What is Colony Morphology of Bacteria?
Colony morphology of bacteria means the visible features seen in bacterial colonies when they grow on solid media like agar plates. Each colony usually comes from one bacterial cell that divides and forms a clump. These clumps or colonies can look very different based on the species and conditions, so their appearance can give hints about what bacteria it is.
Scientists look at things like the shape (round, irregular), size (measured in mm), edge type (smooth or wavy), elevation (flat or raised), texture (dry, mucoid), and color. Some colonies may be shiny or dull, while others might produce pigments that spread in the medium. Sometimes you even notice special smells or color changes on blood agar due to hemolysis. All these features help microbiologists quickly guess which bacteria might be growing before running more complicated tests. It’s a basic but powerful tool in identifying bacteria, spotting contamination, or checking if a culture is pure.
Characteristics of Bacterial Colony
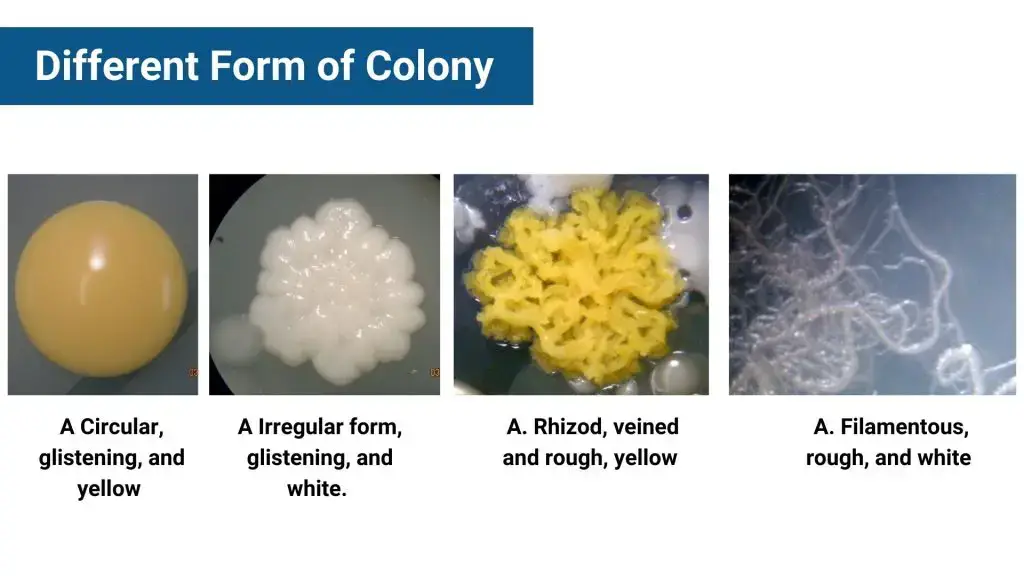
- Shape – colonies can be round, irregular, filamentous, or rhizoid
- Size – ranges from punctiform (less than 1 mm) to large colonies
- Edge/Margin – smooth, undulate, lobate, or filamentous
- Color – varies; some bacteria produce pigments, others do not
- Opacity – transparent, translucent, opaque, or iridescent
- Elevation – flat, raised, convex, pulvinate, umbonate
- Surface – smooth, glistening, rough, dull, or rugose
- Consistency – butyrous, viscid, brittle, or mucoid
- Hemolysis – alpha (green), beta (clear), or gamma (no change) on blood agar
- Odor – some colonies emit distinctive smells, e.g., grape-like or musty
- Motility – some bacteria exhibit swarming or spreading patterns
- Pigmentation – some produce diffusible pigments, others do not
Morphological Characteristics of Bacterial colonies

- Colony shape – includes form, elevation, and margin, each gives vital ID clues
- Form – common shapes are circular, irregular, filamentous, rhizoid, spindle
- Elevation – side view rise of colony; types: flat, raised, umbonate (knobby), crateriform (saucer-like), convex (broader center), pulvinate (cushion-shaped)
- Margin – colony edges, important for ID; can be entire (smooth), irregular, undulate (wavy), lobate (finger-like), curled (spiral), filiform (thread-like), erose (jagged)
- irregular shapes or margins often indicate motile bacteria like Proteus spp.
