The cGMP pathway, short for cyclic guanosine monophosphate pathway, is a crucial signaling mechanism in cells. It involves the synthesis of cGMP from GTP (guanosine triphosphate) through the action of the enzyme guanylate cyclase. cGMP acts as a secondary messenger, similar to cyclic AMP (cAMP), and plays a vital role in various physiological processes.
One of the primary functions of cGMP is in the regulation of ion channels, particularly in the vascular smooth muscles and in the retina. In the vascular system, cGMP leads to vasodilation by relaxing the smooth muscle cells. This is the mechanism behind the action of drugs like nitroglycerin and sildenafil (Viagra), which increase cGMP levels and promote vasodilation.
In the retina, cGMP is involved in the phototransduction pathway, where it controls the opening and closing of ion channels in response to light, thereby playing a critical role in vision.
Additionally, cGMP is involved in the regulation of cell growth and division, and its dysregulation is implicated in various diseases, including cancer and heart disease. The cGMP pathway is tightly regulated by various enzymes, including phosphodiesterases, which break down cGMP, thus modulating its cellular concentration and effects.
What is cGMP Pathway?
- The cyclic guanosine monophosphate (cGMP) pathway is an essential intracellular signaling mechanism in biological systems. This pathway operates through a series of molecular interactions and reactions, primarily involving the synthesis and regulation of the cyclic nucleotide, cGMP. Derived from guanosine triphosphate (GTP), cGMP serves as a secondary messenger, akin to cyclic adenosine monophosphate (cAMP), and is integral in mediating various physiological responses.
- cGMP synthesis is initiated by the enzyme guanylate cyclase. This enzyme is activated by specific stimuli, including nitric oxide (NO) and certain hormones, triggering the conversion of GTP into cGMP. Consequently, the concentration and activity of cGMP within the cell are tightly regulated by phosphodiesterases (PDEs), which degrade cGMP into guanosine monophosphate (GMP), thus modulating its intracellular levels.
- The downstream effects of cGMP are mediated through several mechanisms. One of the primary effectors is protein kinase G (PKG), also known as cyclic GMP-dependent protein kinase (cGK). PKG regulates various cellular processes by phosphorylating target proteins. Besides PKG, cGMP also acts on cyclic nucleotide gated channels (CNG) and interacts with specific PDEs, further influencing cellular responses.
- A key function of the cGMP pathway is the regulation of vasodilation and smooth muscle relaxation. This is particularly evident in the cardiovascular system, where cGMP-mediated relaxation of smooth muscle cells leads to blood vessel dilation and a consequent reduction in blood pressure. This mechanism is exploited in the pharmacological management of conditions such as pulmonary hypertension, erectile dysfunction, and heart failure, where drugs like sildenafil (Viagra) inhibit PDEs, thereby increasing cGMP levels and promoting vasodilation.
- In addition to vascular regulation, cGMP is pivotal in the process of phototransduction in the retina. Here, it controls the opening and closing of ion channels in photoreceptor cells, which is crucial for the conversion of light signals into electrical signals in the visual pathway. Moreover, cGMP influences platelet aggregation and thrombus formation, underlining its diverse roles in various physiological and pathological conditions.
Definition of cGMP Pathway
The cGMP pathway, short for cyclic guanosine monophosphate pathway, is a cellular signaling mechanism where cGMP, synthesized from GTP (guanosine triphosphate), acts as a secondary messenger. It plays a critical role in regulating various physiological processes, such as vasodilation in vascular smooth muscles, phototransduction in the retina, and platelet aggregation. The pathway is tightly regulated by enzymes like guanylate cyclase, which synthesizes cGMP, and phosphodiesterases, which break it down, thereby controlling its cellular concentration and effects.
