What are lipids?
- Lipids, originating from the Greek term λίπος meaning ‘fat’, are a diverse and essential group of organic compounds. These compounds range from fats, waxes, and sterols to fat-soluble vitamins like A, D, E, and K. Additionally, lipids encompass monoglycerides, diglycerides, and phospholipids, among others.
- Primarily, lipids serve critical functions in living organisms. They are instrumental in energy storage and act as pivotal structural components of cell membranes. Therefore, understanding their nature and classification is crucial for both biological and industrial applications.
- To begin with, lipids can be categorized based on their solubility properties. They are broadly recognized as either hydrophobic, repelling or failing to mix with water, or amphiphilic, having both hydrophilic and hydrophobic parts. The amphiphilic characteristic of some lipids makes it possible for them to form structures such as vesicles, liposomes, or membranes in water-based environments. Furthermore, biological lipids can be traced back to two distinct biochemical “building-blocks”: ketoacyl and isoprene groups. Based on this classification approach, lipids can be segmented into eight categories, including fatty acyls, glycerolipids, glycerophospholipids, and more.
- However, it’s essential to differentiate between lipids and fats. While the term “lipid” is occasionally utilized interchangeably with fats, fats are a specific subgroup of lipids known as triglycerides. Besides, lipids also include molecules such as fatty acids, their derivatives, and other sterol-containing metabolites like cholesterol. Although these compounds can be synthesized or broken down through various biosynthetic pathways in mammals, including humans, certain indispensable lipids must be sourced from dietary intake.
- Historically, the classification and understanding of lipids have evolved. In the early 19th century, Henri Braconnot and Michel Eugène Chevreul took pioneering steps in classifying lipids into categories like suifs, huiles, greases, and waxes. Later on, significant discoveries were made, including the synthesis of the first triglyceride by Théophile-Jules Pelouze and the recognition of fats as essential nutrients by William Prout.
- Moreover, over the centuries, various terminologies related to lipids, such as lipoid, lipin, lipide, and lipid, were introduced, with each author attributing slightly different meanings. This linguistic journey culminated in 1923 when Gabriel Bertrand, a French pharmacologist, introduced the term “lipide,” which later became anglicized as “lipid.”
- In conclusion, lipids play indispensable roles in both biological systems and various industries. From their essential functions in organisms to their diverse applications in cosmetics, food, and even nanotechnology, lipids remain a critical focus of scientific study and application. Their detailed classification and rich history underline their significance in the vast domain of organic compounds.
Classification of Lipids Based on the Chemical Composition
1. Simple Lipids
Simple lipids are fundamental components in the realm of biological molecules. They primarily consist of neutral fats and oils, as well as waxes. These lipids play crucial roles in various biological processes, especially in energy storage and insulation.
- Fat and Oil: Composition and Types
- Definition: Fats and oils are classified as triglycerides or triacylglycerols (TAG). In a TAG molecule, three fatty acids are esterified to a single glycerol molecule.
- Classification: When a TAG contains identical fatty acids, it is termed a simple TAG. Conversely, TAGs with varied fatty acids are known as mixed TAGs. It’s noteworthy that most fats and oils found in nature fall under the mixed category.
- Common Fatty Acids: The frequently encountered fatty acids in these lipids have chain lengths of C16 and C18. Examples include palmitic acid, stearic acid, and oleic acid.
- Characteristics: Triglycerides constitute about 98% of dietary lipids. They serve as the primary energy storage molecules and are predominantly found in fat depots. Fats, rich in saturated fatty acids, remain solid at room temperature. Moreover, due to the ester bond formation between the -OH group of glycerol and the -COOH group of fatty acids, fats and oils exhibit non-polar and hydrophobic properties.
- Functions of Fat and Oil
- Energy Provision: Fats and oils are paramount energy sources. The oxidation of a single gram of fat releases approximately 9.3kcal of energy.
- Alternative Energy Source: In scenarios where carbohydrates are scarce, fats and oils step in as vital energy reservoirs.
- Insulation: These lipids offer insulation, especially during colder conditions.
- Role in Seeds: Seeds store these lipids in fat depots, which subsequently assist during germination.
