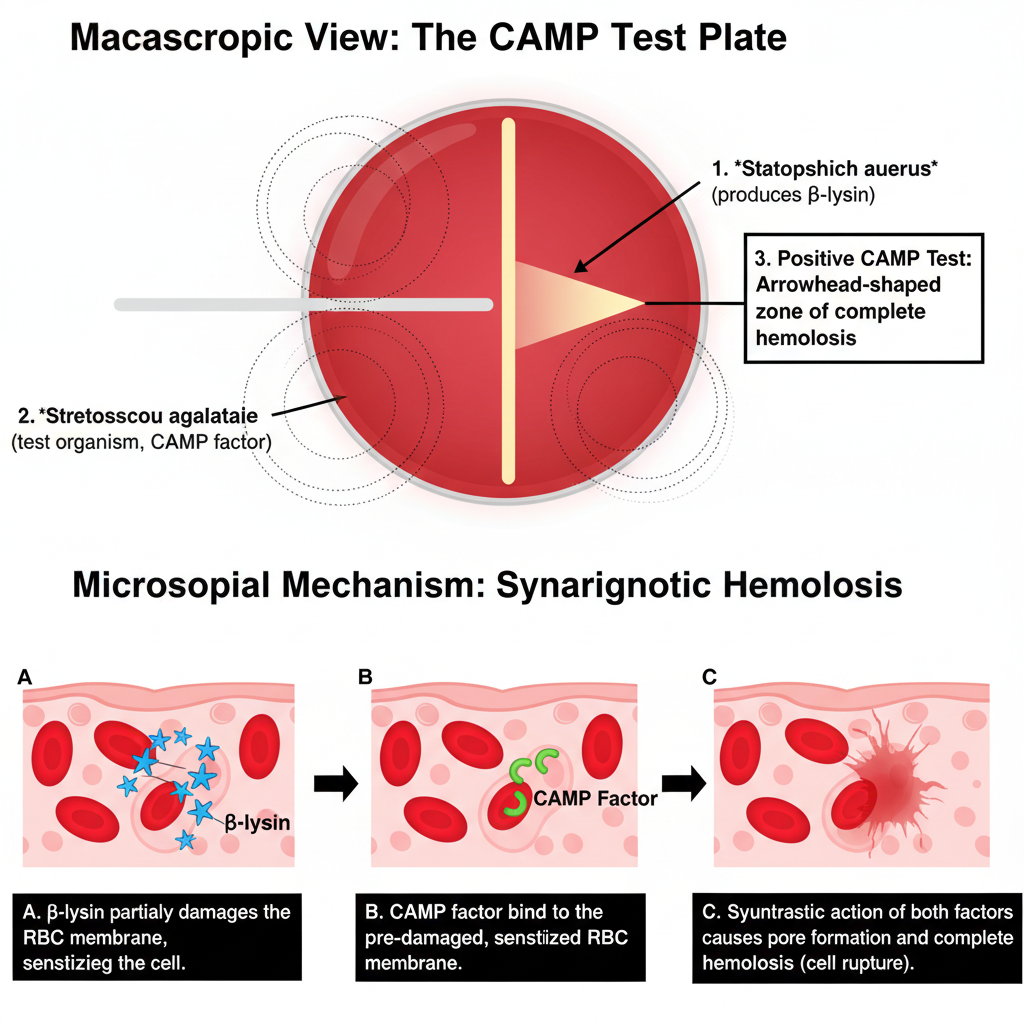
The CAMP test is the process used to identify Streptococcus agalactiae (Group B Streptococcus) by detecting the production of the CAMP factor, which is an extracellular protein showing a synergistic hemolysis with the β-lysin produced by Staphylococcus aureus.
It is performed on sheep blood agar where a β-lysin producing S. aureus strain is streaked in a straight line, and the suspected organism is streaked perpendicular to it, without touching. During incubation the β-lysin sensitizes the red blood cells, and the CAMP factor diffuses from the test organism, producing a clear arrowhead-shaped enhanced hemolysis at the junction.
This is referred to as the CAMP positive reaction and it is used mainly for identifying GBS among the β-hemolytic streptococci. The same principle is used for identifying Listeria monocytogenes which shows a positive CAMP reaction with S. aureus. In the reverse CAMP test the indicator strain is GBS, and the suspected Clostridium perfringens is streaked close to it. It is the process where the alpha-toxin of C. perfringens interacts with the CAMP factor and forms a bow-tie shaped zone of enhanced hemolysis which is the characteristic reaction for this organism.