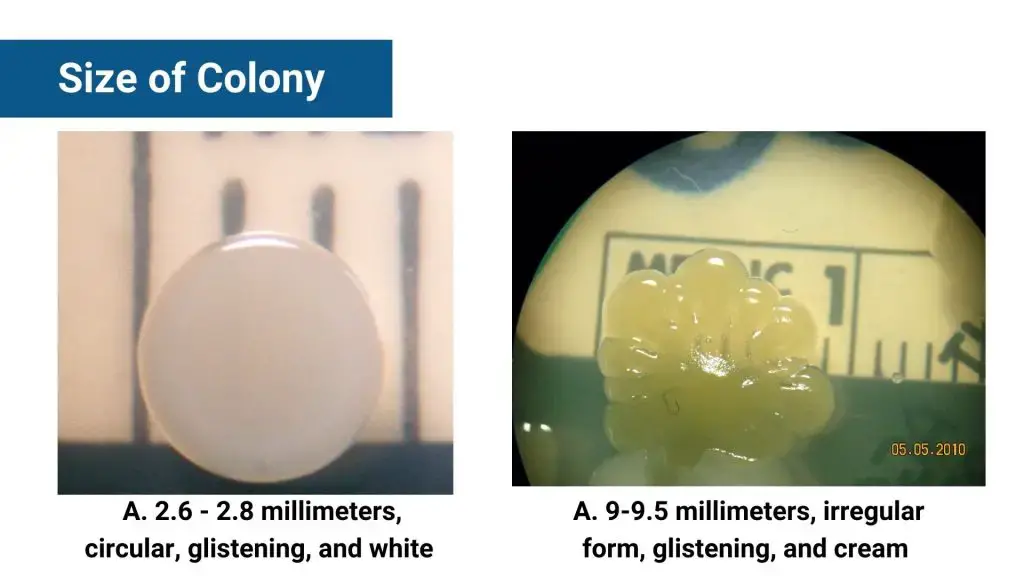
- Colony size – measured by diameter (mm) or relative terms: punctiform (tiny, <0.5 mm), small (<1 mm), medium (~1 mm), large (>1 mm)
- colonies larger than ~5 mm usually motile
- Surface appearance – texture and shine seen with naked eye or magnifier
- common textures: shiny/glistening, dull, rough, veined, wrinkled, smooth
- example: Bacillus species form dry, wrinkled colonies; Pseudomonas stutzeri shows wrinkled colonies too
- Consistency/Texture – felt with inoculating loop; terms include dry, moist, mucoid (sticky), viscid (sticks hard), brittle/friable (breaks apart)
- Colony color (Pigmentation) – varies with bacterial species and medium
- examples:
- Pseudomonas aeruginosa produces green pigment
- Mycobacterium tuberculosis forms buff-colored colonies on L.J medium
- Serratia marcescens shows red-colored colonies
- examples:
- Opacity – how light passes through colony; types are opaque (not see-through), translucent (almost clear but blurry), transparent (clear), iridescent (color changes in reflected light)
- e.g., Streptococcus β-hemolytic colonies are pinpoint translucent on blood agar; Staphylococci colonies usually opaque, smooth, circular
- Special colony types
- Draughtsman colonies of Streptococcus pneumoniae show raised centers when young, later flatten with depressed center and raised edges, forming ringed appearance
- Observation tools – colony morphology viewed by naked eye, magnifying lens, or dissecting microscope for finer detail
- General notes
- Motile organisms often have irregular shapes or margins and larger colonies
- Size and shape combined with other features help bacterial ID
- Texture and color changes can suggest metabolic or growth differences among bacteria




Which Microorganisms can grow on a nutrient agar plate?
Microorganisms like bacteria thrive on solid media as part of colonies. A colony is what? It is an apparent mass of microorganisms that is derived from a single mother cell.
The morphology of the colony of an organism is essential to identify it. Different organisms can thrive within solid substrates. These include:

- Bacteria – A colony made up of bacteria typically appear in colors white cream, yellow, and white. In terms of design, the bacteria colony tends to be quite circular.

