Crimean-Congo Hemorrhagic Fever (CCHF) is a viral disease that is transmitted to humans by ticks. It is a highly infectious and severe disease that can be fatal in up to 50% of cases. The virus is found in many parts of the world, including Africa, Asia, and Europe. CCHF is caused by a virus in the family Bunyaviridae and is transmitted to humans through the bites of infected ticks. The virus can also be transmitted through contact with infected animal tissues or fluids, or through contact with the blood or tissues of infected humans. CCHF symptoms include fever, headache, muscle aches, dizziness, and fatigue, as well as more severe symptoms such as bleeding from the mouth, nose, and gums, and difficulty breathing. There is currently no specific treatment for CCHF, and the best way to prevent the disease is to avoid tick bites.
Crimean-Congo Hemorrhagic Fever Virus Distribution
Crimean-Congo Hemorrhagic Fever (CCHF) is found in many parts of the world, including Africa, Asia, and Europe. It is most commonly found in countries in Africa, including Algeria, Angola, Burkina Faso, Chad, Democratic Republic of Congo, Ethiopia, Kenya, Morocco, and South Africa. In Asia, the virus is found in countries including Afghanistan, Armenia, Azerbaijan, Georgia, Iran, Kazakhstan, Kyrgyzstan, Pakistan, Russia, Tajikistan, Turkey, and Turkmenistan. In Europe, CCHF is found in Albania, Bosnia and Herzegovina, Bulgaria, Kosovo, Montenegro, and Serbia.
CCHF is transmitted to humans through the bites of infected ticks, which are found on a variety of animals, including cattle, sheep, and goats. The virus can also be transmitted through contact with infected animal tissues or fluids, or through contact with the blood or tissues of infected humans. It is important to take precautions to avoid tick bites, such as using insect repellents and wearing long sleeves and pants when spending time in areas where ticks are common.
Reservoirs and vectors
- The CCHF virus cycles through an enzootic cycle of ticks, vertebrates, and ticks. There is no proof, however, that the virus causes disease in animals.
- The CCHF virus can infect a vast array of domestic and wild animals. The viral infection has been observed frequently in smaller animal species, such as hares and hedgehogs, which harbour juvenile tick vectors.
- It is hypothesised that these little creatures serve as amplifying hosts and perpetuate the virus in nature. This virus has been detected in around 31 tick species across seven genera in the family Ixodidae (hard ticks).
- Members of the Hyalomma genus appear to be the most efficient and prevalent vectors for CCHF among the numerous genera of the Ixodidae family. Other ixodid ticks, such as Rhipicephalus, Boophilus, Dermacentor, and Ixodes, may potentially spread the virus.
- These vectors are capable of both trans-ovarial and trans-stadial virus transmission, so contributing to the natural spread of the virus by remaining infected throughout their development and passing it on to the next generation.
- In general, immature ticks (nymphs) inhabit smaller vertebrates, whereas adult ticks transmit the infection to large vertebrates such as livestock.
Structure of Crimean-Congo Hemorrhagic Fever Virus
- Crimean-Congo Hemorrhagic Fever (CCHF) virus is a single-stranded RNA virus that belongs to the family Bunyaviridae.
- It has a spherical shape and measures approximately 90 to 120 nm in diameter with 5-10 nm projections visible on the surface.. The virus is enveloped, meaning it has a lipid bilayer membrane that surrounds it.
- The CCHF virus has three segments of RNA that make up its genome. These segments are designated as small (S), medium (M), and large (L). The S segment codes for the nucleoprotein, which is involved in the replication of the virus. The M segment codes for the viral envelope proteins, which are important for the virus to enter host cells. The L segment codes for the viral polymerase, which is responsible for replicating the viral genome.
- The viral envelope contains proteins called glycoproteins, which are important for the virus to enter host cells and for evading the host immune system. The envelope also contains a protein called the matrix protein, which helps to maintain the shape of the virus.
- Overall, the structure of CCHF virus is complex and essential for its ability to infect and replicate in host cells.
Genome of Crimean-Congo Hemorrhagic Fever Virus
- The genome of Crimean-Congo Hemorrhagic Fever (CCHF) virus is composed of three segments of single-stranded RNA. These segments are designated as small (S), medium (M), and large (L).
- The S segment codes for the nucleoprotein, which is involved in the replication of the virus.
- The M segment codes for the viral envelope proteins, which are important for the virus to enter host cells.