Purpose of CAMP Test
- To identify the Streptococcus agalactiae.
- To distinguish the capability of an organism to generate the CAMP factor.
Principle of CAMP Test
The principle of the CAMP test is based on the synergistic hemolysis produced when two different bacterial factors act together on sheep red blood cells. It is the process where Group B Streptococcus (Streptococcus agalactiae) produces a diffusible thermostable protein called the CAMP factor, and this factor interacts with the β-lysin (sphingomyelinase C) released by a β-lysin producing strain of Staphylococcus aureus. The β-lysin first damages the red blood cell membrane by hydrolyzing sphingomyelin, making the cells sensitive, and then the CAMP factor binds to these sensitized cells which results in complete lysis. The reaction is seen on sheep blood agar as a distinct arrowhead-shaped zone of enhanced hemolysis at the region where the two streaks grow close to each other. This is referred to as the characteristic CAMP positive reaction and forms the basis of identifying CAMP factor producing organisms.
Requirement for CAMP test
- Sheep blood agar plate
- Pure culture of the test organism (suspected Streptococcus agalactiae)
- Standard strain of Staphylococcus aureus (beta-hemolysin producer)
- Inoculating loop or needle
- Incubator set at 35–37°C
- Bunsen burner or sterile workstation
- Marker pen for labeling
- Control organisms (positive and negative controls)
Procedure of CAMP test
Standard Plate Procedure
The CAMP test is carried out on sheep blood agar and it is the process used to detect the CAMP factor produced by Group B Streptococcus. A β-lysin producing strain of Staphylococcus aureus is used as the indicator organism. The steps are as follows–
- The test is performed on a Trypticase soy agar plate containing 5% sheep blood, as sheep erythrocytes have sphingomyelin required for β-lysin activity.