- Buoyancy in Marine Life: In creatures like the sperm whale, triglycerides confer buoyancy.
- Waxes: Composition and Varieties
- Definition: Waxes are esters formed from fatty acids and high molecular weight monohydroxy alcohols.
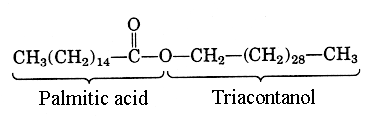
- Examples: Some common waxes include bee’s wax, which comprises myricyl alcohol and palmitic acid, and carnauba’s wax containing tetracosanol and tetra triacosanol.
- Characteristics: Due to their extensive hydrocarbon portions, waxes are more solid and hydrophobic compared to fats and oils.
- Functions of Waxes
- Energy Source in Marine Life: Certain marine organisms, like planktons, utilize waxes as an energy source.
- Water Repellency: Owing to their hydrophobic nature, waxes exhibit water-repelling properties.
- Commercial Uses: Their smooth texture and water-resistant properties make waxes ideal for cosmetics and boot polish production.
2. Compound Lipids
Compound lipids are lipids that contain additional organic molecules beyond the basic components of fatty acids and glycerol. They can be further categorized into specific types based on their molecular structures and components.
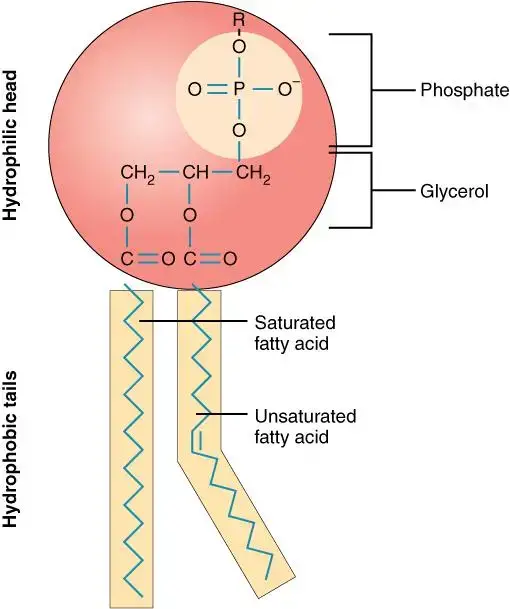
- Phospholipids: Phospholipids are a primary category of compound lipids. They play a crucial role in forming the structural components of cell membranes.
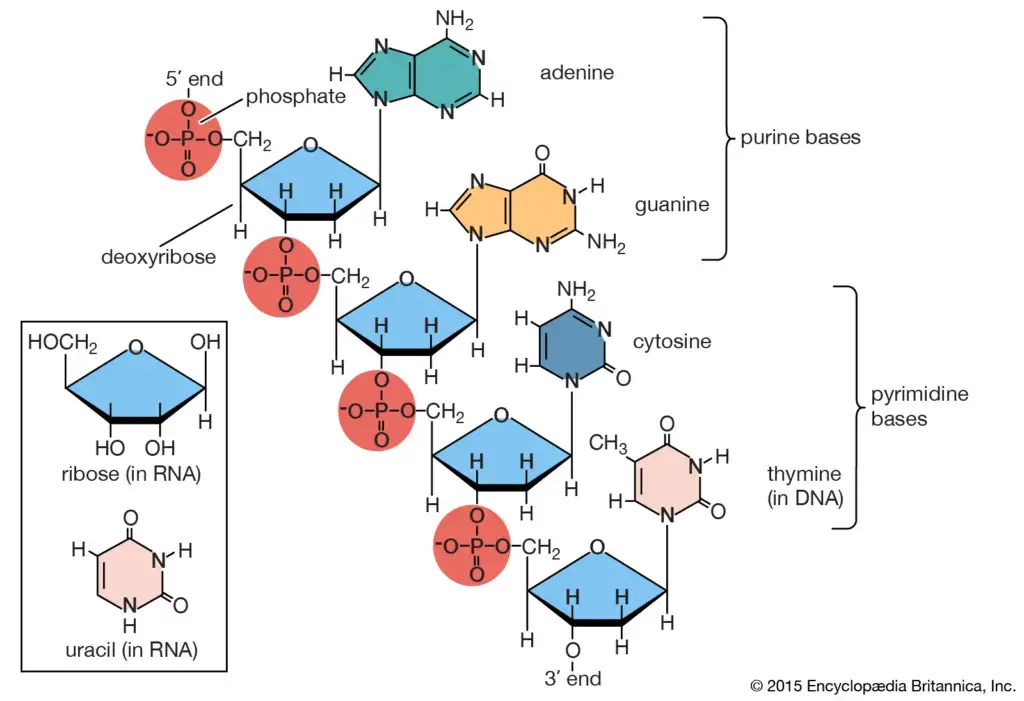
i. Glycerophospholipids:- Glycerophospholipids are composed of glycerol, to which two fatty acids are attached via ester bonds. The third hydroxyl group of glycerol is linked to a phosphate group, which is further connected to various head group substituents.Examples of glycerophospholipids include Phosphatidic acid, Phosphatidylcholine (lecithin), and Phosphatidyl inositol-4,5-bisphosphate.Functions: Glycerophospholipids are integral to the cell membrane’s lipid bilayer. Lecithin, for instance, aids in the transport and metabolism of other lipids within animals.
- Unlike glycerophospholipids, sphingophospholipids contain an amino alcohol called sphingosine instead of glycerol.
- Functions: Sphingomyelin is a significant component of the nervous system in higher animals.
- Glycolipids: Glycolipids are lipids that have a carbohydrate group attached.i. Glyceroglycolipids:
- These contain glycerol, with two fatty acids linked by ester bonds. The third hydroxyl group of glycerol connects to a carbohydrate head group.
