibitors.
Limitations
- Six primers are required
- There is a high risk of cross-contamination
- Multiplexing is subject to limitations
- The subjective nature of visual detection by naked eye is dependent on the perception of color.
6. DNA Microarrays
- The DNA microarray technology has the ability to detect medical viruses.
- For DNA microarray diagnosis, fluorescently-labeled viral nucleic acid in a test specimen is used to screen an array oligonucleotide probles immobilized on solid surfaces (e.g. glass slide).
- These oligonucleotide probes are specific to the genome of the target viruses.
- Fluorescence-based detection allows for the quantification of hybridization results between immobilized probes, target sequences with fluorescent dyes, and is used to detect and quantify them.
- A DNA microarray was used to discover a new coronavirus member during the 2002 SARS outbreak in China.
- DNA microarray technology allows for multiplex detection of potential viruses in clinical specimens. It is a highly-throughput tool.
Limitation
- Routine clinical diagnosis is too expensive.
- It is labor-intensive and
- It is time-consuming (the process of hybridization can take up to several days).
- Only viral pathogens with target probes are detected by the assay.
7. Next-Generation Sequencing (NGS)
- NGS is a very useful tool in diagnosing virology because it can analyze fragments of viral nucleic acids extracted from clinical specimens.
- NGS generally involves the preparation of a test sample, the sequencing of target nucleic acids fragments using one or more NGS platforms and the analysis of sequence data using appropriate bioinformatic tools.
Advantages
- NGS is not dependent on prior knowledge of the genomic sequences of viral pathogens, unlike PCR and DNA microarray.
- You don’t need target-specific PCR primers or oligonucleotide probes.
Limitation
- It takes a long time to get your order back.
- The high volume of samples per run
- Sequencers are expensive.
- Skills in bioinformatics are required.
B. Immunological Diagnostic Techniques of Viruses
In response to viral infections, antibodies are made by the humoral branch. The development of immunological diagnostic techniques is based on this natural immune response to viral infection. There are several immunological diagnostic methods that can detect human viral infections in clinical samples. These include western blotting and immunofluorescence, enzyme-linked immunosorbent, western blotting and immunofluorescence. These assays are based on the formation an antigen-antibody complex. They require clinical specimens, whole viruses or viral antigens and an indicator.
1. Enzyme-Linked Immunosorbent Assay (ELISA)
- ELISA uses enzyme conjugated antibodies to detect specific antiviral antibodies or viral antigens in human specimens.
- A positive sample is one that has an enzyme conjugated to an antibody and a colorless chromogenic substrat. This results in the formation of a colored product.
- No color can be produced if there is no antigen/antibody present in the clinical specimen.
- The amount of antigen-antibody compound formed directly affects the intensity of the color.
- You can observe the color change with your naked eyes or use a spectrophotometer to measure the absorbance.
- ELISA has been performed with several enzymes including horseradish peroxidase and alkaline phosphatase. There are many types of ELISA. The two most common are the antigen-capture ELISA also known as sandwich ELISA and the antibody-capture ELISA.
- Sandwich ELISA detects viral antibodies by immobilizing antigen specific to the viral protein of concern on a microtiter-well; indirect ELISA detects antiviral antibodies in a patient sample through coating whole virus or viral proteins on a microtiter-well.
Advantages
- ELISA is sensitive.
- It is easy to perform.
- It takes a very short time to get results.
2. Western Blotting Analysis
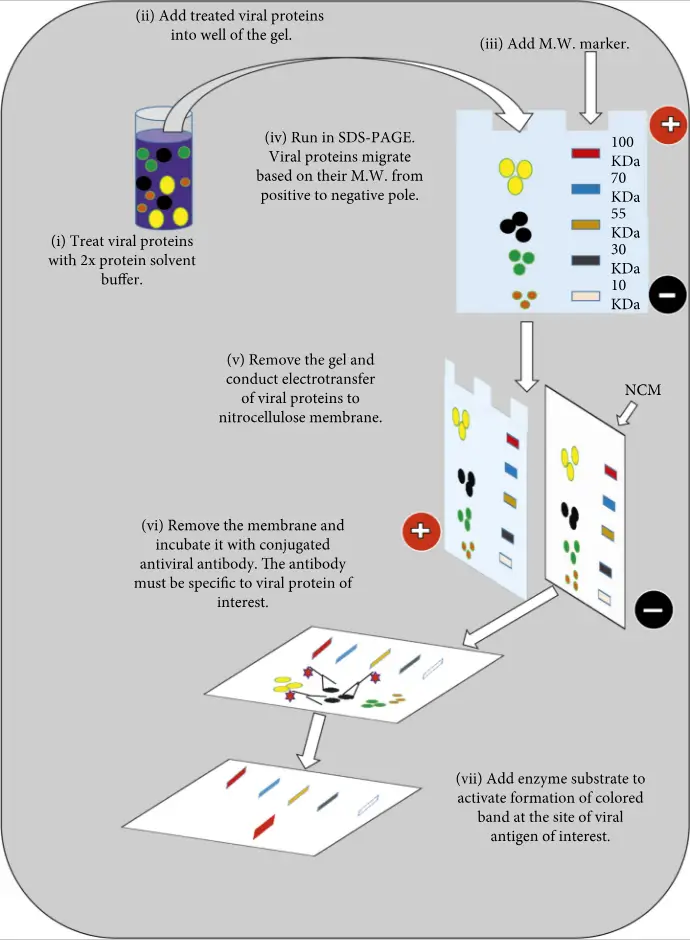
- Western blotting, also known as immunoblotting or assay, is used to detect antiviral antibodies and viral proteins.