- The L segment codes for the viral polymerase, which is responsible for replicating the viral genome.
- The genome of CCHF virus is relatively large compared to other RNA viruses, with a total size of around 19 kb.
- The virus replicates in the cytoplasm of infected cells, and the replication of its genome is dependent on the host cell’s machinery.
- The genetic diversity of CCHF virus is relatively low compared to other viruses, and it has a high level of conservation in its genome.
- However, some genetic variation does exist within the CCHF virus population, and this can affect the virulence and transmission of the virus.
- The size of the L, M, and S segments of Crimean-Congo Hemorrhagic Fever (CCHF) virus is not a fixed value, as there can be some variation in the size of these segments among different strains of the virus. However, in general, the L segment is the largest of the three segments, followed by the M segment and then the S segment. The S segment is typically the smallest of the three segments.
- The size of the L, M, and S segments can vary among different strains of CCHF virus, and this can affect the virulence and transmission of the virus. However, the overall size of the CCHF virus genome is relatively large, with a total size of around 19 kb.
Epidemiology of Crimean-Congo Hemorrhagic Fever Virus
- Crimean-Congo Hemorrhagic Fever (CCHF) is a viral disease that is found in many parts of the world, including Africa, Asia, and Europe.
- It is transmitted to humans through the bites of infected ticks, which are found on a variety of animals, including cattle, sheep, and goats. The virus can also be transmitted through contact with infected animal tissues or fluids, or through contact with the blood or tissues of infected humans.
- CCHF outbreaks typically occur in rural areas where ticks are common, and the disease is more common in the summer months when ticks are more active.
- The disease is rare in the general population, but it can occur in people who work in industries such as agriculture, livestock farming, and healthcare.
- CCHF has a high fatality rate, with up to 50% of cases resulting in death. The best way to prevent CCHF is to avoid tick bites by using insect repellents and wearing long sleeves and pants when spending time in areas where ticks are common.
- It is also important to practice good hygiene, such as washing hands frequently and avoiding contact with the blood or tissues of infected individuals.
Transmission of Crimean-Congo Hemorrhagic Fever Virus
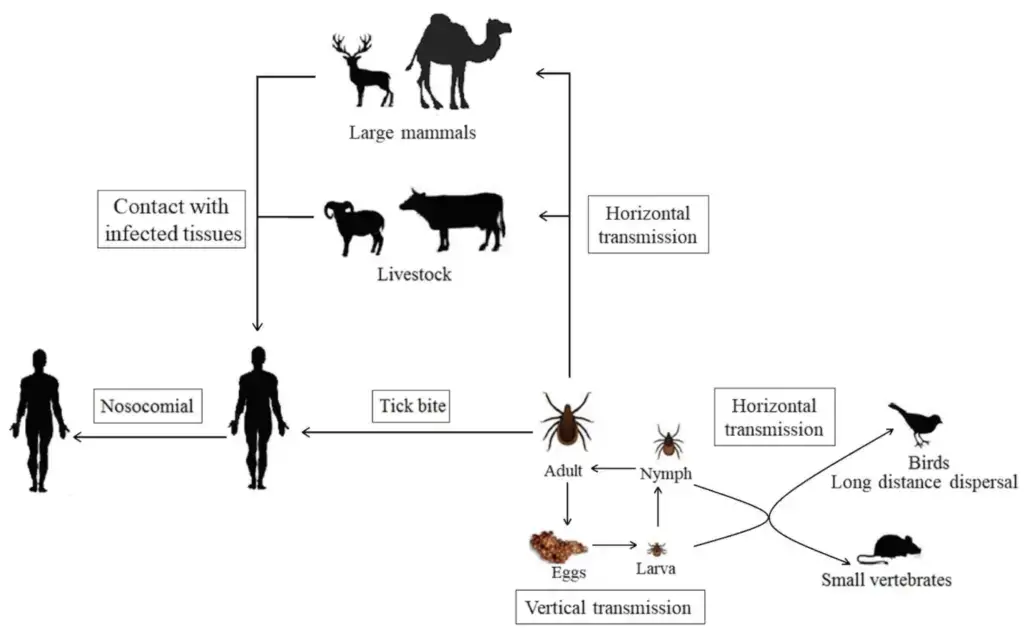
- Crimean-Congo Hemorrhagic Fever (CCHF) is transmitted to humans through the bites of infected ticks. The virus is found in many parts of the world, including Africa, Asia, and Europe, and it is most commonly transmitted to humans through the bites of ticks that are found on a variety of animals, including cattle, sheep, and goats.