- A straight line of β-lysin producing S. aureus is streaked down the center of the plate.
- The suspected organism is streaked perpendicular to the S. aureus streak and kept at a distance of 1–2 mm without touching.
- More than one test organism can be placed on the same plate as long as they remain parallel and perpendicular to the main streak.
- A known GBS strain may be used as the positive control, and Streptococcus pyogenes or Enterococcus species as negative control.
- The plate is incubated at 35–37°C for 18–24 hours in ambient air.
It is called positive when a clear arrowhead-shaped enhanced hemolysis is seen at the junction of the two streaks. A negative result shows no enhanced hemolysis and the organism only produces its normal hemolytic pattern.

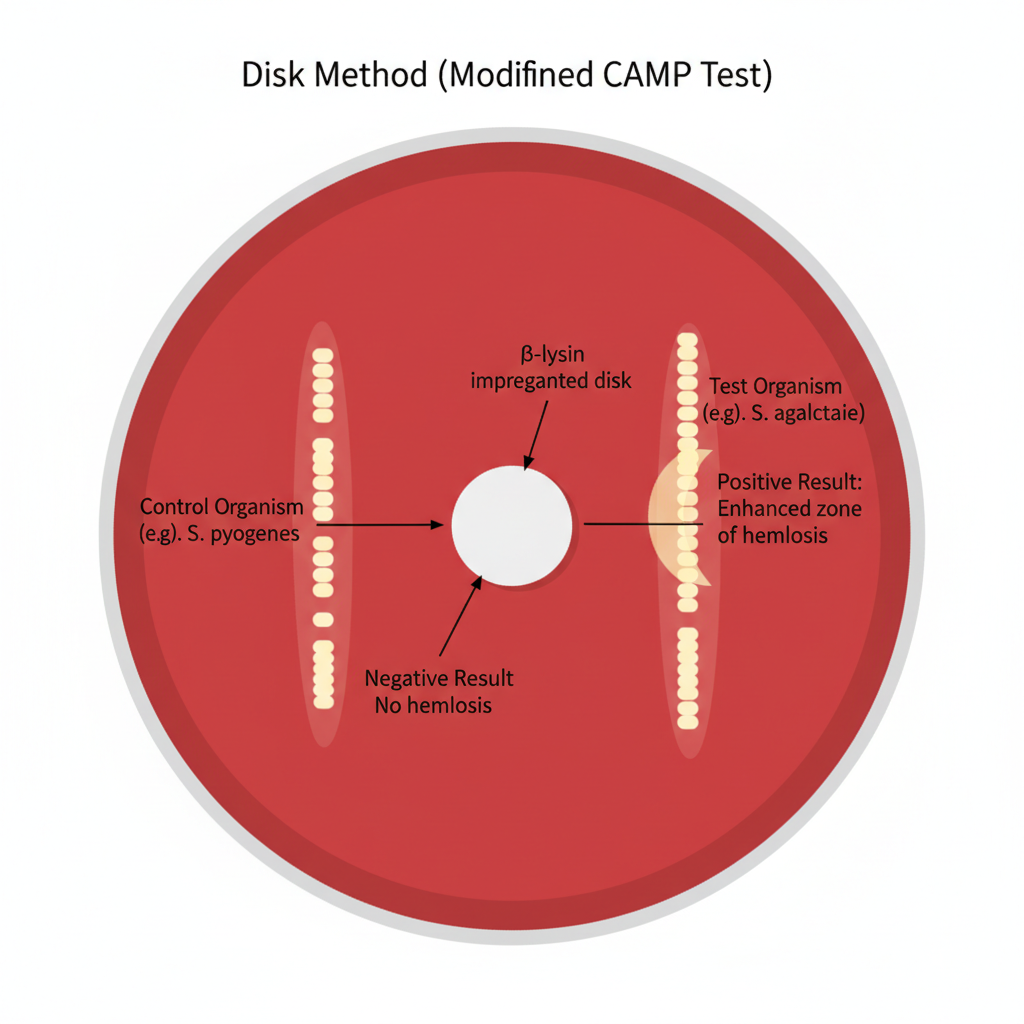
Disk Method (CAMP Test) – Procedure
The disk method is a modified CAMP test where a β-lysin impregnated disk is used instead of a live Staphylococcus aureus streak. It is the process that follows simple steps and is used for detecting CAMP factor production on sheep blood agar.
- A sheep blood agar plate is prepared as the medium for observing hemolysis because sheep erythrocytes contain sphingomyelin needed for β-lysin activity.
- A β-lysin impregnated disk is placed gently on the agar surface using sterile forceps.
- The test organism is streaked in a straight line at a distance of about 2–3 mm from the edge of the disk, keeping the streak parallel to the disk border.
- More than one organism may be tested on the same plate as long as each streak maintains the required spacing from the disk.
- The plate is incubated at 35–37°C for 18–24 hours in ambient air.
- After incubation, the presence of a clear crescent-shaped or arc-shaped enhanced hemolysis adjacent to the disk is taken as the positive reaction, showing the action of CAMP factor with the β-lysin on sheep erythrocytes.
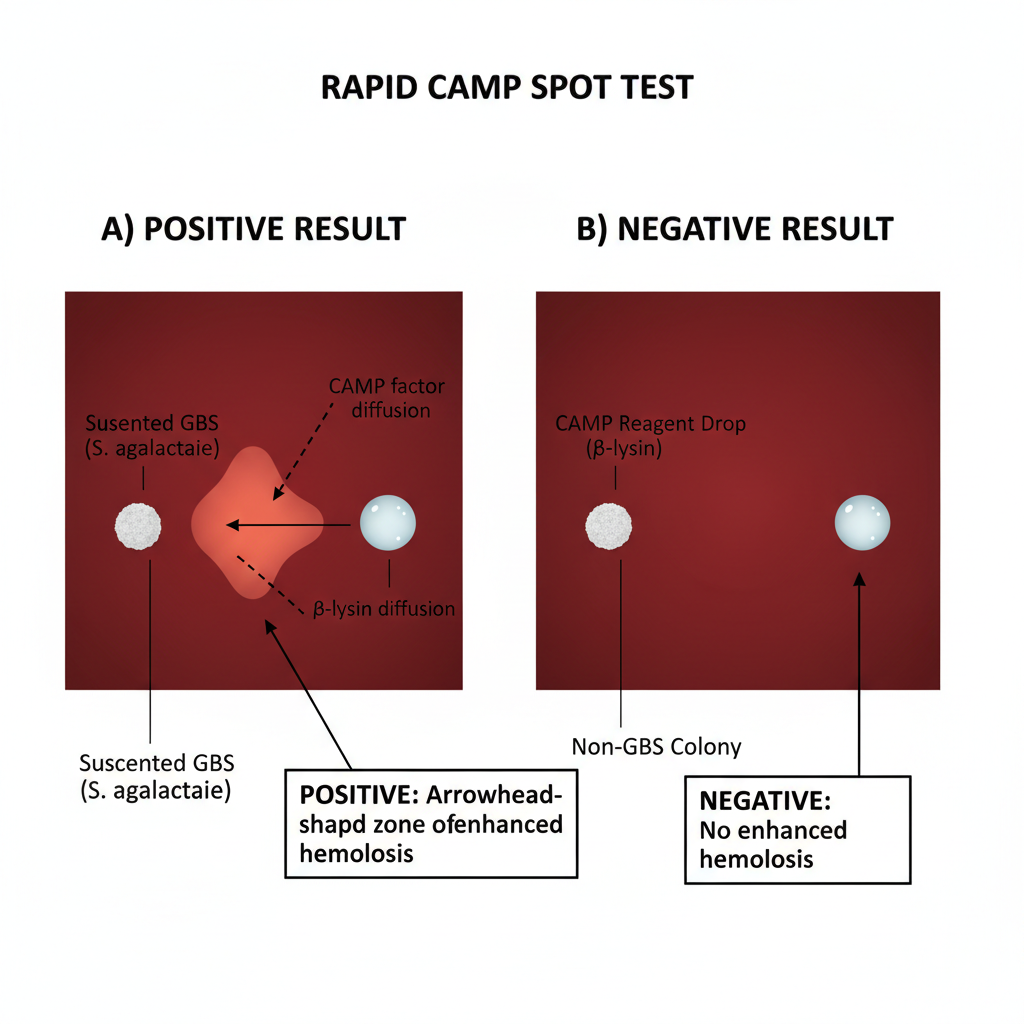