- Yeasts – The colonies of yeast, an fungus type It is a bit like the colonies of bacteria. They may form an uncolored patch that has shiny surfaces.

- Mold – It’s a kind of fungi which usually shows a greyish whitish color and has fuzzy edges. It usually changes to a new hue.

Colony morphology of bacteria on nutrient agar
- Shape – Circular, irregular, rhizoid, filamentous
- Size – Punctiform (<1 mm), small (1–2 mm), medium (2–4 mm), large (>4 mm)
- Margin – Entire (smooth), undulate (wavy), lobate (lobed), filamentous, rhizoid (root-like)
- Elevation – Flat, raised, convex, umbonate (raised center), pulvinate (very convex)
- Surface – Smooth, rough, glistening, dull, wrinkled, moist, mucoid (slimy), dry
- Opacity – Transparent, translucent, opaque
- Color – White, cream, yellow, red, pink, brown, greenish (due to pigments)
- Texture – Brittle, creamy, sticky, dry, mucoid, viscid (slimy), friable (crumbly)
- Odor – Sweet, putrid, fruity, grape-like, musty
- Hemolysis – Alpha (greenish zone), beta (clear zone), gamma (no change)
These features aid in preliminary bacterial identification and differentiation on nutrient agar. For example, Staphylococcus aureus forms golden-yellow, smooth, convex colonies with entire margins and a creamy texture. In contrast, Pseudomonas aeruginosa produces large, flat, smooth colonies that may secrete blue-green pigments and emit a characteristic grape-like odor.
Examples;
- Staphylococcus aureus – golden-yellow, circular, convex colonies with entire margins, smooth texture
- Escherichia coli – greyish-white, large, smooth, opaque discs; some strains may produce mucoid colonies
- Pseudomonas aeruginosa – flat, smooth colonies; may produce blue-green pigments and emit a characteristic grape-like odor
- Bacillus subtilis – cream-colored, irregular colonies with undulate margins; may exhibit a rough texture
- Bacillus mycoides – white, opaque, rhizoid colonies with characteristic swirling patterns
- Staphylococcus epidermidis – white, raised, cohesive colonies approximately 1–2 mm in diameter
- Burkholderia pseudomallei – wrinkled, metallic colonies with an earthy odor
Colony morphology of bacteria on blood agar
- Hemolysis type
- α‑hemolysis – partial lysis, greenish zone (e.g., Streptococcus pneumoniae, viridans streptococci)
- β‑hemolysis – clear zone from complete lysis (e.g., Staphylococcus aureus, Streptococcus pyogenes, E. coli)
- γ‑hemolysis – no hemolysis (e.g., S. epidermidis, Moraxella catarrhalis)
- Shape & size
- Circular colonies generally seen in S. aureus, E. coli, S. epidermidis (~1 mm)
- Irregular or spreading forms seen with Pseudomonas aeruginosa, Proteus mirabilis (swarming)
- Elevation & margin
- Raised convex with smooth entire margins – S. aureus
- Flat or low convex with irregular margins – P. aeruginosa
- Texture & surface
- Glistening, smooth – Haemophilus influenzae satellites, Corynebacterium striatum
- Moist, shiny, opaque – P. aeruginosa, E. coli
- Wrinkled or rough – Burkholderia pseudomallei on blood agar, B. anthracis “ground-glass”
- Color & pigmentation
- Golden yellow – S. aureus β‑hemolytic colonies
- Gray-white – E. coli, Klebsiella
- Non‑pigmented translucent – H. influenzae microcolonies
- Odor & other traits
- P. aeruginosa has grape‑like scent; Proteus mirabilis gives a pungent odor and shows swarming on agar
- B. cereus appears large, irregular, sometimes with β-hemolysis
Examples-
- Staphylococcus aureus – circular, 1 mm, raised smooth golden colonies, clear β-hemolysis zone
- Streptococcus pyogenes – small grey colonies, β-hemolysis, regular margins
- S. pneumoniae – raised, mucoid, α-hemolysis, sometimes donut‑shaped
- Escherichia coli – circular, moist, opaque, white-grey, β‑hemolysis on blood agar
- Pseudomonas aeruginosa – irregular, moist, shiny, flat, produces pigments, grape odor
- Moraxella catarrhalis – circular white, γ‑hemolytic, smooth on blood agar
- Corynebacterium striatum – 1‑2 mm, smooth moist white colonies, non‑hemolytic
Colony morphology of bacteria on macconkey agar
- Lactose fermenters (pink/red colonies)
- Escherichia coli – flat, dry, pink colonies, ~2–3 mm, non-mucoid, with darker pink precipitate of bile salts
- Klebsiella spp. – large (4–6 mm), mucoid, pink-red colonies with surrounding diffuse pigment
- Enterobacter spp. – smaller than Klebsiella, pink mucoid colonies.