- CCHF can also be transmitted through contact with infected animal tissues or fluids, such as when a person handles an infected animal or comes into contact with its blood or tissues. The virus can also be transmitted through contact with the blood or tissues of infected humans, such as through medical procedures or through contact with infected blood or body fluids.
- CCHF is not transmitted through the air, and it is not highly contagious. The risk of transmission can be reduced by practicing good hygiene, such as washing hands frequently and avoiding contact with the blood or tissues of infected individuals. It is also important to avoid tick bites by using insect repellents and wearing long sleeves and pants when spending time in areas where ticks are common.
Replication of Crimean-Congo Hemorrhagic Fever Virus
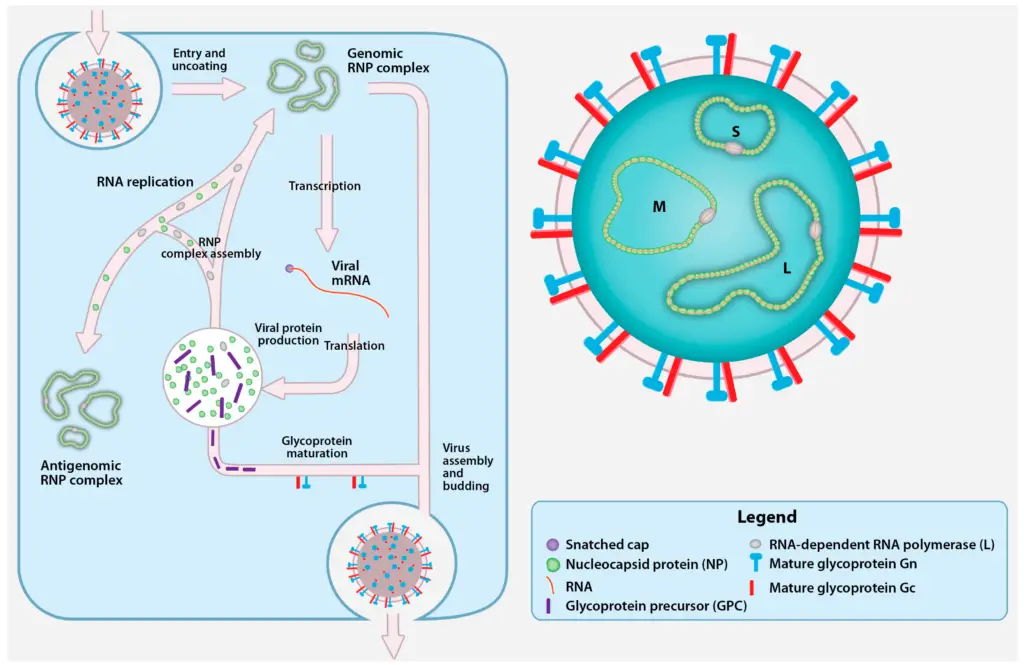
Cell Entry
- Initial binding of CCHFV to the cell surface is mediated by Gn and/or Gc glycoproteins. However, the precise glycoproteins involved in the attachment, ingestion, and fusion of viruses remain unknown.
- As monoclonal antibodies targeting Gc can counteract CCHFV infection of mammalian cells, it is suggested that Gc is responsible for attaching to the cellular receptors. The Gc of CCHFV is believed to likewise mediate fusion; the anticipated fusion loop of CCHFV shares considerable similarity with the Gc fusion loop of Rift Valley fever virus (RVFV).
- Not yet found are the receptors essential for CCHFV entrance into cells. A functional connection between CCHFV Gc and cell surface nucleolin, a protein found mostly in nucleoli, has been postulated [40]. Nucleolin is a receptor for respiratory syncytial virus, an entry factor for Japanese encephalitis virus, and an HIV entry enhancer. To confirm the role of nucleolin in CCHFV cell binding and/or fusion, however, more research is required within the context of CCHFV infection.
- Following attachment to the cell surface, CCHFV is endocytosed by clathrin-mediated endocytosis. Clathrin and the clathrin pit adaptor protein-2 complex are required for entry, but not caveolin-1.
- Cholesterol and a low pH are also required for CCHFV entrance. After endocytosis, CCHFV particles are transported to early endosomes and then to multivesicular bodies (MVB) via a Rab5-dependent process.