Rapid Spot Test
The rapid spot test is the process used to detect CAMP factor in a shorter time by using a reagent containing extracted β-lysin instead of a live Staphylococcus aureus streak. It is performed directly on a blood agar plate near the suspected colony.
- A sheep blood agar plate with well-isolated colonies of the suspected organism is selected as the test surface.
- A small drop of CAMP reagent containing β-lysin is placed close to the presumptive GBS colony, keeping the drop near but not touching the colony.
- The plate is kept at room temperature or slightly warmed conditions to allow diffusion of the reagent toward the colony.
- In this step the CAMP factor diffuses from the colony and interacts with the β-lysin in the reagent, producing localized enhanced hemolysis.
- The plate is observed after 20 minutes to 1 hour for the presence of a clear hemolytic zone around the reagent drop near the colony.
- A positive reaction is indicated when enhanced hemolysis appears next to the suspected colony, while the absence of such hemolysis is taken as the negative reaction.
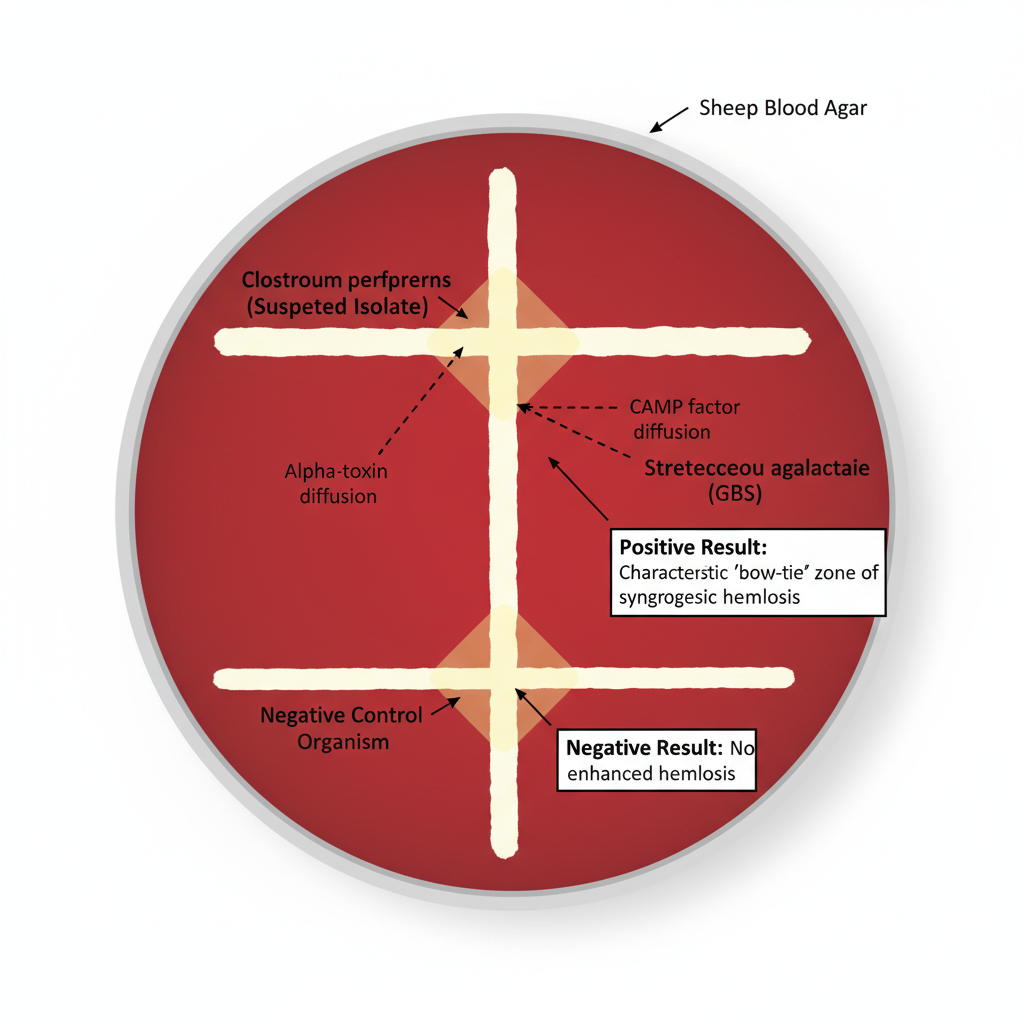
Reverse CAMP Test
The reverse CAMP test is the process used mainly for identifying Clostridium perfringens by observing its interaction with the CAMP factor produced by a known GBS strain. It is performed on sheep blood agar and depends on the formation of the characteristic bow-tie shaped hemolysis.
- A sheep blood agar plate is selected as the medium because sheep erythrocytes contain sphingomyelin required for β-lysin activity and synergistic lysis.
- A known CAMP-positive Streptococcus agalactiae strain is streaked in a straight line down the center of the plate.
- The suspected Clostridium perfringens isolate is streaked perpendicular to the central GBS streak, keeping a small gap without touching the lines.
