The Biuret test is a chemical test used for detecting the presence of proteins. It is the reaction in which proteins are identified because peptide bonds present in the protein react with the Biuret reagent in an alkaline condition. It is carried out by using an alkaline solution of copper sulphate (CuSO₄) together with sodium hydroxide (NaOH) and the colour change is the main indicator for the presence of protein. When the reagent is added to a solution that contain proteins, the copper ions form a coordination complex with nitrogen atoms of the peptide bonds and the solution is changed from blue to violet. This is referred to as a positive Biuret reaction.
It is the process where at least two peptide bonds must be present for the colour change to occur, so free amino acids does not show the reaction. The intensity of violet colour is increased with the concentration of the protein and this makes the test useful for estimating protein quantity by spectrophotometry. The reagent does not contain actual biuret compound but the name is used because the biuret molecule also shows similar peptide-like bonds that give the same reaction.
Purpose of Biuret Test
- To detect the protein in the given solution.
- To confirm the presence of the peptide bond.
Principle of Biuret Test
The principle of the Biuret test is based on the reaction between peptide bonds and cupric ions in an alkaline condition. It is the process in which proteins give a violet-coloured complex when copper ions (Cu²⁺) interact with the nitrogen atoms present in peptide bonds. The alkaline medium is usually provided by sodium hydroxide or potassium hydroxide, and in this condition the copper ions are able to form a stable chelate with the peptide linkage. This is referred to as the Biuret reaction.
It is required that at least two peptide bonds must be present, so tripeptides and larger proteins show a positive reaction while free amino acids or dipeptides does not respond. The colour intensity increases with the number of peptide bonds in the solution and it is directly proportional to the protein concentration. Because of this the test is also used in quantitative estimation by measuring absorbance at 540 nm.
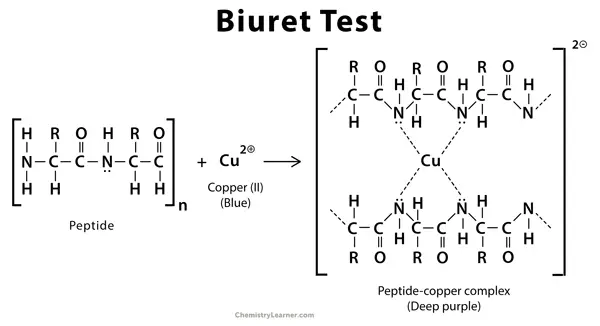
Reaction of Biuret Test
The Biuret test is the process where peptide bonds react with copper ions in alkaline medium. It is the reaction forming a violet coloured complex. The colour depends on the number of peptide bonds present.
The reaction is as follows–
Peptide bonds + Cu²⁺ (alkaline) → Cu–peptide complex (violet colour)
In this step the copper (II) ions is changed into a complex state by the nitrogen atoms of the peptide linkage. This complex gives the violet colour which is referred to as the positive Biuret reaction. The reaction occurs only when the sample has two or more peptide bonds.
Requirements for Biuret Test
Chemical reagents
– Copper(II) sulphate (CuSO₄·5H₂O), which is the source of cupric ions for forming the coloured complex.
– Alkaline solution (NaOH or KOH) for creating the basic medium needed for the reaction.
– Potassium sodium tartrate (Rochelle salt) used as stabilizer to prevent copper hydroxide precipitation.
– Potassium iodide (KI) sometimes added to keep copper ions in solution.
Laboratory equipment
– Clean and dry test tubes for preparing the reaction mixture.
– Pipettes for transferring reagent and sample.
– Spectrophotometer or colorimeter for quantitative measurement at 540–550 nm.
– Cuvettes for holding the reagent–sample mixture inside the instrument.
– White tile or paper sheet for observing colour change in qualitative tests.
– Water bath if incubation is required in some procedures.
Sample specifications
– Sample must contain peptide bonds (at least two) for giving positive reaction.
– Proteins should be soluble in alkaline medium.
– Suitable concentration of protein solution (generally 0.5–20 mg/mL or more).
– Solid samples need to be crushed and mixed with water before testing.
Experimental controls
– Positive control containing protein (like albumin or BSA).
– Negative control lacking protein, usually distilled water or buffer.
– Standard curve prepared from known protein dilutions for quantitative estimation.