- In contrast, inhibiting Rab7-dependent trafficking (from early to late endosomes or out of the MVB) has no effect on infection or CCHFV association with the MVB.
- Interfering with the formation of functional MVBs, such as by depleting endosomal sorting complex required for transport (ESCRT) pathway components, decreased CCHFV infection levels. This suggests that the MVB is the primary organelle where the CCHFV envelope unites with host membranes.
Transcription and Replication
- The genomic RNPs are released into the cytosol upon cell entrance and fusion, and the encapsulated vRNA serves as a template for the L protein to synthesis viral mRNA. No studies have characterised the 3′ termini of nairovirus mRNA or the components involved in transcription termination.
- Dugbe virus, a similar nairovirus, contains a 7-methylguanylate (m7G) cap containing sequences acquired from cellular mRNA in its 5′ terminal sections. To activate viral mRNA synthesis, the L protein utilises m7G-capped primers that are extracted from cellular mRNA by an endonuclease domain within the L protein.
- As reported for endonucleases of other L proteins, the CCHFV L protein contains a residue (D693) anticipated to coordinate a Mn2+ that is essential for the cap snatching action. Mutating D693 eliminates L protein transcription activity selectively, but has no effect on its capacity to replicate CCHFV genome analogues. This suggests that CCHFV replication does not begin with capped primers.
- The replication of genomic RNPs requires the replication and encapsidation of uncapped, negative sense vRNA and complementary positive sense RNA (cRNA). The L protein and NP are essential for the replication of RNPs.
- The encapsulated versions of cRNA and vRNA are referred to as antigenomic and genomic RNPs, respectively. Since CCHFV is a negative strand RNA virus, genomic RNPs are utilised as a template to create capped mRNA and antigenomic RNPs.
- During replication of the genomic RNPs, the L protein synthesises a cRNA and NP subunits are added to the elongating strands to produce antigenomic RNPs, which are then employed as a template to generate genomic RNPs.
Glycoprotein Maturation, Viral Assembly, and Egress
- RNP replication is followed and eventually accompanied by the processing and maturation of viral proteins. The maturation of CCHFV GPC is unusually complicated and has little in common with the maturation of glycoproteins by other bunyaviruses.
- GPC maturation produces the structural glycoproteins Gn and Gc, the secreted non-structural proteins GP160, GP85, and GP38, and the non-structural M protein (NSM. Nairoviruses are the only known bunyaviruses to encode non-structural glycoproteins that are secreted.
- The GPC is highly glycosylated and then cleaved by host proteases, including the proprotein convertases, a family of mammalian serine proteases known to degrade a wide range of cellular proteins, viral glycoproteins, and bacterial toxins.
- The GPC contains an N-terminal signal peptide that directs its production to the secretory route. After elongation and translocation into the endoplasmic reticulum (ER), the signal peptide is eliminated, the GPC is N-glycosylated and folded, intramolecular disulfide bridges are created, and the precursor protein’s transmembrane domains cross the ER membrane five times.
- Prior to translation, GPC is cleaved into the Gn precursor (PreGn), the Gc precursor (PreGc), and the NSM. This phase is believed to need the signal peptidase and intramembrane cleaving proteases (I-CLiPs), as the production of these three proteins requires cleavage after the signal peptide and at or within transmembrane domains-2 and -4.
- PreGn and PreGc are transported from the ER to the Golgi complex, where the PreGn mucin-like domain (MLD) acquires many O-linked glycans. PreGn can reach the Golgi complex, the putative site of CCHFV assembly, without PreGc, whereas PreGc cannot exit the ER without PreGn.
- Mutating the N577 glycosylation site of PreGn, which is positioned in the mature Gn region, inhibits PreGn from leaving the ER and hinders the secretion of GP160, GP85, and GP38, indicating that glycosylation of N577 plays a crucial role in folding and proper trafficking to the Golgi complex.
- PreGn and PreGc must undergo limited endoproteolysis to complete GPC maturation. Unidentified is the host protease necessary to convert PreGc to Gc. Considering that the PreGc cleavage motif of CCHFV (RKPL) is identical to the GPC cleavage pattern of Guanarito virus, an arenavirus that is cleaved by the subtilisin kexin isozyme-1/site-1 protease (SKI-1/S1P), this protease or a protease with similar specificity (SKI/S1P-like) might clea PreGn is cleaved by SKI-1/S1P early in the secretory route, either in the endoplasmic reticulum (ER) or after its escape from the cis-Golgi apparatus.