- In this step the alpha-toxin (phospholipase C) produced by C. perfringens diffuses toward the CAMP factor from the GBS strain, setting the condition for synergistic hemolysis.
- The plate is incubated anaerobically at 35–37°C for 18–24 hours because C. perfringens requires anaerobic conditions for proper toxin expression.
- A positive reaction is observed as a bow-tie or reverse-arrow shaped enhanced hemolysis formed at the junction of the two streaks. The absence of this pattern is taken as the negative reaction.
Quality Control of CAMP test
- Positive control Streptococcus agalactiae ATCC 13813 is used to show arrowhead shaped enhanced hemolysis
- Negative control Streptococcus pyogenes ATCC 19615 or Enterococcus faecalis ATCC 29212 is included with no arrowhead formation
- Indicator strain beta-lysin producing Staphylococcus aureus ATCC 25923 is streaked for synergistic reaction
- Sheep blood agar plates with 5% sheep blood are prepared as media
- Beta-lysin stability in S. aureus strain is checked to avoid loss of toxin production
- Test organism is streaked within 1 to 2 mm of S. aureus without touching
- Plates are incubated at 35–37°C for 18 to 24 hours in ambient air
- Anaerobic incubation or candle jar is avoided to prevent false positives
- CAMP-negative GBS strains due to cfb gene mutations are considered in interpretation
- Listeria monocytogenes positive reaction is differentiated by catalase test and Gram staining
Result of CAMP test

Positive Result
It is indicated when an arrowhead-shaped zone of enhanced β-hemolysis is seen. This is formed at the region where the test organism is streaked perpendicular and close to the β-lysin producing Staphylococcus aureus. The arrow points toward the S. aureus line. It is the process where the CAMP factor produced by the organism acts synergistically with the β-lysin to lyse red blood cells. This is referred to as the characteristic reaction of Group B Streptococcus (GBS).