Environmental conditions
– Reaction must be kept at alkaline pH.
– Standing time of 3–5 minutes for qualitative tests or 20–30 minutes for stable colour development in quantitative tests.
Biuret Reagent Preparation
Standard 1-Liter Preparation
- It is prepared by dissolving 1.5 g hydrated copper sulfate (CuSO₄) and 6 g sodium potassium tartrate in 500 mL distilled water.
- In this step 375 mL of 2 M sodium hydroxide solution is added to the mixture.
- The final volume is adjusted to 1000 mL with distilled water.
Rice University Method (with Potassium Iodide)
- It is started with 400 mL of 0.2 M sodium hydroxide solution.
- The salts are dissolved in a fixed order i.e. 9 g sodium potassium tartrate, 3 g copper sulfate pentahydrate, and then 5 g potassium iodide.
- Distilled water is then added to make the final volume 1 L.
Small Batch Preparation (≈100 mL)
- 1 g copper(II) sulfate is dissolved in 100 mL distilled water.
- 1.2 g sodium potassium tartrate is added for stabilization of copper ions.
- 10 mL of 10% sodium hydroxide solution is mixed to make the reagent alkaline.
Important Precautions
- The tartrate is important because it stabilizes the copper ions. It is added so the copper does not precipitate as copper hydroxide when alkali is introduced.
- Any black precipitate forming during preparation or storage is an indication of reagent deterioration and it is discarded.
Procedure of Biuret Test
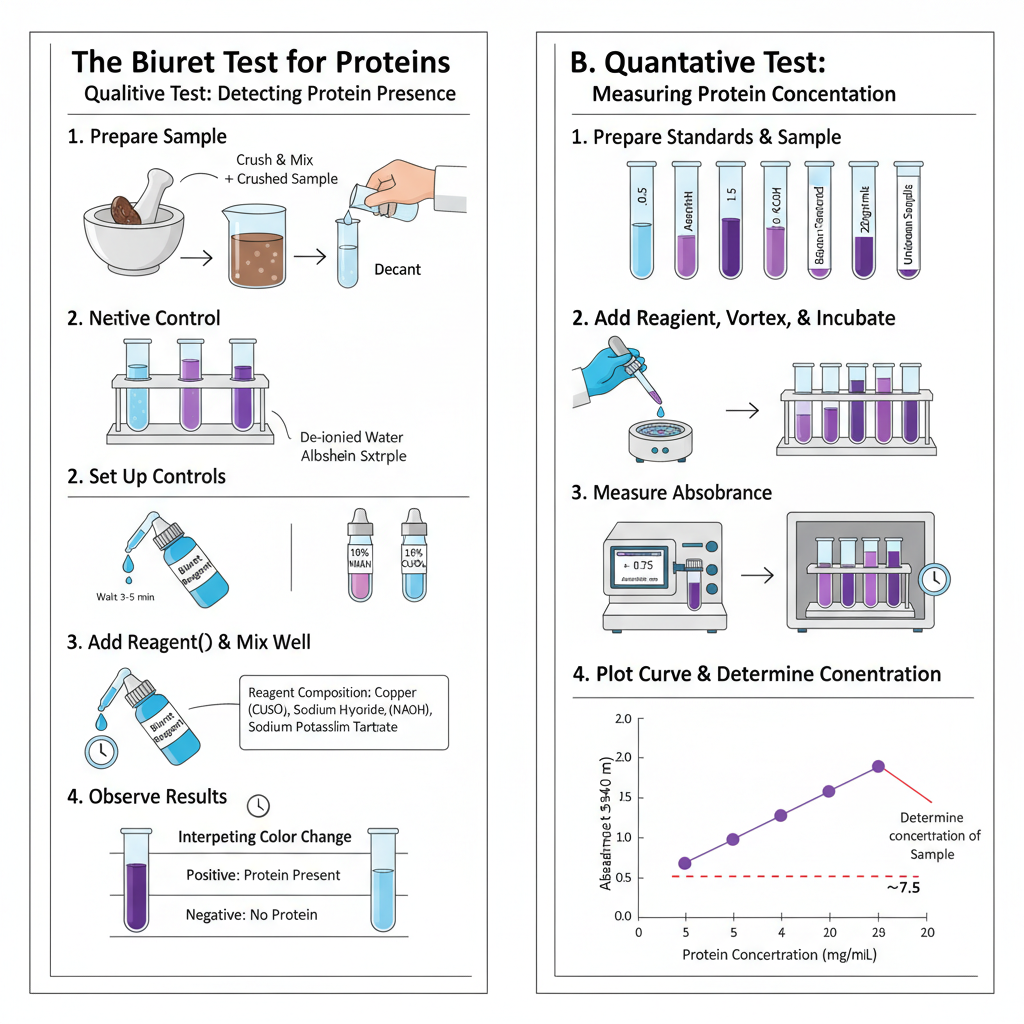
Qualitative Biuret Test
- Sample is prepared by crushing solid material and mixing with de-ionized water. The liquid is decanted to get aqueous extract. Liquid samples are used directly if aqueous.