Characteristics of cGMP
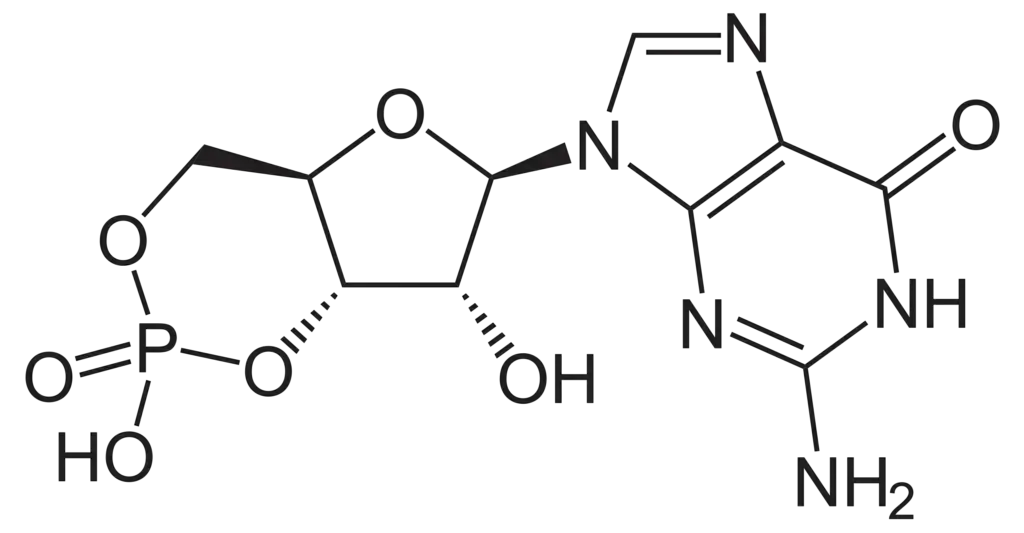
- Chemical Structure and Origin: Cyclic guanosine monophosphate (cGMP) is structurally similar to cyclic AMP (cAMP), differing primarily in its nucleobase; cGMP contains a guanine base instead of the adenine found in cAMP. It is synthesized from guanosine triphosphate (GTP), indicating its nucleotide origin and cyclic structure.
- Synthesis Pathways: The synthesis of cGMP occurs via two major pathways. The first involves a membrane-bound guanylyl cyclase, which is linked to a natriuretic peptide receptor. The second pathway utilizes soluble guanylyl cyclase activated by nitric oxide (NO). These pathways highlight the diverse regulatory mechanisms governing cGMP production.
- Degradation by Phosphodiesterases: Similar to cAMP, cGMP is degraded by phosphodiesterases. Specific phosphodiesterases, like PDE-5A (the target of sildenafil), selectively affect cGMP. Others, such as PDE-2 and PDE-3, can hydrolyze both cAMP and cGMP, underlining the interconnectedness of these secondary messenger systems.
- Downstream Effects of cGMP – Protein Kinase G Activation: cGMP activates Protein Kinase G (PKG), which leads to several physiological effects:
- Smooth muscle relaxation is achieved by decreasing intracellular calcium availability.
- A negative inotropic effect is produced by reducing myofilament calcium responsiveness.
- Promotion of angiogenesis, crucial for new blood vessel formation.
- Effects via cGMP-gated Ion Channels: cGMP affects mainly unselective cation channels, which are relevant to the movement of sodium and calcium ions. These channels are primarily expressed in the retinal and olfactory neuroepithelium and in nephrons, indicating their significant roles in sensory perception and renal function.
- Interaction with Phosphodiesterases: cGMP can bind to phosphodiesterases, increasing their activity against both cGMP and cAMP. This binding results in the inhibition of both secondary messenger systems, demonstrating cGMP’s role in modulating cellular responses by influencing the concentration of key signaling molecules.
Components of cGMP pathway
- cGMP Generators: The cyclic guanosine monophosphate (cGMP) pathway involves specific enzymes for cGMP generation. Soluble guanylyl cyclases (sGC) and particulate guanylyl cyclases are the key types. The sGC, stimulated by nitric oxide (NO) and carbon monoxide (CO), are heterodimers comprising different subunits like α1, β1 (NOGC1), and α2, β1 (NOGC2). These cyclases have a heme-binding site crucial for NO and CO interaction, leading to GTP conversion into cGMP.