- Sphingoglycolipids consist of sphingosine, where the amino group is linked to a fatty acid via a peptide bond, and the hydroxyl group connects to a carbohydrate head group.
- Functions: Glycolipids play a structural role in the cell membrane. They also have roles in signal transduction and tissue differentiation.
- Other Types of Compound Lipids
- Sulpholipids: These are sulfate esters of glycolipids, predominantly found in chloroplasts and certain bacteria.
- Aminolipids: Commonly found in bacteria, these lipids contain amino acids. An example is the lipid containing serine.
- Proteolipids: These are lipids attached to proteins.
3. Derived Lipids
Derived lipids are substances that are produced as a result of the hydrolysis of simple and compound lipids. They encompass a range of molecules, including steroids and certain fatty acids, which play crucial roles in various biological processes.
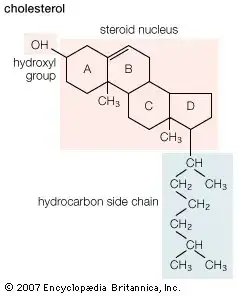
- Steroids: Steroids are a prominent category of derived lipids characterized by their unique structure.
i. Structural Features:- Steroids are composed of four interconnected rings, forming a structure known as the steroid nucleus. This nucleus is a complex derivative of triterpenes.An illustrative example of steroids is cholesterol, which is a vital component of animal cell membranes. Besides, cholesterol is stored within cells as fatty acid esters and serves as a precursor for the synthesis of steroid hormones and bile salts.Notably, cholesterol is absent in fungi and plants. However, plants contain other steroids such as stigmasterol, sitosterol, and campesterol. In fungi, the cell membrane comprises ergosterol.
- Cholesterol plays a pivotal role in maintaining the integrity and fluidity of animal cell membranes.
- It also acts as a precursor for the production of various steroid hormones and bile salts, which are essential for digestion and absorption of dietary fats.
- Eicosanoids: Eicosanoids are biological signaling molecules that function as short-range messengers in the body.
i. Origin and Structure:- Eicosanoids originate from a 20-carbon fatty acid known as arachidonic acid. This family of molecules includes prostanoids and leukotrienes.Their formation involves two primary molecular pathways: the cyclooxygenase (cyclic) pathway and the lipoxygenase (linear) pathway. The former leads to the production of prostanoids, encompassing prostaglandins, prostacyclins, and thromboxanes. In contrast, the lipoxygenase pathway results in the synthesis of leukotrienes.