Negative Result
It is shown when no enhanced hemolysis is produced near the junction of the two streaks. The organism only shows its normal hemolytic pattern (gamma or weak beta). The distinct arrowhead is absent.
Results of Modified CAMP Procedures
Rapid Spot Test
A positive reaction is indicated when enhanced hemolysis appears within 20 minutes to 1 hour after a drop of CAMP factor reagent is placed near the suspected GBS colony.
Disk Method
A positive result shows a clear crescent-shaped or arc-shaped hemolytic zone at the interface of the test organism and the β-lysin impregnated disk.
Reverse CAMP Test
It is used mainly for identifying Clostridium perfringens. The reaction is as follows– a bow-tie or reverse-arrow zone of enhanced hemolysis appears where C. perfringens intersects with the central GBS streak. These are typical findings for reverse CAMP positive organisms.
Anomalies and False Results
CAMP-Negative GBS
Some isolates of GBS do not produce CAMP factor. These strains may show negative CAMP results because deletions or mutations occur in the cfb gene. These are important because they reduce the sensitivity of this test.
False Positives
Some of the important organisms showing false results are–
- Streptococcus pyogenes: It may show a false positive if incubated in anaerobic conditions or candle jar due to activity of Streptolysin O.
- Listeria monocytogenes: It produces a CAMP-like reaction. It is differentiated by Gram reaction (rods) and catalase test (positive).
Other Positive Organisms
Some organisms like Rhodococcus equi, Propionibacterium species, and some Corynebacterium species can also produce positive CAMP reactions.
List of CAMP test positive Organisms
- Streptococcus agalactiae.
- Rhodococcus equi
- Listeria monocytogenes
- Propionibacterium avidum/granulosum,
- Actinomyces neuii
- Turicella otitidis
- Corynebacterium glucuronolyticum
- Corynebacterium colyeae
- Corynebacterium imitans, and some strains of Corynebacterium striatum and Corynebacterium afermentans group.
Uses of CAMP Test
- It is used for the presumptive identification of Group B Streptococcus (Streptococcus agalactiae).
- It helps in differentiating GBS from other β-hemolytic streptococci like Streptococcus pyogenes and non-group B isolates.
- It is used in clinical screening of pregnant women to detect GBS colonization which is important because neonatal sepsis and meningitis is commonly caused by GBS.
- It is applied in veterinary diagnosis to identify GBS in milk samples where it is the major source of bovine mastitis.
- It is the process used to identify Listeria monocytogenes since this organism shows CAMP positive reaction with Staphylococcus aureus.
- It helps in differentiating Listeria monocytogenes from Listeria ivanovii by using Rhodococcus equi as the indicator strain in the modified CAMP reaction.
- It is used in the Reverse CAMP test for identification of Clostridium perfringens.
- It helps in distinguishing C. perfringens from other Clostridium species as only C. perfringens shows the characteristic bow-tie shaped enhanced hemolysis.
- It is used to detect other CAMP-positive organisms like Rhodococcus equi, Propionibacterium species, some Corynebacterium species, and Actinomyces neuii.
- It is the process that also detects certain Gram-negative organisms like Aeromonas, Vibrio and Pasteurella haemolytica showing CAMP-like reactions.
- It is used in identifying Corynebacterium pseudotuberculosis and Arcanobacterium haemolyticum by Reverse CAMP testing.
- It is important in understanding diagnostic limitations because some GBS strains is CAMP-negative due to mutations in the cfb gene which can affect sensitivity.
- It is now supplemented with molecular diagnostic tests like NAAT or PCR in laboratories to ensure detection of CAMP-negative variants.
Limitations of CAMP Test
- It is limited because some GBS strains is CAMP-negative and do not produce the CAMP factor, resulting in false-negative reactions.
- It is the process where deletions or mutations in the cfb gene disturb CAMP factor formation, so the reaction is not seen even though the organism is GBS.
- It is affected when partial deletions of the cfb gene occur, where the N-terminal region is present but the C-terminal functional region is absent, leading to mismatch between phenotypic and PCR results.
- It may miss GBS colonization in pregnant women if the strain is CAMP-negative, increasing the risk of undetected neonatal infection.
- It can show false-positive reactions with organisms like Listeria monocytogenes, Rhodococcus equi, Propionibacterium species, S. porcinus, S. pseudoporcinus and others.