- NO-GC Stimulators, Activators, and Inhibitors: Various compounds, including nitroglycerin and newer molecules like Riociguat, stimulate or activate NO-GCs, influencing cGMP production. These compounds are used in treating diseases like pulmonary arterial hypertension. Additionally, specific inhibitors like ODQ (quinoxalin derivative) target NO-GCs, offering a method to understand cGMP-dependent and independent NO effects.
- Particulate Guanylyl Cyclases: Particulate guanylyl cyclases, identified as pGC-A to pGC-G, possess a domain arrangement significant for their function. They are activated by different ligands, including natriuretic peptides and heat-stable enterotoxins. Their activity involves phosphorylation and subsequent dephosphorylation upon ligand binding, playing roles in cardiovascular and renal functions.
- cGMP Degradation: Phosphodiesterases: The degradation of cGMP is primarily facilitated by phosphodiesterases (PDEs). Eleven PDE families exist, with specific ones like PDE1A, PDE1B, PDE5, PDE6, and PDE9 specializing in cGMP hydrolysis. Each family has unique characteristics and regulatory domains, contributing to the precise control of cGMP levels in various cellular contexts.
- PDE Inhibitors: Inhibitors for most PDE families are available, with clinical significance lying in PDE5 inhibitors like sildenafil, vardenafil, and tadalafil. These inhibitors are used to treat conditions like erectile dysfunction and pulmonary arterial hypertension, highlighting their role in modulating cGMP levels for therapeutic purposes.
- Alternative Pathway for cGMP Reduction: Besides degradation, cGMP can be reduced through export from the cell by transporters like the ABC transporter multidrug resistance-associated protein 4. This pathway plays a role in tissues including vascular smooth muscle.
- cGMP Effectors: CNG Channels and cGMP-dependent Protein Kinases: The primary effectors of cGMP are cyclic nucleotide-gated (CNG) ion channels and cGMP-dependent protein kinases (cGK or PKG). CNG channels, activated by cGMP, are crucial in sensory transduction processes like vision and olfaction. cGMP-dependent protein kinases, encoded by prkgI and prkgII, are key players in various cellular functions, including vasodilation, platelet function, and synaptic transmission.
Each component of the cGMP pathway plays a distinct yet interconnected role in maintaining cellular homeostasis and response to various stimuli. This pathway’s complexity and widespread impact underscore its significance in physiology and pharmacology.
Synthesis Of Cyclic GMP
Cyclic guanosine monophosphate (cGMP) is synthesized from guanosine triphosphate (GTP) by the enzyme guanylate cyclase (GC). This process is crucial for the generation of cGMP, a second messenger molecule that plays pivotal roles in various cellular processes.
- Guanylate Cyclase – The Catalytic Enzyme: Guanylate cyclase belongs to the lyase enzyme category and is a key enzyme in the synthesis of cGMP. It catalyzes the conversion of GTP into 3′-5′-cyclic guanosine monophosphate. There are two forms of GC: membrane-bound guanylate cyclase (pGC) and soluble guanylate cyclase (sGC).
- Activation of Membrane-bound GC (pGC): Membrane-bound GC, or particulate GC, is typically activated by peptide hormones like atrial natriuretic factor. This subfamily of GC includes various isoforms such as guanylate cyclase A/natriuretic peptide receptor 1 (GC-A/Npr1), guanylate cyclase B/natriuretic peptide receptor 2 (GC-B/Npr2), and guanylate cyclase 2D (GC-D/GUCY2D), all expressed in the brain. These receptors bind natriuretic peptides, playing a role in processes associated with the senses of taste, smell, and vision.