- Eicosanoids play a crucial role in mediating various physiological responses, including inflammation, blood clotting, and immune responses. Their short-range signaling ensures rapid and localized effects on target cells.
- Other Derived Lipids
- Derived lipids also include other hydrolyzed products from simple and compound lipids. These can combine with different compounds such as alcohols, ketones, vitamin D, sex-hormone steroids, terpenes, and carotenoids.
Classification of Lipids Based on Fatty Acids
Lipids, essential components of living organisms, can be classified based on the type of fatty acids they contain. Broadly, they are categorized into two groups: saturated and unsaturated fatty acids.
- Saturated Fatty Acidsi. Structural Features:
- Saturated fatty acids are characterized by the absence of double or triple bonds between carbon atoms. They possess a straightforward, unbranched, and linear chain of CH2 groups connected by carbon-carbon single bonds. At one end of this chain is a carboxylic acid group.The general formula for saturated fatty acids is CH3 – (CH2)n – COOH, where ‘n’ denotes the number of methylene groups.
- Common saturated fatty acids include lauric, myristic, palmitic, stearic, behenic, and lignoceric acids.
- Unsaturated Fatty Acidsi. Structural Features:
- Unsaturated fatty acids contain one or more double or triple bonds in their structure. Based on the number of these bonds, they can be further classified as monounsaturated (one double bond) or polyunsaturated (multiple double bonds).
- Most naturally occurring unsaturated fatty acids adopt the cis configuration, as opposed to the trans configuration.
- A few unsaturated fatty acids with triple bonds are found in nature, primarily in plants, such as stearolic acid.
- Unsaturated fatty acids are named based on the number of carbon atoms they contain. The suffix “-anoic” is used for saturated fatty acids, while “-enoic” is used for unsaturated ones. For instance, stearic acid, which has 18 carbon atoms, is termed octadecanoic acid (18:0). Here, “18:0” indicates an 18-carbon fatty acid with no double bonds.
- Another naming convention employs the delta numeric system. For example, cis-Δ9 signifies a cis double bond between the 9th and 10th carbon atoms. Similarly, trans-Δ4 represents a trans double bond between the 4th and 5th carbon atoms.
- Monounsaturated fatty acids include palmitoleic acid, oleic acid, gadoleic acid, erucic acid, and nervonic acid.
- Polyunsaturated fatty acids encompass linoleic acid, linolenic acid, and arachidonic acid.
Classification of Lipids Based on Requirements by the Human Body
Lipids, an indispensable component of our diet, play various crucial roles in the human body. Based on the body’s requirements and synthesis capabilities, lipids are systematically categorized into two distinct groups: essential and non-essential fatty acids.
- Essential Fatty Acids
- Definition: Essential fatty acids refer to those specific fatty acids that the human body cannot synthesize on its own. Therefore, it becomes imperative to obtain them from dietary sources to ensure optimal metabolic functions.[
- Examples and Importance: The primary members of this category are linoleic acid, linolenic acid, and arachidonic acid.Their significance lies in the fact that they serve as precursors to various bioactive molecules and play a vital role in maintaining cellular structure, brain functions, and overall health.
- Non-Essential Fatty Acids
- Definition: Contrary to essential fatty acids, non-essential fatty acids are those lipids that the human body can produce internally. Thus, their dietary intake isn’t mandatory for metabolic processes, as the body can sufficiently generate them as needed.
- Examples and Roles: Some notable examples of non-essential fatty acids include palmitic acid, oleic acid, and butyric acid. Besides forming cell membrane structures, they also contribute to energy production and other cellular functions.
Biological Significance of Lipids
- Chemical MessengerLipids serve as crucial signaling molecules in cellular communication. They participate in various signaling pathways, ensuring the proper functioning of cells. Therefore, understanding the role of specific lipid molecules in signaling is vital.
- Sphingosine-1-phosphate: This molecule functions as a potent messenger, playing roles in calcium mobilizing regulations, cell growth, and apoptosis.
- Diacylglycerol and phosphatidylinositol phosphate: These lipids are involved in the calcium-mediated activation of protein kinase C.