- It is affected by Streptococcus pyogenes when incubated anaerobically, because Streptolysin O becomes active and may mimic the CAMP effect.
- It is sometimes difficult to differentiate the nonspecific “matchstick” hemolysis from the true arrowhead zone, leading to interpretation errors.
- It depends on correct incubation conditions (35–37°C for 18–24 hours). Too short or too long incubation may give false results.
- It requires a β-lysin producing Staphylococcus aureus indicator strain which may lose β-toxin production due to bacteriophage integration, leading to weak or absent synergistic hemolysis.
- It must be performed on sheep blood agar because synergistic hemolysis depends on sphingomyelin content. Blood from other animals will not produce the correct reaction.
- It is less sensitive than molecular methods because CAMP-negative GBS strains cannot be detected by phenotypic testing alone.
- It cannot provide rapid results, since it needs overnight incubation, whereas NAATs can detect GBS in a short time.
- It cannot serve as a standalone confirmatory test and is recommended to be used along with PCR-based or multi-target molecular methods for reliable diagnosis.
Advantages of CAMP Test
- It is a low-cost method and can be used easily in routine laboratories because the test needs simple materials like sheep blood agar.
- It is the process that is technically simple to perform and does not need advanced instruments or skilled handling.
- It uses reagents that is stable for long periods, especially in the modified tests where extracted toxins can be stored without losing activity.
- It helps in differentiating Streptococcus agalactiae (GBS) from other β-hemolytic streptococci which is usually CAMP-negative.
- It is used to identify Listeria monocytogenes by its characteristic positive reaction with Staphylococcus aureus while helping in distinguishing it from other Listeria species.
- It helps in identifying Clostridium perfringens through the Reverse CAMP test where the bow-tie pattern is produced as the typical reaction.
- It provides a clear visual zone of enhanced hemolysis which indicates the production of CAMP factor by the organism.
- It has rapid testing variations like the spot test that can show hemolysis within 20 minutes to 1 hour.
- It can also be done as a tube-based rapid method giving results within 4 hours, so it reduces the time needed for identification.
- It is important in veterinary screening to detect GBS in milk samples which is the major cause of bovine mastitis.
- It remains a useful screening tool even when molecular methods are available because it shows an actual phenotypic expression instead of just gene detection.
- Aryal, S. (2022, August 10). CAMP test- Principle, uses, procedure and result interpretation. Microbiology Info.com. https://microbiologyinfo.com/camp-test-principle-uses-procedure-result-interpretation/
- Boyanov, V. S., Alexandrova, A. S., & Gergova, R. T. (2025). Diagnostic challenges and genotypic characteristics of CAMP-negative phenotype GBS isolates. Journal of Pure and Applied Microbiology, 19(4), 10231. https://doi.org/10.22207/JPAM.19.4.16
- Buchanan, A. G. (1982). Clinical laboratory evaluation of a reverse CAMP test for presumptive identification of Clostridium perfringens. Journal of Clinical Microbiology, 16(4), 761–762. https://doi.org/10.1128/jcm.16.4.761-762.1982
- de-Paris, F., Machado, A. B. M. P., Gheno, T. C., Ascoli, B. M., de Oliveira, K. R. P., & Barth, A. L. (2011). Group B Streptococcus detection: comparison of PCR assay and culture as a screening method for pregnant women. The Brazilian Journal of Infectious Diseases, 15(4), 323–327.
- Gase, K., Ferretti, J. J., Primeaux, C., & McShan, W. M. (1999). Identification, cloning, and expression of the CAMP factor gene (cfa) of Group A streptococci. Infection and Immunity, 67(9), 4725–4731. https://doi.org/10.1128/iai.67.9.4725-4731.1999
- Geng, B. (2020). The cost-effectiveness of culture vs NAAT based screening of pregnant women for Group B Streptococcus to reduce early-onset sepsis of the newborn [Doctoral thesis, Yale University]. Yale Medicine Thesis Digital Library. https://elischolar.library.yale.edu/ymtdl/3904
- Gubash, S. M. (1978). Synergistic hemolysis phenomenon shown by an alpha-toxin-producing Clostridium perfringens and streptococcal CAMP factor in presumptive streptococcal grouping. Journal of Clinical Microbiology, 8(5), 480–488. https://doi.org/10.1128/jcm.8.5.480-488.1978
- Guo, D., Xi, Y., Wang, S., & Wang, Z. (2019). Is a positive Christie-Atkinson-Munch-Peterson (CAMP) test sensitive enough for the identification of Streptococcus agalactiae? BMC Infectious Diseases, 19(1), 7. https://doi.org/10.1186/s12879-018-3561-3
- HajiAhmadi, P., Momtaz, H., & Tajbakhsh, E. (2025). Molecular characterization of Streptococcus agalactiae strains isolated from pregnant women. Scientific Reports, 15, 5887. https://doi.org/10.1038/s41598-025-86565-z
- Hanson, A. (2006, October 9). CAMP test protocols. American Society for Microbiology. https://asm.org/asm/media/protocol-images/camp-test-protocols.pdf
- Khafri, A., & Nazari, A. (2004). The rapid CAMP test for identification of Streptococcus agalactiae using alpha toxin. Archives of Razi Institute, 58, 119–124.