- PreGn cleavage at the RRLL motif releases the MLD and GP38 domain-containing N-terminal products (GP160 and GP85) [58]. Furin can further cleave GP160/85 in the trans-Golgi network (TGN) at a conserved RSKR motif at the junction of the MLD and the GP38 domain.
- This cleavage is hypothesised to liberate the GP38 domain from the MLD, despite the fact that current antibodies can only detect GP38 and the uncleaved MLD-containing glycoproteins (GP160/85), but not the cleaved MLD.
- Initial speculation suggested that GP160 was a dimer of GP85 due to the presence of MLD and GP38 epitopes in both glycoproteins; however, denaturation with urea and reducing agents did not alter the mobility of either GP160 or GP85. In addition, the sensitivity of glycosidases specific for N- and O-linked glycans indicates that the glycan composition of the non-structural glycoproteins (GP160, GP85, and GP38) is likely comparable.
- Consequently, additional studies are required to explain the difference in SDS-PAGE mobility between GP85 and GP160.
- We have a limited understanding of CCHFV assembly and outflow. Similar to other bunyaviruses, CCHFV RNPs are most likely located in the cytoplasm, and NP is situated close to the Golgi complex.
- The subcellular localization of viral glycoproteins frequently determines the location of virus budding; accumulation of glycoproteins in the Golgi complex and TGN and NP in perinuclear regions is compatible with budding and assembly of CCHFV particles in the Golgi complex and/or TGN.
- Following assembly, CCHFV particles are released by exocytosis and egress occurs from the basolateral membrane in polarised epithelial cells. Often, there is no detectable cytopathology.
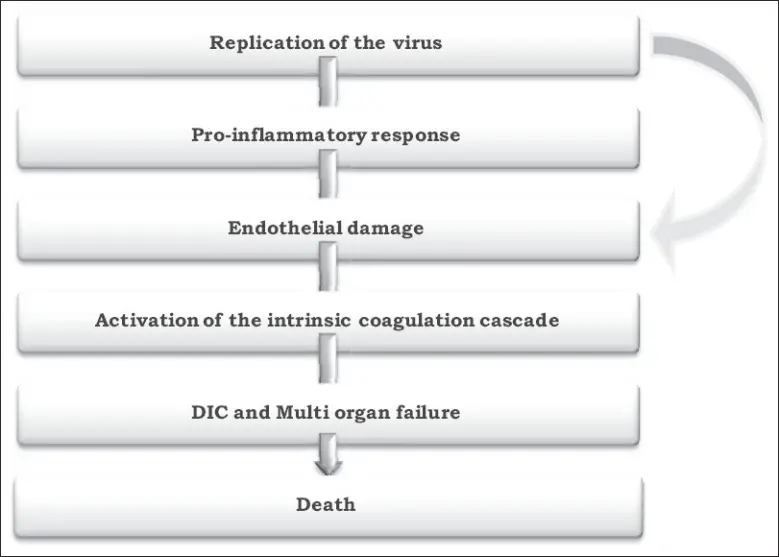
Pathogenesis of Crimean-Congo Hemorrhagic Fever
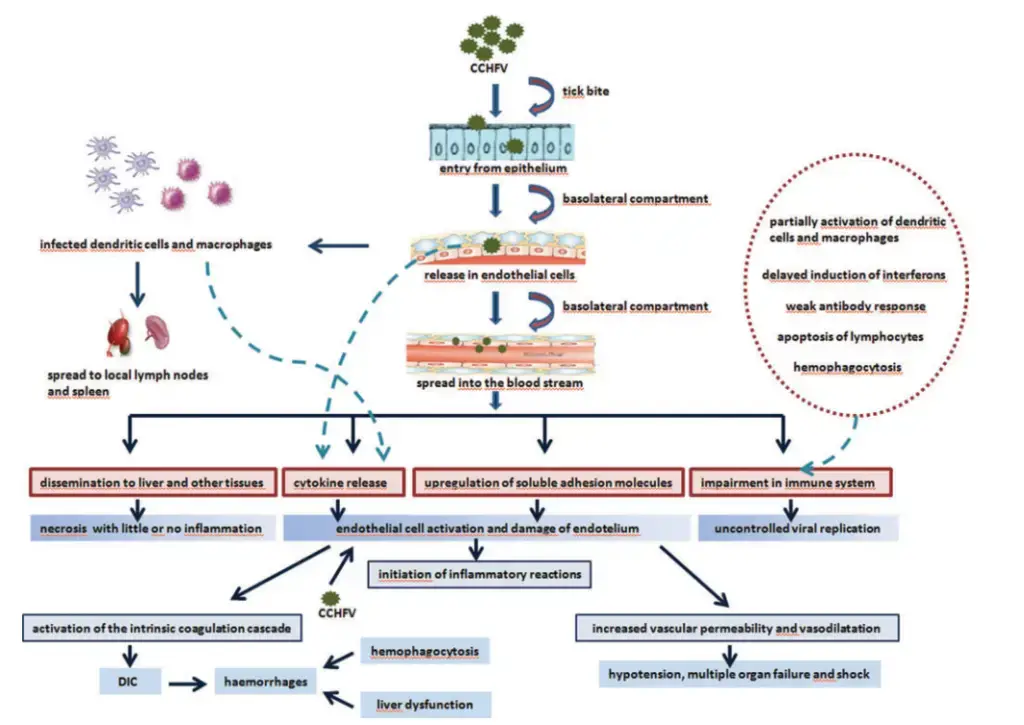
- Initially, the vector’s gut is infected, and after a few days or weeks, the virus manifests in the vector’s saliva.
- When the vector takes a blood meal, the infective saliva penetrates the human or other vertebrate host’s tiny capillaries or lymph nodes.
- A few days of incubation follow, following which the vertebrate host develops viremia.
- The host develops a fever, exhibiting the more severe signs and symptoms typical of the infecting virus.
- Immunoglobulin M (IgM) first predominates, followed by immunoglobulin G (IgG), and the host recovers unless a specific target organ is harmed by a typical humoral immune response.
- In Crimean-Congo hemorrhagic fever, the liver and vascular endothelium are the target organs.