- Control tubes are set up. Negative control contains de-ionized water. Positive control contains known protein like egg albumin. Test tube contains sample solution of 1–2 mL.
- Biuret reagent is added. Equal volume of reagent is mixed with sample. Reagent contains copper sulfate, sodium hydroxide and sodium potassium tartrate.
- Alternative method uses separate solutions. 1 mL of 1–10% sodium hydroxide is added first. Then few drops of 1% copper sulfate solution are added.
- Mixture is shaken well. It is allowed to stand at room temperature for 3 to 5 minutes.
- Color is observed against white background. Blue color means negative result. Violet or purple color means positive result for proteins. Pink color indicates short peptides.
Quantitative Biuret Test
- Protein standards are prepared. Bovine serum albumin is used in concentrations from 0.5 to 20 mg/mL.
- Biuret reagent is added to standards and samples. Ratio is 1 part sample to 9 parts reagent.
- Mixture is vortexed. It is incubated for 20 minutes for full color development.
- Absorbance is measured at 540 nm or 550 nm using spectrophotometer.
- Standard curve is plotted from absorbance of standards. Protein concentration of sample is calculated from the curve.
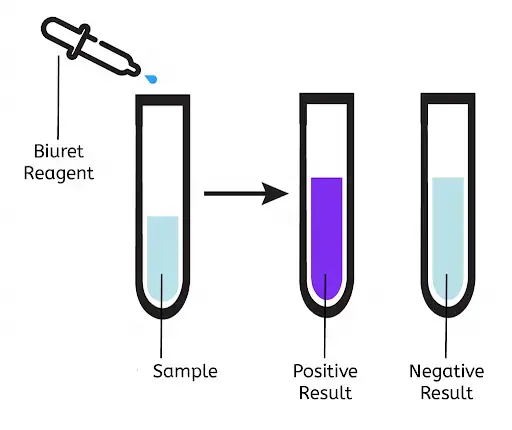
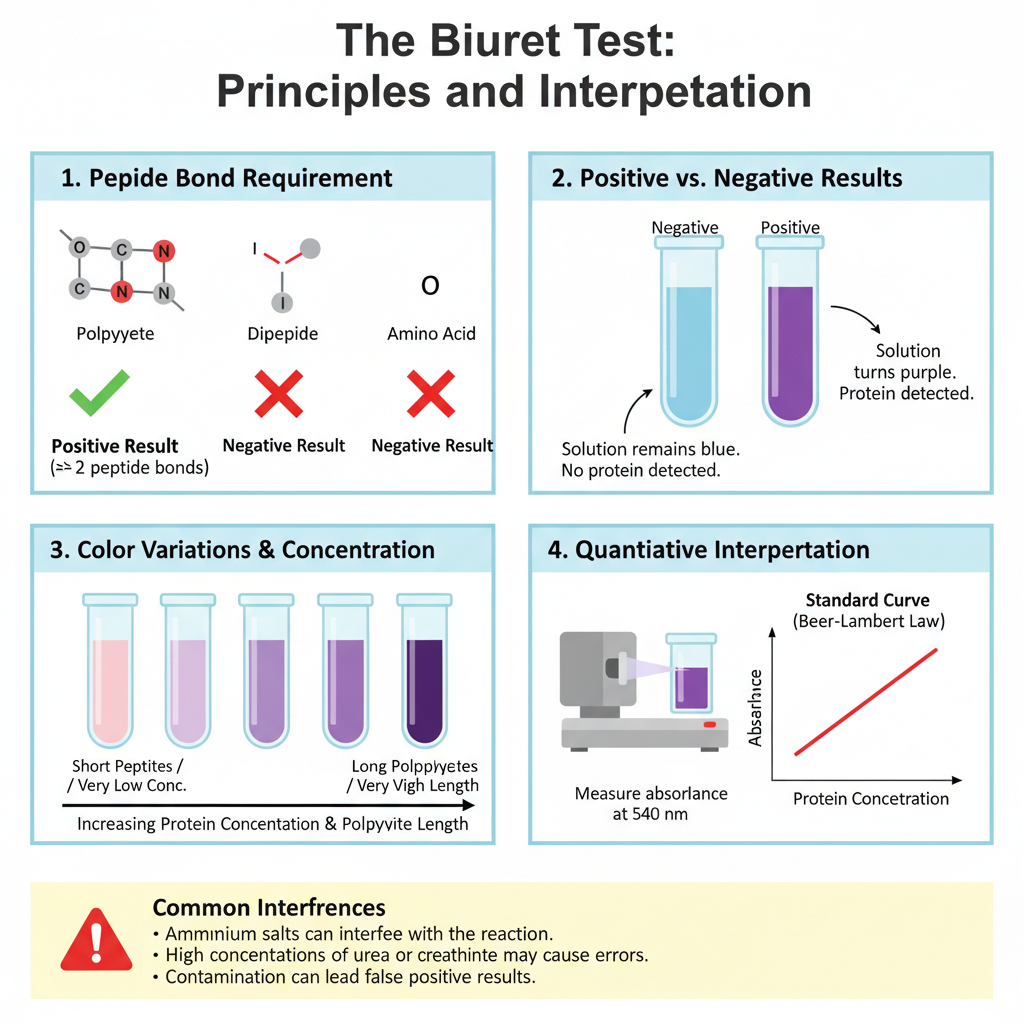
Result and Interpretation of Biuret Test

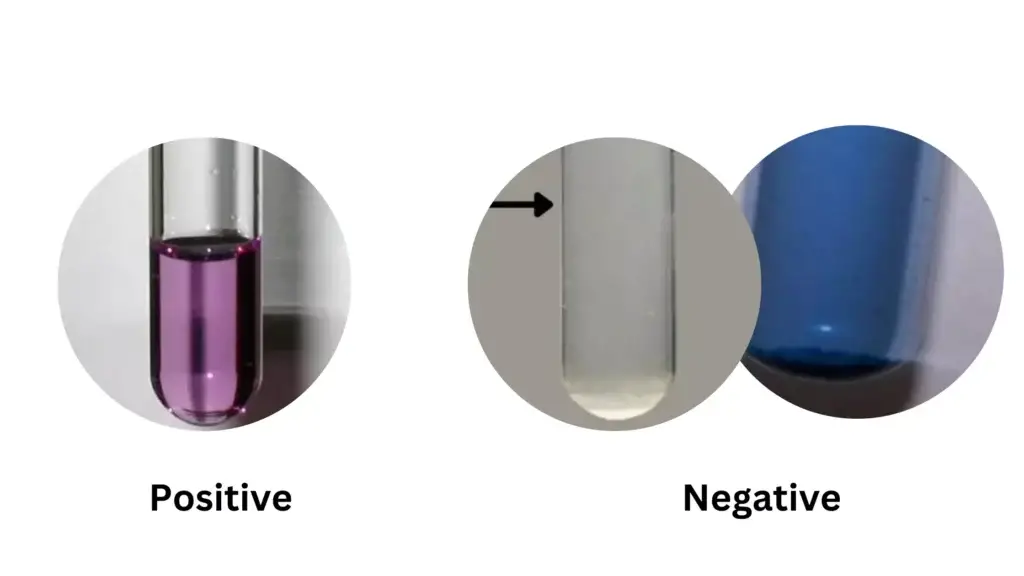
Positive Result
- Solution changes from blue to violet or purple color.
- This indicates presence of proteins or polypeptides.
- Reaction needs at least two peptide bonds.
- Darker color shows higher protein concentration.
Negative Result
- Solution remains blue.
- This means no proteins or peptides are present.
- Free amino acids or dipeptides give negative result.