- Late/slow lactose fermenters
- Citrobacter spp. – appear non‑fermenting for ~24 h but then turn light pink after ~48 h.
- Serratia marcescens – may produce red pigment at lower temp, slow lactose fermenter.
- Non‑lactose fermenters (colorless/white colonies)
- Salmonella spp. – colorless, convex 2–3 mm colonies with serrated margins.
- Shigella spp. – flat, jagged 1–2 mm colonies, colorless; S. sonnei slow fermenter.
- Proteus spp. – pale non‑fermenter colonies, swarming growth, foul odor.
- Pseudomonas spp. – colorless, smooth, flat colonies often with greenish/brownish pigment.
- Yersinia spp. – colorless to peachy colonies
- Others
- Mucoid lactose fermenters (MLF) – colony variants; mucoid due to capsule (e.g. Klebsiella, Enterobacter)
- Lactose non‑fermenters (NLF) – remain pale even after incubation.
- No growth – most Gram‑positive bacteria inhibited by bile salts and crystal violet.
MacConkey agar thus helps distinguish gram-negative bacteria based on lactose metabolism, with key morphological clues like color, size, margin, mucoidity & growth patterns guiding preliminary ID.
Colony morphology of different bacteria
- Pseudomonas aeruginosa – flat, smooth colonies on nutrient agar often with greenish pigment, grape‑like odor, flat elevation with entire margins and moist surface.
- Serratia marcescens – small circular colonies, sometimes weakly umbonate, brick‑red pigment at ~25 °C.
- Staphylococcus aureus – circular, entire margins, convex, smooth & creamy, golden‑yellow pigment, “old socks” odor.
- Bacillus mycoides – large white opaque, rhizoid/hairy colonies with swirling pattern, rapid spreading.
- Micrococcus luteus – lemon‑yellow, circular, smooth colonies (~yellow dots on TSA).
- Corynebacterium xerosis – circular colonies with entire margins on nutrient agar.
- Neisseria spp. – sticky, moist, circular, translucent colonies on nutrient agar.
- Streptomyces albus – filamentous margin, convex elevation, chalky colonies that may depress agar.
- Mycobacterium smegmatis – irregular, rough textured colonies on agar.
- Bacillus anthracis – irregular shape, weakly undulate margin, ground‑glass appearance.
On MacConkey agar (selective for Gram‑negatives)
- Escherichia coli – flat, dry pink to rose‑red, ~2‑3 mm, non‑mucoid with bile‑salt precipitate.