- Prostaglandins: As an eicosanoid, it plays roles in inflammation and immunity.
- Hormones such as estrogen, testosterone, and cortisol: These lipids modulate various functions, including metabolism, reproduction, and blood pressure regulation.
- Oxysterol: This lipid regulates biological responses by interacting with liver X receptors, which are essential for maintaining cholesterol, fatty acid, and glucose homeostasis.
- Phosphatidylserine: This lipid signals the phagocytosis of apoptotic cells by exposing itself on the outer leaflet of the bilayer cell membrane.
- Energy StorageLipids, specifically triacylglycerols or triglycerides, are primary energy reservoirs in both plants and animals. Stored in adipose tissues, their complete breakdown releases significant energy, approximately 38 kJ/g (9 kcal/g). Besides, the enzyme lipase controls the breakdown of these triglycerides in the body.
- Structural Component of the Cell MembraneThe cell’s plasma membrane comprises a lipid bilayer with embedded proteins. This bilayer consists of amphipathic glycerophospholipid molecules. Then, glycolipids and phospholipids in the cell membrane serve as its structural components. Additionally, the cellular membrane contains non-glyceride lipids like sphingomyelin and sterols, contributing to membrane flexibility.
- Other FunctionsBeyond the functions mentioned above, lipids have various other roles in biological systems:
- Pigments: Lipids like carotene serve as pigments.
- Hormones: Some lipids function as hormones, such as the vitamin D derivative and sex hormones.
- Cofactors: Lipids like vitamin K act as cofactors in enzymatic reactions.
- Detergents: Bile salt, a lipid, functions as a detergent in the digestive system.
- Insulation and Protection: A subcutaneous layer of lipids provides insulation, protecting the body from cold temperatures and aiding in temperature regulation.
- Prostaglandins: These lipids stimulate uterine contraction, reduce blood pressure, promote vasodilation, inflammation, and pain.
- Thromboxanes: They function as vasoconstrictors
References
- McNamara, J. R., Warnick, G. R., & Cooper, G. R. (2006). A brief history of lipid and lipoprotein measurements and their contribution to clinical chemistry. Clinica Chimica Acta, 369(2), 158–167. doi:10.1016/j.cca.2006.02.041.
- Lipid. Retrieved from https://en.wikipedia.org/wiki/Lipid.
- Kumar, Pranav & Mina, Usha. (2016). Life Sciences, Fundamentals, and Practice, Part I.
- Soult Allison (2020). Lipids and Triglycerides. Retrieved from https://chem.libretexts.org/Courses/University_of_Kentucky/UK.
- Beeswax. Retrieved from https://www.toppr.com/ask/question/bees-wax-consists-of/.
- Lipids. Retrieved from https://www.slideshare.net/mbgk1983/chem-134-unit-8-lipids.
- Ether Lipids. Retrieved from https://en.wikipedia.org/wiki/Ether_lipid#/media/File:Ether_lipid.png.
- Lipids. Retrieved from http://www.chem.latech.edu/~deddy/chem121/Lipids.htm.
- Roy, Arpita. (2014). Production and characterization of biosurfactant from bacterial isolates. 10.13140/RG.2.2.25144.34562.
- Lipoproteins. Retrieved from https://healthjade.net/lipoprotein/.
- Cholesterol. Retrieved from https://www.britannica.com/science/cholesterol.
- About Eicosanoids. Retrieved from http://msrblog.com/assign/science/biology/about-eicosanoid.html.
- Saturated fatty acids. Retrieved from https://www.britannica.com/science/lipid/Saturated-fatty-acids.
- Acetylenic fatty acids. Retrieved from http://cyberlipid.gerli.com/description/simple-lipids/fatty-acids/acetylenic-fa/
- Phukan Luma. Classification and biological significance of Lipids. Retrieved from http://dhingcollegeonline.co.in/attendence/classnotes/files/1601818679.pdf.
- Busatto, S., Walker, S. A., Grayson, W., Pham, A., Tian, M., Nesto, N., Wolfram, J. (2020). Lipoprotein-based drug delivery. Advanced Drug Delivery Reviews. doi:10.1016/j.addr.2020.08.003.