- This further contributes to hemostatic failure by inducing platelet aggregation and degranulation, followed by the activation of the intrinsic coagulation cascade.
- Important regulators of the aetiology and death of CCHF patients are proinflammatory cytokines.
- Significantly elevated levels of Interleukin (IL)-6 and Tumor Necrosis Factor (TNF)- are observed in patients with fatal CCHF.
Clinical manifestations of Crimean-Congo Hemorrhagic Fever
- Humans are the only host in which CCHFV clinical symptoms are detectable. Four unique phases characterise the typical progression of CCHF infection: the incubation period, the prehemorrhagic phase, the hemorrhagic phase, and the convalescent phase.
- The incubation time for the CCHF virus ranges from 3 to 7 days. The mean duration is significantly impacted by the route of infection, viral load, and source of infection, which may be blood or animal tissue. The minimum viral load necessary for disease transmission is 1-10 organisms.
- The disease begins with a prehemorrhagic phase marked by vague prodromal symptoms that mimic those of other viral illnesses. The most prominent symptoms include high temperature, myalgia, headache, nausea, abdominal pain, and diarrhoea that is not bloody.
- This condition is associated by hypotension, relative bradycardia, tachypnea, conjunctivitis, pharyngitis, and flushing or rash of the skin.
- In the majority of patients, the prehemorrhagic phase evolves into the hemorrhagic phase after a duration of 4-5 days. The hemorrhagic phase is often brief and progresses rapidly, manifesting as increasing haemorrhage and diathesis.
- Petechiae, conjunctival haemorrhage, epistaxis, hematemesis, hemoptysis, and melena are examples of these. Additionally, certain patients may have hepatosplenomegaly.
- Forty to sixty percent of cases are deadly. In severe situations, multiorgan failure, disseminated intravascular coagulation, and circulatory shock culminate in death. During hemorrhagic presentations, acute respiratory distress syndrome (ARDS) and diffuse alveolar bleeding, accompanied by systemic inflammatory reaction, have also been reported.
- Convalescence develops 10 to 20 days following the onset of sickness in survivors. During this period, patients may experience a weak pulse, tachycardia, hearing loss, memory loss, and hair loss. However, only a few epidemics have recorded severe aftereffects.
Diagnosis of Crimean-Congo Hemorrhagic Fever Virus
Multiple laboratory tests are capable of diagnosing CCHF virus infection:
- ELISA,
- antigen detection,
- serum neutralisation,
- reverse transcriptase polymerase chain reaction (RT-PCR), and
- cell culture-based virus isolation.