- Activation of Soluble GC (sGC): Soluble GC, present in the cytoplasm, is typically activated by nitric oxide (NO). This form of GC functions as a heterodimer consisting of various combinations of α (α1 or α2) and β (β1 or β2) subunits. However, in the brain, the β2 subunit mRNAs are not produced, indicating a specific subunit composition for brain sGC.
- Inhibition of sGC: Soluble guanylate cyclase can be inhibited by specific compounds such as ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one), providing a method to regulate cGMP synthesis and study the pathway’s physiological and pathological roles.
- Functional Implications: The synthesis of cGMP by guanylate cyclase, whether membrane-bound or soluble, is fundamental for various cellular signaling pathways. The differential activation and regulation of GC subtypes allow for diverse physiological responses, underlying the importance of precise control in cGMP synthesis.
cGMP pathway steps
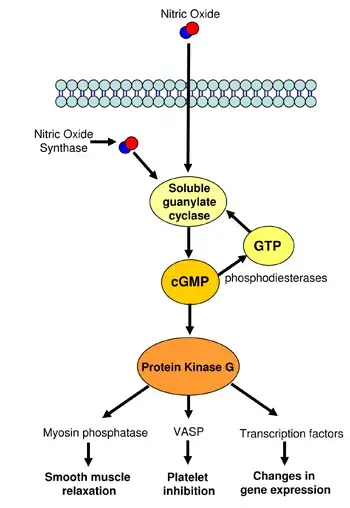
Cyclic GMP pathway consist of five steps;
- Receptor Activation: The cGMP pathway initiates with receptor activation on the cell membrane. This activation is triggered by various extracellular signals such as hormones, neurotransmitters, and other ligands. The nature of these signals varies widely, reflecting the diverse physiological contexts in which the cGMP pathway operates.
- Activation of Guanylate Cyclase: Following receptor activation, guanylate cyclase is stimulated. This enzyme is crucial for the conversion of guanosine triphosphate (GTP) into cyclic guanosine monophosphate (cGMP). The enzymatic reaction involves the cyclization of GTP, forming the ring structure that characterizes cGMP.
- cGMP Production: The production of cGMP marks its role as a second messenger within the cell. Once synthesized, cGMP diffuses throughout the cell, where it can interact with and activate various downstream signaling pathways. This step is pivotal in transducing the initial extracellular signal into a specific intracellular response.
- Cellular Responses: The presence of cGMP in the cell leads to the activation of protein kinases, most notably the cGMP-dependent protein kinase (PKG). PKG phosphorylates target proteins within the cell, thereby modulating their activity. This phosphorylation event is key to eliciting a range of cellular responses, depending on the cell type and the nature of the initial stimulus.
- Termination of Signal: The termination of the cGMP signal is as crucial as its initiation and propagation. This is accomplished by phosphodiesterases (PDEs), which hydrolyze cGMP to its inactive form, guanosine monophosphate (GMP). This enzymatic breakdown of cGMP ensures that the cellular response is appropriately regulated and does not persist beyond the necessary timeframe.
Factors Affecting of cGMP pathway
The cGMP (cyclic guanosine monophosphate) pathway is influenced by several factors, which can either enhance or inhibit its activity. These factors play a vital role in the regulation of various physiological processes such as vasodilation, vision, and intestinal fluid secretion. Here are some key factors affecting the cGMP pathway:
- Nitric Oxide (NO) Levels: NO is a major activator of soluble guanylate cyclase (sGC), which catalyzes the synthesis of cGMP from GTP. Factors that increase or decrease NO production will directly affect cGMP levels. For instance, endothelial dysfunction can reduce NO availability, leading to decreased cGMP synthesis.
- Natriuretic Peptides: These peptides, including atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), activate membrane-bound guanylate cyclases, thereby increasing cGMP production. Their levels can be influenced by cardiovascular health and fluid balance in the body.