- Klebsiella spp. – large (4‑6 mm), mucoid pink‑red colonies with diffuse pigment
- Enterobacter spp. – smaller mucoid pink colonies
- Citrobacter spp. – pale initially, light pink after ~48 h (late fermenter)
- Serratia spp. – late fermenter, may show red pigment if incubated at lower temperatures
- Salmonella/Shigella/Proteus/Providencia/Pseudomonas/Yersinia – colorless/non‑lactose fermenters; Salmonella: convex, serrated margin ~2‑3 mm; Shigella: flat, jagged; Proteus: pale, swarming; Pseudomonas: smooth, flat, possible pigment; Yersinia: colorless to peach
Colony morphology of gram negative bacteria
- Escherichia coli – circular, smooth, moist, grey‑white colonies on nutrient agar, ~2–3 mm; ferments lactose, often pink on MacConkey but here on general medium appears colorless; useful clue for enterobacteria
- Klebsiella pneumoniae – large mucoid, raised, glistening colonies; encapsulated, so appear sticky; prominent on nutrient agar too
- Salmonella spp. – medium convex colonies, smooth, colorless on MacConkey; on nutrient agar appear pale, ~2–3 mm, often with serrated margins
- Proteus mirabilis – swarming growth on non-selective agar: concentric rings of spreading, pale, irregular colonies; often give fishy odor
- Pseudomonas aeruginosa – flat to slightly raised, smooth colonies; may secrete blue‑green pyocyanin or yellow pyoverdine pigments; grape‑like odor is characteristic
- Eikenella corrodens – small greyish colonies that often pit/dent the agar; may give greenish discoloration and bleach‑like odor
General morphological traits of Gram‑negative colonies
- shape: mostly circular or irregular (in swarming species)
- elevation: flat, raised, convex depending on species
- margin: entire in E. coli, serrated in Salmonella, filamentous in Proteus
- surface: smooth and moist in E. coli & Klebsiella; mucoid for encapsulated types; dry & wrinkled for Proteus swarms
- color: generally non‑pigmented pale; pigment‑producing Pseudomonas stands out
- odor: Pseudomonas (grape‑like), Proteus (foul/fishy), Eikenella (bleach‑like)
Colony morphology of gram positive bacteria
- Staphylococcus aureus – circular, 1–3 mm, convex, smooth‑glistening, golden‑yellow colonies; often with clear β‑hemolysis on blood agar, on nutrient agar appears creamy opaque
- Staphylococcus epidermidis – white, raised, cohesive ~1–2 mm, non‑hemolytic colonies on blood agar; smooth and glossy
- Corynebacterium striatum – small (1–2 mm), moist, smooth, white colonies on blood agar; club‑shaped pleomorphic bacilli
- Micrococcus luteus – bright yellow, circular, smooth, glistening colonies on nutrient agar; ~1 mm in diameter
- Bacillus subtilis – large, irregular, rough colonies on nutrient agar; produce wrinkled, sometimes spreading margins
- Bacillus cereus – creamy‑white to yellowish, irregular, often waxy or ground‑glass colonies on blood agar; spore‑forming
- Bacillus licheniformis – rough cream‑colored colonies; may turn reddish with pulcherrimin pigment in iron‑rich media
- Streptococcus pyogenes – small (0.5–1 mm), circular, translucent to grey, convex colonies with clear β‑hemolysis
- Streptococcus pneumoniae – small, umbonate, mucoid, translucent to grey colonies; partial α‑hemolysis (greenish zone)
- Streptomyces albus – dry chalky white colonies with filamentous margins; may depress agar surface
General traits – Gram‑positives often produce:
- circular shape, raised or convex elevation
- smooth or glistening surfaces for staphylococci and micrococci
- rough, filamentous margins in bacilli like B. subtilis and actinobacteria
- pigmentation (yellow, cream, golden)
- hemolysis patterns on blood agar distinguish types (β, α, or γ)
Colony morphology of lactic acid bacteria
- Shape & size – typically small, circular, 0.1–1 mm, but some strains form pinpoint colonies only after 24–48 h on MRS or M17 agar
- Colour – whitish to creamy or off‑white; some appear milky‑white on CaCO₃‑supplemented MRS
- Elevation & margin – convex, raised, with regular entire edges, smooth glossy surface
- Clear zone formation – distinct halo or clear zone around colony where acid dissolves CaCO₃ (on GYC or CaCO₃ agar), indicating strong acid producers like Lactococcus or Lactobacillus
- Texture & appearance – shiny, sometimes glistening; heterofermentative LAB may produce minimal gas bubbles but colonies stay intact
- Growth rate – slow relative to respiring bacteria; produce colonies visible ~24‑48 h, often up to 2‑3 mm in diameter
- Hemolysis – mostly non‑hemolytic (γ‑hemolysis) on blood agar, though L. acidophilus may show weak β‑hemolysis
Common examples:
- Lactobacillus spp. – small, round, milky‑white colonies, convex, non‑hemolytic; strong acid producers often create clear zones on CaCO₃ agar
- Lactococcus lactis – circular, off‑white, shiny convex colonies around 0.5–1 mm, entire margins
- Leuconostoc mesenteroides – small grayish round colonies under 1 mm on MRS; appears as cocci chains microscopically
- Streptococcus thermophilus – yellowish, convex round colonies 0.7–0.9 mm on selective media
How to identify of bacteria based on colony morphology?