- Kumari, S. (2020). CAMP tests (Standard and rapid) and reverse CAMP test [Practical Class Notes]. Bihar Veterinary College. https://basu.org.in/wp-content/uploads/2021/01/CAMP_Tests__Standard_and_Rapid__and_Reverse_CAMP_test.pdf
- Lai, X., Chen, M., Wang, J., Wang, J., Lv, H., Xie, H., He, W., Chen, D., Huang, Y., Cai, P., & Zheng, L. (2025). CAMP-negative Streptococcus agalactiae strains exhibited complete or partial chromosomal deletions of the CAMP-factor encoding gene cfb. Microbiology Spectrum, 13(5), e03257-24. https://doi.org/10.1128/spectrum.03257-24
- Li, Y., Zeng, W., Li, Y., Fan, W., Ma, H., Fan, X., Jiang, J., Brefo-Mensah, E., Zhang, Y., Yang, M., Dong, Z., Palmer, M., & Jin, T. (2019). Structure determination of the CAMP factor of Streptococcus agalactiae with the aid of an MBP tag and insights into membrane-surface attachment. Acta Crystallographica Section D: Structural Biology, 75(Pt 8), 772–781. https://doi.org/10.1107/S205979831901057X
- McKellar, R. C. (1993). Novel mechanism for the CAMP reaction between Listeria monocytogenes and Corynebacterium equi. International Journal of Food Microbiology, 18(1), 77–82. https://doi.org/10.1016/0168-1605(93)90010-e
- Murray, P. R., Wold, A. D., Schreck, C. A., & Washington, J. A., 2nd. (1976). Effects of selective media and atmosphere of incubation on the isolation of group A streptococci. Journal of Clinical Microbiology, 4(1), 54–56. https://doi.org/10.1128/jcm.4.1.54-56.1976
- Phillips, E. A., Tapsall, J. W., & Smith, D. D. (1980). Rapid tube CAMP test for identification of Streptococcus agalactiae (Lancefield group B). Journal of Clinical Microbiology, 12(2), 135–137. https://doi.org/10.1128/jcm.12.2.135-137.1980
- Pokhrel, P. (2015, September 24). Reverse CAMP test for the identification of Clostridium perfringens. Microbiology Notes. https://microbiologynotes.com/reverse-camp-test-for-the-identification-of-clostridium-perfringens/
- Sapkota, A. (2022, January 27). CAMP test- Principle, procedure, types, results, uses, limitations. Microbe Notes. https://microbenotes.com/camp-test-principle-procedure-and-result-interpretation/
- Tapsall, J. W., & Phillips, E. A. (1984). Streptococcus pyogenes streptolysin O as a cause of false-positive CAMP reactions. Journal of Clinical Microbiology, 19(4), 534–537. https://doi.org/10.1128/jcm.19.4.534-537.1984
- Watson, R. (n.d.). Tests used to identify Gram negative bacteria. University of Wyoming. Retrieved from https://www.uwyo.edu/molb2210_lab/info/biochemical_tests.htm
- Wikipedia. (n.d.). CAMP test. Retrieved from Wikipedia.
- Zhou, J., Zhang, L., Zhang, Y., Liu, H., Xu, K., Zhang, B., Feng, T., & Yang, S. (2023). Analysis of molecular characteristics of CAMP-negative Streptococcus agalactiae strains. Frontiers in Microbiology, 14, 1189093. https://doi.org/10.3389/fmicb.2023.1189093