- For detection of viral proteins, denatured whole viral proteins are first separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
- The viral proteins are then electrotransferred onto the nitrocellulose membrane.
- After that, the membrane is incubated with specific enzyme conjugated antibodies for the viral proteins.
- The addition of a chromogenic substance to viral antigens will cause colored bands to form if the proteins are bound by an enzyme-labeled antibody.
- After being subjected to SDS/PAGE, the viral specific denatured protein are electrophoretically blocked onto nitrocellulose membrane in order to detect antiviral antibodies.
- The membrane is incubated with the patient’s serum.
- Antibodies against viral proteins in patient serums will bind to specific viral proteins.
- The addition of an enzyme conjugated secondary antibody anti-human antibody to a chromogenic substrat results in the formation of colored bands at the sites of viral proteins.
- The use of immunoblotting in clinical diagnosis has been made possible by serosurveillance, as well as to confirm a human viral infection.
3. Immunofluorescence Assay
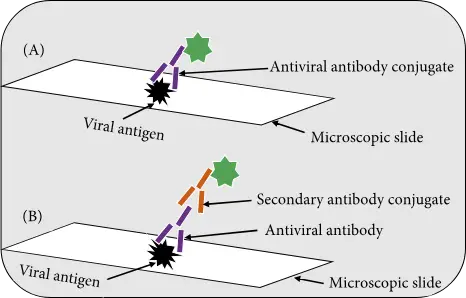
- For the detection of antiviral antibodies or viral antigens in clinical samples, immunofluorescence assays are commonly used.
- There are two types of assays: the direct immunofluorescence (DFA), which detects virus antigens in patient samples, and the indirect immunofluorescence (IFA), which detects antiviral antibodies or viral antigens within clinical specimens.
- The DFA uses an antibody that recognizes virus antigen to be directly conjugated with a fluorescent dye. The IFA detects viral antigen specific antibodies with a second fluorescently-labeled anti-human antibody.
- IFA is more sensitive that DFA, because multiple fluorescently labeled anti–immunoglobulin antibody bind to each antiviral antibodies increasing the intensity fluorescence at each site.
- Fluorescein isothiocyanate, also known as FITC, is the most commonly used fluorescent dye in diagnostic virusology. It emits an intense yellow-green fluorescence. However, rhodamine emits a deep, red fluorescence.
- After staining, the specimen can be examined under a fluorescence microscope using incident UV light.
- SARS was diagnosed using IFA.
4. Hemagglutination Inhibition (HI) Assay
- A number of viruses, including dengue virus and rubella virus, adenovirus and rubella virus, have hemagglutinin antibodies on their surfaces. This binds to RBCs and is known as hemagglutination. For the development of HI assays, the virus’ ability to agglutinate RBCs can be inhibited.
- Serial dilutions are made of the serum sample in the HI assay using a microtiter plate.
- Next, a specific amount of viral Hemagglutinin will be added.
- Finally, the appropriate RBCs can be added.
- A positive reaction is indicated by the absence of HA.
- This can be done by tilting the microtiter plates, which allows RBCs to flow freely.
- Recorded is the dilution rate at which complete inhibition of RBC agglutination occurred.
- The HI titer is therefore the reciprocal of last serum dilution that completely inhibits HA.
- This assay was used to serosurveillance influenza A (H1N1) virus pdm09 and measles virus.
- The effectiveness of pandemic influenza vaccines can be assessed using HI assay.
Advantages of Immunological Diagnostic Techniques
- High level of sensitivity and specificity
- It is relatively easy to do.
- Rapidity and the possibility to test multiple specimens simultaneously.
Limitations of Immunological Diagnostic Techniques
- Interferences can occur. The presence of cross-reactive agents that have similar or identical epitopes to the viral antigen may cause interferences in immunoassays.
- Even though there is no evidence of viral antigen in the sample, endogenous antigens may interact with detection antibodies or antiviral antibodies and cause false positive results.
- When immunoassays are performed in areas where malaria is endemic, their specificity may be compromised.
- The HI assay can be time-consuming and laborious.
- Because there are no standard reagents for assays, the interpretation of the results may differ between laboratories.
- If the specimen is being used for IF, it may be exposed to ultraviolet light for a prolonged time. This can cause fluorescence to fade and could lead to false-negative results.
- Some immunoassays require expensive equipment and reagents.
References
- Daniel Hussien Reta, Tesfaye Sisay Tessema, Addis Simachew Ashenef, Adey Feleke Desta, Wajana Lako Labisso, Solomon Tebeje Gizaw, Solomon Mequanente Abay, Daniel Seifu Melka, Fisseha Alemu Reta, “Molecular and Immunological Diagnostic Techniques of Medical Viruses”, International Journal of Microbiology, vol. 2020, Article ID 8832728, 19 pages, 2020. https://doi.org/10.1155/2020/8832728
- https://agrilife.org/vetmed/files/2012/10/LS_5_4_sample_lesson.pdf
- https://virology-online.com/general/Tests.htm