Here is the step by step guid for identification of bacteria based on colony morphology;
- Observe shape – check if colonies are circular, irregular, rhizoid, or filamentous
- Check size – classify as punctiform (<1 mm), small (1–2 mm), medium (2–4 mm), or large (>4 mm)
- Look at margins – smooth edges suggest Staphylococcus, while undulate, lobate, or serrated hint Bacillus, Salmonella, or Proteus
- Note elevation – flat, raised, convex, or umbonate help narrow species; e.g., Streptococcus pneumoniae is umbonate
- Assess surface texture – smooth, rough, wrinkled, or mucoid; mucoid often indicates encapsulated strains like Klebsiella
- Record opacity – colonies may appear transparent, translucent, or opaque depending on thickness, media, and pigments
- Identify color – pigments like golden-yellow (S. aureus), green (P. aeruginosa), red (Serratia), or cream (E. coli) help species-level clues
- Check consistency – touch with sterile loop: buttery, sticky, dry, brittle, or viscous
- Look for hemolysis (if blood agar used)
- β-hemolysis – clear zone, complete lysis (S. pyogenes, S. aureus)
- α-hemolysis – green zone, partial lysis (S. pneumoniae)
- γ-hemolysis – no lysis (Enterococcus faecalis)
- Odor detection – not always used but helpful
- Pseudomonas smells fruity or grape-like
- Proteus has a putrid, fishy odor
- Medium-specific changes
- MacConkey: lactose fermenters turn pink (E. coli), non-fermenters stay pale (Salmonella)
- MRS + CaCO₃: LAB produce clear halos
- XLD, EMB, blood agar, etc., reveal selective or differential traits
- Compare with known database – use colony traits alongside gram stain, biochemical tests, and genetic methods for full ID
- Repeat on multiple media – confirm morphology consistency across nutrient agar, MacConkey, blood agar, or specialized media
Colony Morphology in Fungi
- Growth form and hyphal structure – most filamentous fungi grow by hyphae forming a fluffy, cottony, wool‑like or powdery mat on agar; yeast‑like fungi remain smooth and structureless but may form pseudohyphae giving wrinkles or fluffiness
- Colony shape and size – colonies can be circular, irregular, spreading or restricted; size varies with species, medium, and age (e.g., Penicillium roqueforti ~40–50 mm on standard media)
- Surface texture – descriptors include velvety, granular, floccose (cottony), powdery, chalky, glabrous (smooth), silky or waxy
- Margin characteristics – colony edges may be filiform, lobate, scalloped, ragged or smooth reflecting hyphal growth patterns
- Elevation – colonies can be flat, raised, convex, umbonate; filamentous molds often appear raised or fluffy
- Pigmentation (obverse & reverse) – surface colors can be white, green, black, orange, yellow; reverse side often shows distinct pigmentation (e.g., olive‑brown Penicillium roqueforti)
- Opacity/transparency – fungal mats are typically opaque or translucent not clear
- Age‑related changes – colonies may change color, texture, diameter with maturity; e.g., green center with white fringe in Aspergillus versicolor, then yellow/orange Biolog
- Sporulation patterns – sporulating structures often form characteristic textures or pigmentation like brush‑shaped phialides in Penicillium and conidiophore patterns in Aspergillus
- Differentiation from bacteria – fungal colonies appear hairy, filamentous, larger and powder‑like, while bacteria are small, smooth, creamy or damp glossy spots
- Taxonomic and diagnostic value – macromorphology aids preliminary identification (e.g. Microsporum gypseum cottony buff with scalloped edges), supports further microscopy/sequencing
Factors affects the colony morphology of bacteria
The following important factors influence the colony morphology of bacteria;
- Temperature – affects growth rate, colony size, pigment production (e.g., Serratia marcescens makes red pigment at ~25 °C but not at 37 °C)
- pH – influences enzyme activity and colony appearance; extreme pH slows growth and can alter texture
- Nutrient composition – rich media yield larger, smoother, opaque colonies; minimal media produce smaller, sometimes translucent colonies.