- Phosphodiesterase (PDE) Activity: PDEs are enzymes that degrade cGMP. The most well-known is PDE5, targeted by drugs like sildenafil (Viagra). The activity of PDEs can be influenced by hormonal levels, drugs, and disease states.
- Ion Channel Function: cGMP regulates the activity of certain ion channels, especially in the retina and in smooth muscle cells. Alterations in ion channel function can affect the cellular responses to cGMP.
- G Protein-Coupled Receptor (GPCR) Signaling: Certain GPCRs can influence cGMP levels indirectly by modulating the activity of guanylate cyclase or affecting NO production.
- Oxidative Stress and Inflammation: These conditions can impair NO synthesis and sGC function, thereby affecting cGMP levels.
- Pharmacological Agents: Various drugs, including nitrates (which release NO) and PDE inhibitors, can significantly impact the cGMP pathway.
- Hormonal Regulation: Hormones like insulin and glucagon can indirectly influence cGMP levels through their effects on NO production and vascular function.
- Dietary Factors: Certain dietary components, such as nitrates found in vegetables, can enhance NO production and thus affect cGMP synthesis.
- Genetic Factors: Genetic variations in the enzymes related to the cGMP pathway, like guanylate cyclase or PDEs, can impact the efficiency and regulation of this pathway.
| Factor | Impact on cGMP Pathway |
|---|---|
| Nitric Oxide (NO) Levels | Increased NO enhances cGMP production by activating soluble guanylate cyclase (sGC). Reduced NO leads to lower cGMP levels. |
| Natriuretic Peptides | Activate membrane-bound guanylate cyclases, increasing cGMP production. |
| Phosphodiesterase (PDE) Activity | High PDE activity, especially PDE5, leads to increased degradation of cGMP, reducing its levels. |
| Ion Channel Function | Altered ion channel function can impact cellular responses to cGMP. |
| G Protein-Coupled Receptor (GPCR) Signaling | Can modulate cGMP levels indirectly by affecting guanylate cyclase activity or NO production. |
| Oxidative Stress and Inflammation | Impair NO synthesis and sGC function, thereby affecting cGMP levels. |
| Pharmacological Agents | Drugs like nitrates and PDE inhibitors significantly impact cGMP levels. |
| Hormonal Regulation | Hormones can indirectly influence cGMP levels through effects on NO production and vascular function. |
| Dietary Factors | Components like dietary nitrates can enhance NO production, affecting cGMP synthesis. |
| Genetic Factors | Variations in genes related to guanylate cyclase or PDEs can impact the efficiency of the cGMP pathway. |
Inhibitors of cGMP pathway
Inhibitors of the cGMP (cyclic guanosine monophosphate) pathway play a significant role in regulating various physiological processes. These inhibitors can act at different points in the pathway to reduce the production or action of cGMP. Here are some notable types of inhibitors:
- Nitric Oxide Synthase (NOS) Inhibitors: Since nitric oxide (NO) is a major activator of soluble guanylate cyclase (sGC), which produces cGMP, inhibiting NOS can decrease cGMP levels. Examples of NOS inhibitors include L-NAME (N^G-Nitroarginine methyl ester) and L-NMMA (N^G-Monomethyl L-arginine).
- Soluble Guanylate Cyclase (sGC) Inhibitors: These compounds directly inhibit sGC, the enzyme responsible for converting GTP to cGMP. Methylene blue is a well-known example that non-selectively inhibits sGC.
- Phosphodiesterase (PDE) Activators: Although not direct inhibitors of cGMP synthesis, PDE activators increase the degradation of cGMP. An increase in PDE activity, especially PDE5, leads to a decrease in cGMP levels. While PDE inhibitors like sildenafil are used to enhance cGMP signaling, factors that upregulate PDE activity would have the opposite effect.
- Endothelial Dysfunction Agents: Any factor that leads to endothelial dysfunction can indirectly inhibit the cGMP pathway. This includes oxidative stress, hyperglycemia, and certain inflammatory cytokines, which can reduce NO availability and thus lower cGMP synthesis.