Virus or RNA detection in blood or tissue samples is used to diagnose patients with terminal diseases or within the initial few days of sickness, as these patients typically do not generate a detectable antibody response.
Tests on patient samples pose a high risk of biohazards and should only be undertaken under conditions of maximal biological containment. If samples have been inactivated, however (through virucides, gamma rays, formaldehyde, heat, etc.), they can be handled in a basic biosafety setting.
Treatment of Crimean-Congo Hemorrhagic Fever
There is no specific antiviral treatment for Crimean-Congo Hemorrhagic Fever (CCHF). Treatment is mainly supportive and involves the following:
- Providing fluids and electrolytes to prevent dehydration
- Administering blood transfusions to replace lost blood
- Treating complications, such as low blood pressure, organ failure, and bleeding
- Providing oxygen therapy if the patient has difficulty breathing
It is important to practice infection control measures to prevent the spread of CCHF, especially in hospitals and other healthcare settings. This includes wearing protective clothing, such as gloves and masks, and properly disinfecting surfaces and equipment.
Research is ongoing to develop effective therapies for CCHF, such as vaccines and antiviral drugs. Some studies have shown that the antiviral drug ribavirin may be effective in treating CCHF, but more research is needed to confirm this.
Prevention and control of Crimean-Congo Hemorrhagic Fever Virus
There are several measures that can be taken to prevent and control the spread of Crimean-Congo Hemorrhagic Fever (CCHF). These include:
- Avoiding contact with ticks: Ticks are the main vectors of CCHF, so it is important to take measures to prevent tick bites. This includes wearing protective clothing, using insect repellent, and checking for ticks regularly.
- Practicing infection control measures: In hospitals and other healthcare settings, it is important to practice infection control measures to prevent the spread of CCHF. This includes wearing protective clothing, such as gloves and masks, and properly disinfecting surfaces and equipment.
- Educating the public: Raising awareness about CCHF and how it is transmitted can help to prevent the spread of the disease.
- Controlling ticks: Implementing measures to control the tick population, such as using insecticides or removing tall grass and brush, can help to reduce the risk of CCHF.
- Developing vaccines and antiviral drugs: Research is ongoing to develop vaccines and antiviral drugs to prevent and treat CCHF.
- Implementing quarantine measures: In cases where CCHF is spreading within a community, quarantine measures may be implemented to contain the outbreak.
References
- Makwana D, Yadav PD, Kelaiya A, Mourya DT. First confirmed case of Crimean-Congo haemorrhagic fever from Sirohi district in Rajasthan State, India. Indian J Med Res. 2015 Oct;142(4):489-91. doi: 10.4103/0971-5916.169221. PMID: 26609042; PMCID: PMC4683835.
- Akıncı, E., Bodur, H., & Leblebicioglu, H. (2013). Pathogenesis of Crimean-Congo Hemorrhagic Fever. Vector-Borne and Zoonotic Diseases, 13(7), 429–437. doi:10.1089/vbz.2012.1061
- Appannanavar SB, Mishra B. An update on crimean congo hemorrhagic Fever. J Glob Infect Dis. 2011 Jul;3(3):285-92. doi: 10.4103/0974-777X.83537. PMID: 21887063; PMCID: PMC3162818.
- https://www.ecdc.europa.eu/en/crimean-congo-haemorrhagic-fever/facts/factsheet
- https://journals.asm.org/doi/10.1128/JVI.00752-06
- https://africacdc.org/disease/crimean-congo-haemorrhagic-fever/
- https://www.frontiersin.org/articles/10.3389/fcimb.2017.00213/full
- https://www.mdpi.com/2076-2607/9/9/1907
- https://www.science.org/doi/10.1126/science.abl6502
- https://www.sciencedirect.com/topics/medicine-and-dentistry/crimean-congo-hemorrhagic-fever
- https://www.who.int/news-room/fact-sheets/detail/crimean-congo-haemorrhagic-fever#:~:text=Crimean%2DCongo%20haemorrhagic%20fever%20(CCHF)%20is%20a%20widespread%20disease,rate%20of%2010%E2%80%9340%25.
- https://www.who.int/health-topics/crimean-congo-haemorrhagic-fever#tab=tab_1
- https://www.cdc.gov/vhf/crimean-congo/index.html
- https://www.cdc.gov/vhf/crimean-congo/pdf/factsheet.pdf
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.