- Agar concentration & gelling agent – high agar gel strength restricts nutrient flow, giving smaller colonies; gelatin melts at 37 °C limiting growth, agar alternatives affect morphology.
- Incubation time – longer incubation increases colony size, can enhance pigment and morphological traits.
- Oxygen/atmospheric conditions – aerobic vs anaerobic growth alters elevation, texture, and margin of colonies.
- Moisture/water activity (a_w) – low water activity slows growth and may produce rough, wrinkled colonies.
- Salt concentration/osmotic pressure – high NaCl or sugar raises osmotic stress, affects size and colony boundary
- Interactions with other microbes – competition and quorum sensing can cause irregular or filamentous growth patterns.
- Stressors (antibiotics, toxins, temperature extremes) – can induce morphological changes like filamentation, spheroplasts, altered pigment and texture.
- Genetic/organism-specific factors
- Capsule production yields mucoid/glossy colonies
- Pigment genes activated only under certain environmental triggers (e.g., temperature, nutrients).
- Motility traits cause spreading/swarming (e.g., Proteus) or filamentous margins (e.g., Bacillus, actinobacteria).

How to Observe the Colony Morphology?
Since bacteria were first grown on solid media, describing their colony appearance has been an important tool for microbiologists, especially clinical ones, who often use colony morphology to identify bacteria. This practice, taught early to microbiology students, involves examining plate cultures to note colony shape, color, texture, and growth speed, among other features. Various systems and terms have been developed over time to classify colonies, from simple categories like conglomerate or rhizoid to more detailed descriptions including granular, filamentous, and arborescent. Common modern protocols group observations by color, form, elevation, margin, opacity, and texture, following widely accepted lab manuals. Although photographic examples are rare, simple drawings help demonstrate key colony features, keeping colony morphology a fundamental exercise in microbiology labs.
Purpose
Determining the morphology of a single colony growing on the surface of a plate culture can be an important tool in the description and identification of microorganisms.
Principle
A bacterial colony on solid media usually grows from a single cell, so if colonies are well separated, each will show a distinct shape, size, color, elevation, margin, and texture. These features are mostly observed with the naked eye, though dissecting microscopes can be used for more detail. Colony morphology helps to superficially distinguish different microorganisms, for example, rough and smooth colonies of Streptococcus pneumoniae look different. Pigmented colonies also show clear differences, making colony observation a useful initial step in identification.
Requirement
- suitable growth medium – bacteria gotta grow on right agar, otherwise colonies won’t show proper shapes or colors, and yeah the type of medium always affects appearance so note it down always
- controlled incubation – temp, pH, humidity, oxygen… all these factors kinda mess with how fast they grow, how big they get, even how shiny or pigmented they look sometimes
- perfect streaking technique – no sloppy streaking here, cuz if colonies touch each other, their shapes merge and you can’t tell anything… separation’s key for morphology
- media details must be logged – like what agar it was, any sugars or dyes inside, how long it was incubated, how warm it was, was it in CO₂? these all matter more than ppl think
- observation tools – sure, you can use naked eye, but dissecting microscope or even a magnifier helps when the colonies are small or edges ain’t very clear
- measurement and shape notes – diameter in mm, color, edge pattern, how much it’s raised, if pigment spreads, if it looks see-thru or not, etc. everything’s gotta be described
- test consistency – use a sterile needle or loop, touch the colony gently and see if it’s like buttery (butyrous), sticky (viscous), or dry and crumbles
- pure culture only – mixed colonies ruin the observation, always use a pure strain or else you’re just lookin at a mess, nothin reliable in that
Protocol
- Prepare agar plates and label them – use sterile media suited for the target bacteria and clearly write species name or code on bottom of dish
- Inoculate using streak plate technique – flame‑sterilized loop to spread sample over agar in quadrants or T‑streak to achieve well‑isolated colonies.