- GPCR Antagonists: Certain G-protein coupled receptors, when activated, can enhance cGMP production. Thus, antagonists for these receptors can indirectly reduce cGMP levels.
- Hormonal Regulators: Hormones that negatively affect NO production or endothelial function can indirectly inhibit cGMP synthesis. For example, high levels of certain stress hormones might impair NO-mediated cGMP production.
- Anion Channel Blockers: In some cases, cGMP functions by activating anion channels. Inhibitors of these channels can therefore attenuate the effects of cGMP.
| Type of Inhibitor | Examples | Point of Action in cGMP Pathway |
|---|---|---|
| Nitric Oxide Synthase (NOS) Inhibitors | L-NAME, L-NMMA | Inhibit the production of NO, reducing activation of soluble guanylate cyclase (sGC) |
| Soluble Guanylate Cyclase (sGC) Inhibitors | Methylene Blue | Direct inhibition of sGC, reducing cGMP synthesis |
| Phosphodiesterase (PDE) Activators | – (Not specific compounds, but conditions or factors increasing PDE activity) | Increase the degradation of cGMP |
| Endothelial Dysfunction Agents | Oxidative stress factors, hyperglycemia | Indirectly reduce NO availability, thus lowering cGMP synthesis |
| GPCR Antagonists | – (Specific to the type of GPCR involved) | Indirectly reduce cGMP levels by inhibiting receptors that enhance cGMP production |
| Hormonal Regulators | High levels of stress hormones | Negatively affect NO production or endothelial function, indirectly inhibiting cGMP synthesis |
| Anion Channel Blockers | – (Specific to the type of anion channel involved) | Attenuate the effects of cGMP by blocking its downstream action |
2 / 2
Regulation of cGMP pathway
The regulation of the cyclic guanosine monophosphate (cGMP) pathway is a complex process that plays a crucial role in various physiological functions, including vasodilation, platelet aggregation, and perception of light:
- Synthesis of cGMP: cGMP is synthesized from guanosine triphosphate (GTP) by the enzyme guanylate cyclase. There are two types of guanylate cyclases:
- Soluble guanylate cyclase (sGC): This is typically activated by nitric oxide (NO), a signaling molecule. When NO binds to sGC, it stimulates the conversion of GTP to cGMP.
- Membrane-bound guanylate cyclase: This is activated by natriuretic peptides (like ANP and BNP) and some other hormones. These cyclases are part of the receptor complexes for these peptides.
- Effects of cGMP: Once synthesized, cGMP acts as a secondary messenger and activates various downstream targets, including:
- Protein kinase G (PKG): PKG phosphorylates various proteins, leading to smooth muscle relaxation (vasodilation), inhibition of platelet aggregation, and changes in ion channel conductance.
- Phosphodiesterases (PDEs): These enzymes regulate the degradation of cGMP. PDE5, for instance, specifically degrades cGMP in the smooth muscle cells of the penis and other tissues, which is targeted by drugs like sildenafil (Viagra).
- Degradation of cGMP: cGMP is hydrolyzed to guanosine monophosphate (GMP) by PDEs. The activity of PDEs is a key regulatory mechanism for controlling the levels of cGMP.
- Feedback Mechanisms: The cGMP pathway is also regulated by feedback mechanisms. High levels of cGMP can modulate the activity of guanylate cyclase or NO synthesis, thus forming a regulatory loop.
Function Of Cyclic GMP
- Visual Transduction in the Retina: Cyclic GMP plays a pivotal role in visual transduction in the retina. In the dark, cGMP levels are high, keeping CNG channels open, allowing Na+ and Ca2+ influx, leading to depolarization of photoreceptor membranes and glutamate release. Upon light exposure, rhodopsin activation triggers a reduction in cGMP levels, closing CNG channels, reducing Na+ and Ca2+ concentration, and hyperpolarizing the membrane. This alteration in glutamate release signals the perception of light to the brain. Additionally, mutations affecting cGMP levels or CNG channel functions can lead to retinal degeneration.