- One peptide bond is not enough for color change.
Variations in Color
- Pink or pale violet color appears with short peptides.
- Low protein amount also gives pale color.
- Deep purple color indicates long polypeptide chains.
- High protein concentration produces deep color.
Quantitative Interpretation
- Absorbance is measured at 540 nm or 550 nm.
- Spectrophotometer is used for reading.
- Standard curve helps calculate protein amount.
- Beer-Lambert law is followed in measurement.
Interferences
- Ammonium salts in buffer interfere with test.
- Biuret compound gives false positive result.
- High urea or creatinine in urine causes error.
- Protein amount may be overestimated.
Uses of Biuret Test
- It is used for measuring total protein concentration in a solution by observing the violet colour formed in alkaline condition.
- The test is suitable for samples having high protein concentration as the reaction is less sensitive compared to other assays.
- It is used for analysing whole tissue samples because free amino acids do not interfere with the reaction.
- The Biuret reaction is the basic chemical principle for more sensitive assays like the Lowry method and BCA method.
- It is used in clinical laboratories to detect and estimate protein level in blood serum and urine.
- It is helpful in checking kidney function where urine protein is measured for detecting nephrotoxicity.
- Abnormal protein levels detected by this test may indicate inflammation and certain clinical conditions.
- It is used in food science for detection of proteins in food materials especially in liquid samples.
- The test helps in quality control of food by checking protein content and detecting adulteration.
- It is used in some industrial processes for monitoring protein amount during fermentation.
- It is a common practical test in school and college laboratories for teaching peptide bonds and qualitative protein detection.
Advantages of Biuret Test
- The reaction takes place with the peptide bonds so most proteins give a uniform colour response at the same concentration.
- It does not react with free amino acids or dipeptides as minimum two peptide bonds is needed, so it helps in distinguishing proteins from small nitrogen compounds.
- The test is stable and not affected by many detergents or surfactants present in crude samples.
- The violet coloured complex formed in the reaction remains stable for a long time giving reliable readings.
- It is suitable for whole tissue samples because free amino acids in crude extract do not interfere with colour formation.
- The procedure is simple which usually needs only mixing of reagent and incubation for some time.
- The reagents required are cheap and easy to prepare in laboratory conditions.
- It is suitable for samples having high protein concentration since the linear range extends up to very high values.
Limitation of Biuret Test
- The test has low sensitivity and needs high protein concentration for giving proper colour.
- It consumes large sample volume, so it is not suitable when the sample amount is very limited.
- Ammonium salts and Tris buffer interfere with the reaction because these ions compete with copper ions.
- High magnesium ions and pigments like bile pigments may disturb the colour formation.
- Reducing agents such as DTT or β-mercaptoethanol affect the copper reduction process and give wrong estimation.
- Some non-protein nitrogen compounds like urea, creatinine and biuret itself can react and give false positive result.
- The colour formed may vary with different proteins and peptides making interpretation difficult.
- Free amino acids and dipeptides do not react as the test needs minimum two peptide bonds for forming the violet complex.
- The protein must be soluble in the reagent because insoluble or turbid samples do not give correct result.
- Proteins having high proline content may show reduced reactivity leading to underestimation.
Precautions
- The Biuret reagent should not contain black precipitate as it shows the stabilizer is failed and the reagent must be discarded.
- It is important that copper sulphate and the alkaline components is mixed properly to avoid immediate copper hydroxide formation.
- Samples containing ammonium salts must be avoided because ammonium ions interfere with the reaction and prevent violet colour formation.
- Tris and other basic buffers should not be used since these interfere with the test and reduce colour intensity.
- The protein solution must be completely soluble in the alkaline reagent as insoluble or turbid samples do not give correct result.
- In biological samples compounds like urea, creatinine and free amino acids may cross-react giving overestimation of protein.
- Reducing agents like DTT or β-mercaptoethanol should not be present in high amount as these disturb copper ions and the reaction.
- The recommended ratio of sample and reagent should be maintained for proper colour development.
- Excess reagent should not be added because the deep blue of unreacted copper sulphate can hide the violet colour.
- The mixture should be kept for the required incubation time so that the colour is developed completely.
- The sample must be mixed immediately after adding the reagent for a uniform reaction condition.
- A negative and a positive control should be used in the test for comparing the result.