Incubate plates – invert them and incubate at an appropriate temperature (usually 18–24 h at 35–37 °C for bacteria); incubation time may vary. - Select well‑isolated colony – pick one colony separated from others to avoid mixed morphology observations.
- Observe with naked eye first – note form, overall shape (circular, irregular, rhizoid), size (measure in mm), and color/pigmentation.
- Use magnification – place plate lid‑on under dissecting microscope or magnifier; observe margin (smooth, undulate, lobate, filamentous), elevation (flat, raised, convex, umbonate), surface (glistening, wrinkled).
- Assess opacity and texture
- opacity as transparent, translucent, or opaque
- texture (consistency) by touching colony gently with sterile loop/needle: butyrous, viscous, mucoid, dry/brittle
- Optionally note hemolysis or odor – on blood agar record alpha/beta/gamma hemolysis; carefully smell from a distance for characteristic odors.
- Record all details systematically – include medium type, incubation conditions, and precise descriptors of size, form, margin, elevation, surface, color, opacity, texture, hemolysis, odor
- Sketch colony views – draw top and side profile (agar line and colony shape) for visual record

Colony Morphology of Bacteria Infographic


Importance of Observing Bacterial Colony Morphology
- Identification of microorganisms – colony morphology provides macroscopic clues (shape, color, size, margin, elevation, texture, opacity) that help narrow down species or genus before further tests.
- Detection of contamination – observing unusual colony types alerts when cultures are mixed or contaminated, critical in clinical or industrial settings
- Support for presumptive diagnosis – features like hemolysis patterns on blood agar or pigment production can quickly suggest pathogenic species
- Quality control and purity check – consistent colony morphology ensures culture integrity, important in research, biotech or pharma production
- Evaluating growth conditions – colony appearance reflects suitable or stressful environments, aiding optimization of incubation parameters.
- Guide for next-step testing – morphological observations help determine which biochemical, molecular or antibiotic tests should follow.
- Epidemiological tracking – during outbreaks, comparing morphology across isolates assists in linking cases and tracking pathogen spread
- Educational and historical value – teaching basic skills and traditionally foundational in microbiology curricula, historically vital diagnostic tool
Colony Morphology of bacteria pdf
Download this pdf to see the colony morphology of different bacteria – Colony Morphology, Authors: Donald Breakwell, Bryan MacDonald, Christopher Adams, Kyle Smith, Richard Robison
- https://hudsonrobotics.com/bacterial-colony-morphology-101/
- https://microbiologysociety.org/why-microbiology-matters/what-is-microbiology/bacteria/observing-bacteria-in-a-petri-dish.html
- https://www.cdc.gov/labtraining/docs/job_aids/biochemicals_gram_positive_organism_id/Colonial_Characteristics_Branded_508.pdf
- https://bio.libretexts.org
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4671912/
- https://www.ruf.rice.edu/~bioslabs/BIOC318/morphology.asp
- https://www.slideshare.net/HiwrHastear/culture-characteristic-of-bacteria
- https://www.scienceprofonline.com/microbiology/bacterial-colony-morphology-identification-unknown-bacteria.html
- https://guides.baker.edu/c.php?g=303096&p=2022446
- https://www.ruf.rice.edu/~bioslabs/BIOC318/morphology.asp
- https://en.wikipedia.org/wiki/Colonial_morphology
- https://www.sciencebuddies.org/science-fair-projects/references/interpreting-agar-plates
- http://www.uwyo.edu/molb2021/virtual-edge/lab01/colony_morphology.html
- https://microbiologyclass.com/microorganisms-and-their-colonial-characteristics/
- https://www.brainkart.com/article/Growth-and-Colony-Characteristics-of-Bacteria-and-Fungi_35237/
- http://www.medical-labs.net/bacterial-colony-morphology-2-887/
- https://www.pathelective.com/micromeded/bacterial-colony-morphologies
- https://www.researchgate.net/figure/Phenazine-production-modulates-colony-morphology-in-P-aeruginosa-PA14-P-aeruginosa_fig1_23222373
- https://universe84a.com/colony-characteristics/
- https://laboratoryinfo.com/colony-morphology-of-bacteria/
- https://hudsonrobotics.com/bacterial-colony-morphology-101/