- Olfactory Signal Transduction: In olfactory transduction, a subset of neurons uses cGMP-stimulated CNG channels, contrasting with the majority that are activated by cAMP. cGMP in these neurons induces membrane depolarization, playing a role in olfactory signaling. However, the relevance of this pathway in humans remains unclear, as pGC-D, involved in this process, is a pseudogene in humans.
- Signaling in the Ear: cGMP, through the cGMP-cGKI pathway, is protective in the inner ear. It plays a role in reducing vulnerability to noise-induced hearing loss (NIHL) and aids in recovery. This suggests the therapeutic potential of manipulating the cGMP pathway in ear health, although its relevance in human auditory processing needs further investigation.
- Signaling in the CNS: cGMP is integral in the regulation of synaptic plasticity, learning, and behavior. The brain contains components of the cGMP signal transduction pathway, including NO-GCs, PDEs, cGK isoforms, and CNG channels. However, the global modulation of this pathway in the brain presents challenges due to its complex involvement in various neurotransmitter systems.
- PDEs and Their Role: Specific PDEs, such as PDE5, PDE6, and PDE9, play roles in cognitive functions, retinal health, and mood disorders. The complexity of their functions in the CNS underlines the intricate role of cGMP in various behavioral and physiological phenomena.
- Axon Branching: cGMP-mediated signaling via cGKIα is essential in axon branching during embryogenesis, contributing to the formation of complex neuronal networks.
- Synaptic Plasticity: cGMP influences synaptic plasticity, particularly in processes like long-term potentiation (LTP) and long-term depression (LTD), which are crucial for learning and memory. The modulation of key receptors like AMPA by cGK, influenced by cGMP levels, is central in these processes.
- Circadian Rhythm Regulation: The cGMP pathway contributes to the regulation of circadian rhythms. It interacts with circadian clock genes in the suprachiasmatic nucleus, influencing the body’s internal clock through mechanisms that involve several signaling molecules, including NO and Ca2+.
- Pain Regulation: cGMP, particularly through cGKIα, is significantly involved in pain sensitivity and processing of nociceptive signals in the spinal cord. Its modulation could influence pain perception and response.
- Behavioral Implications: Studies in drosophila have shown that cGMP-dependent protein kinase levels can influence foraging behavior, suggesting a possible role for cGMP in modulating specific behaviors in higher organisms, including humans.
Clinical impotence of cGMP pathway
- Cardiovascular Health: The cGMP pathway is significantly involved in cardiovascular health through its vasodilatory effects. Medications that enhance cGMP levels, such as phosphodiesterase type 5 (PDE5) inhibitors or nitric oxide donors, are employed to treat conditions like hypertension and erectile dysfunction. Furthermore, cGMP plays a role in cardioprotection by reducing hypertrophy and protecting the heart during reperfusion injury, indicating its therapeutic potential in various cardiovascular diseases.
- Neurological Disorders: In the nervous system, cGMP regulates neurotransmitter release and synaptic plasticity. Dysregulation of the cGMP pathway is linked to neurological disorders, suggesting that targeting this pathway could offer new therapeutic approaches. Additionally, alterations in the cGMP pathway may contribute to cognitive disorders such as Alzheimer’s disease, highlighting its importance in cognitive function and the potential for developing treatments targeting this pathway.
- Gastrointestinal Function: cGMP is involved in the relaxation of gastrointestinal smooth muscles, impacting conditions like irritable bowel syndrome (IBS) or constipation. The modulation of smooth muscle function by cGMP is crucial for proper gut motility, and disruptions in the cGMP pathway may lead to various gastrointestinal disorders.
- Ophthalmic Health: In ophthalmic health, cGMP levels influence intraocular pressure. Medications affecting the cGMP pathway are used in treating conditions like glaucoma, demonstrating the pathway’s significance in ocular health.