- In spectrophotometric reading the instrument should be warmed before use to stabilize the reading.
- Absorbance readings should be taken within a short time difference because long delay can affect accuracy.
FAQ
Q. What is the Biuret Test?
A. The Biuret test is a chemical test used for detecting proteins by forming a violet coloured complex in alkaline condition.
Q. What does the Biuret test for?
A. It tests for the presence of peptide bonds and thus shows proteins or long polypeptide chains.
Q. What does a positive result in the Biuret test indicate?
A. A positive result indicates that proteins or polypeptides is present in the sample.
Q. Why does the Biuret test give a purple color?
A. The purple colour is formed when peptide bonds react with copper (II) ions in alkaline medium forming a coordination complex.
Q. What is the principle of the Biuret Test?
A. It is the process where peptide bonds bind with copper ions in alkaline condition and the complex gives violet colour.
Q. What reagent is used in the Biuret test?
A. The Biuret reagent is used which contains copper sulphate in alkaline solution.
Q. What is the composition of Biuret reagent?
A. It is made of copper sulphate, sodium hydroxide, and potassium sodium tartrate for stabilizing the copper ions.
Q. What ions does Biuret reagent contain?
A. It contains Cu²⁺ ions which react with peptide bonds.
Q. What is the procedure for the Biuret test?
A. The sample is mixed with Biuret reagent, shaken, and left for few minutes to observe violet or blue colour.
Q. How does the Biuret test help in distinguishing between proteins and amino acids?
A. Proteins have many peptide bonds which react, but free amino acids do not react so they cannot give the violet colour.
Q. What are the limitations of the Biuret Test?
A. The test has low sensitivity, needs high protein concentration, and is affected by ammonium salts, pigments, and reducing agents.
Q. Why is the Biuret solution blue initially?
A. It is blue because of the copper (II) ions present in the reagent before reacting with peptide bonds.
Q. What is the role of sodium hydroxide in the Biuret test?
A. Sodium hydroxide provides strong alkaline medium which is required for the copper–peptide reaction.
Q. Why is the Biuret test needed?
A. It is needed for quick detection and estimation of proteins in biological and laboratory samples.
Q. What are high sensitivity variants of the Biuret test?
A. The Lowry method and the BCA method are high sensitivity assays based on the Biuret reaction.
- Aaron. (2023, June 5). Which protein assay is best for you? Azure Biosystems. https://azurebiosystems.com/blog/protein-assay-best/
- Biology Online. (2023, March 3). Biuret test. Biology Online Dictionary. https://www.biologyonline.com/dictionary/biuret-test
- Biswas, N., Chaudhuri, A., Chakraborty, S., & Choudhury, C. R. (2019). Example of square planar copper(II) biuret complex: Crystal structure, DNA and protein binding activity and molecular docking study. Inorganic and Nano-Metal Chemistry, 48(10), 1–13. https://doi.org/10.1080/24701556.2019.1572623
- Brilliant Biology Student. (2016). Biuret test for protein. https://brilliantbiologystudent.weebly.com/biuret-test-for-protein.html
- BYJU’S. (n.d.). Biuret test. https://byjus.com/chemistry/biuret-test/
Expert Analytical Report: The Biuret Test for Protein Quantification and Detection. (n.d.). [Internal Report]. - Hortin, G. L., & Meilinger, B. (2005). Cross-reactivity of amino acids and other compounds in the biuret reaction: Interference with urinary peptide measurements. Clinical Chemistry, 51(8), 1411–1419. https://doi.org/10.1373/clinchem.2005.052019
- Rice University. (n.d.). Biuret protein assay. Experimental Biosciences. https://www.ruf.rice.edu/~bioslabs/methods/protein/biuret.html
- Savory, J., Pu, P. H., & Sunderman, F. W., Jr. (1968). A biuret method for determination of protein in normal urine. Clinical Chemistry, 14(12), 1160–1171. https://doi.org/10.1093/clinchem/14.12.1160
- Thermo Fisher Scientific. (n.d.). Chemistry of protein assays. https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/chemistry-protein-assays.html
- Vedantu. (2023). Understanding the biuret test for protein detection. https://www.vedantu.com/chemistry/biuret-test
- Wikipedia contributors. (n.d.). Biuret test. Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Biuret_test