- Pulmonary Function: The cGMP pathway plays a vital role in pulmonary vasodilation. This is particularly relevant in conditions like pulmonary hypertension, where medications enhancing cGMP levels are used to manage pulmonary vascular disorders.
- Urogenital Function: cGMP is critical in urogenital functions, especially in erectile function. PDE5 inhibitors, which increase cGMP levels, are commonly used to treat erectile dysfunction. Moreover, the smooth muscle regulation in the lower urinary tract through the cGMP pathway suggests its involvement in conditions like overactive bladder.
FAQ
What is cGMP Pathway?
cGMP stands for cyclic Guanosine Monophosphate, a secondary messenger used in many cellular processes. The cGMP pathway involves the synthesis, function, and degradation of cGMP within cells.
How is cGMP synthesized?
cGMP is synthesized from guanosine triphosphate (GTP) by the enzyme guanylate cyclase. This can be triggered by various signals including nitric oxide (NO) and natriuretic peptides.
What is the role of cGMP in the body?
cGMP plays a vital role in several physiological processes including vasodilation, regulation of fluid and electrolyte homeostasis, phototransduction in the retina, and modulation of synaptic transmission.
How does cGMP cause vasodilation?
cGMP induces vasodilation by relaxing vascular smooth muscle cells. It activates protein kinase G, which leads to the phosphorylation of myosin light chains and a reduction in intracellular calcium levels, causing relaxation.
What are PDE inhibitors and their relation to cGMP?
Phosphodiesterase (PDE) inhibitors are drugs that prevent the degradation of cGMP, thereby enhancing its effects. For example, sildenafil (Viagra) is a PDE5 inhibitor used to treat erectile dysfunction by increasing cGMP levels.
What diseases are associated with the cGMP pathway?
Disorders related to cGMP include hypertension, heart failure, and erectile dysfunction. Also, certain rare genetic diseases can affect the components of the cGMP pathway.
How is cGMP regulated?
cGMP levels are regulated through a balance between its synthesis by guanylate cyclase and its degradation by phosphodiesterases. Additionally, cellular receptors and signaling pathways modulate the activity of guanylate cyclase.
What is the difference between cGMP and cAMP?
Both are cyclic nucleotides acting as secondary messengers, but cAMP (cyclic Adenosine Monophosphate) is derived from adenosine triphosphate (ATP) and is involved in different signaling pathways such as those mediated by adrenaline.
Can cGMP pathway be targeted for therapeutic purposes?
Yes, several drugs target the cGMP pathway. Vasodilators and certain drugs for treating heart failure and pulmonary hypertension work by modulating cGMP levels or its downstream effects.
What is the role of cGMP in drug manufacturing?
In this context, cGMP stands for Current Good Manufacturing Practice. It refers to the regulations enforced by the FDA to ensure proper design, monitoring, and control of manufacturing processes and facilities in the pharmaceutical industry. This is not directly related to the cyclic Guanosine Monophosphate pathway but shares the same acronym.
References
- https://www.ncbi.nlm.nih.gov/books/NBK542234/
- W. Reierson, G. et al. (2011) ‘CGMP signaling, phosphodiesterases and major depressive disorder’, Current Neuropharmacology, 9(4), pp. 715–727. doi:10.2174/15701591179837627.
- Cyclic guanosine monophosphate (2023) Wikipedia. Available at: https://en.wikipedia.org/wiki/Cyclic_guanosine_monophosphate (Accessed: 12 November 2023).
- Hofmann, F. (2019) ‘The cgmp system: Components and function’, Biological Chemistry, 401(4), pp. 447–469. doi:10.1515/hsz-2019-0386.
- Denninger, J.W. and Marletta, M.A. (1999) ‘Guanylate cyclase and the ⋅no/cgmp signaling pathway’, Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1411(2–3), pp. 334–350. doi:10.1016/s0005-2728(99)00024-9.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.