A biosafety level (BSL) refers to a specific set of biocontainment measures designed to isolate hazardous biological agents within a controlled laboratory environment.
This document outlines the necessary protective measures required in a laboratory environment to safeguard employees, the ecosystem, and the community from infectious agents.
Historical Overview
The classification of four levels of biosafety was established in the mid-1970s during discussions regarding the safety of recombinant DNA.
The initial prototype of a Class III biosafety cabinet was developed in 1943 at Camp Detrick, Maryland.
On April 18, 1955, a meeting at Camp Detrick facilitated the exchange of biosafety knowledge among high-security laboratories.
The system consists of four ascending levels of containment, ranging from BSL-1, which represents minimal risk, to BSL-4, the highest risk level. Each level is characterised by distinct engineering controls, work practices, and safety equipment.
What do you mean by Biosafety and Biosafety levels?
Biosafety involves the implementation of safety measures, the use of specialised equipment, and the design of facilities to safeguard individuals, communities, and the environment from inadvertent exposure to infectious agents or toxins.
Biosafety involves the implementation of containment principles, engineering and administrative controls, the use of personal protective equipment, and adherence to responsible laboratory practices to mitigate the risks of exposure, contamination, unauthorised access, or accidental release.
Biosafety levels (BSLs) consist of four ascending tiers, ranging from BSL-1 to BSL-4, which are designed to implement containment protocols according to the risk associated with particular biological agents.
Each Biosafety Level (BSL) delineates the necessary safety measures, encompassing laboratory practices, protective equipment, safety cabinets, facility design, and access restrictions, all determined by factors such as infectivity, disease severity, transmissibility, and the procedures employed.
BSL-1: This level involves working with agents that are not recognised as causing disease in healthy adults. It requires minimal precautions such as handwashing, the use of gloves, and basic personal protective equipment (PPE), without the necessity for specialised facility features.
BSL-2: designated for moderate-risk agents; incorporates laboratory access control, biosafety cabinets for aerosol procedures, and requires specialised training.
BSL-3 is designated for the handling of serious or lethal pathogens that can be transmitted through inhalation. This level of biosafety necessitates the implementation of comprehensive biosafety manuals, medical surveillance protocols, and specific facility features, including sealed laboratories, directional airflow systems, and double-door access points.
BSL-4 represents the highest level of containment for highly dangerous agents for which there are no available treatments or vaccines. This level of containment necessitates the use of full-body positive-pressure suits or sealed cabinets, chemical showers, multiple airlocks, and stringent decontamination procedures.
Purposes of Biosafety levels
Here are the core purposes of Biosafety Levels (BSL):
- Prevent laboratory-acquired infections.
- Protect personnel and the community from exposure to pathogens.
- Contain hazardous biological agents within designated facilities.
- Mitigate environmental contamination risks.
- Standardize procedures based on pathogen risk groups.
- Ensure compliance with national and international regulations.
- Provide clear operational frameworks for handling biological materials.
- Minimize accidental release of pathogens during research or diagnostics.
- Define appropriate engineering controls and safety equipment.
- Establish mandatory training and emergency response protocols.
Biosafety Definition
Biosafety defines as the application of safety precautions which reduces a laboratorians risk of exposure to potentially infectious material and limits contamination of the work environment and ultimately the community.
Why we need Biosafety?
- Stop people and lab workers from accidentally coming into contact with pathogens to protect their health.
- To stop zoonotic spillover and cross-species transmission, protect the health of animals in study.
- Keep the environment clean and the ecology healthy.
- Avoiding sample contamination will help make sure that scientific data is reliable and reproducible.
- Follow rules and standards set by the Cartagena Protocol and other national and international bodies.
- Use structured risk assessments to manage and evaluate biosafety concerns in a methodical way.
- To keep the health and productivity of the staff, stop illnesses from spreading in the lab.
- Lower the chances of inadvertent or planned release that could cause outbreaks or bioterrorism.
- Show that you are responsible in your work to keep the public’s trust in biotechnology and research organisations.
- Enforce biosafety rules in research on new pathogens to help stop pandemics around the world.
Different Level of Biosafety
There are 4 Biosafety Levels;
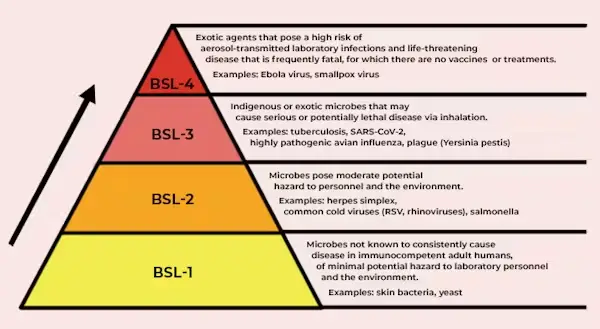
- Biosafety Level 1 (BSL-1)
- Microorganisms that are not recognized as pathogenic in healthy adult humans.
- Practices involve standard microbiological techniques, implemented without the use of specific primary or secondary barriers.
- An open bench workspace, a handwashing sink, and surfaces that can be easily cleaned.
- E.g: Non-harmful variants of Escherichia coli.
- Biosafety Level 2 (BSL-2)
- Agents that present a moderate risk, linked to human diseases of differing levels of severity.
- Practices include restricted access, biohazard warning signage, precautions for “sharps,” and a biosafety manual outlining waste decontamination and medical surveillance policies.
- Utilize Class I or II Biological Safety Cabinets (BSCs) for any procedures that have the potential to generate splashes or aerosols.
- Availability of an autoclave, an eyewash station, and self-closing doors.
- E.g: Staphylococcus aureus, Salmonella spp.
- Biosafety Level 3 (BSL-3)
- Agents that are either indigenous or exotic can lead to serious or potentially lethal diseases when inhaled.
- Practices include controlled access, thorough decontamination of all waste, and ensuring lab clothing is decontaminated prior to laundering.
- Utilize Class I or II BSCs for all open manipulations of agents.
- Ensure physical separation from access corridors, implement self-closing double-door access, and ensure that exhausted air is not recirculated.
- E.g: Examples include Mycobacterium tuberculosis and West Nile virus.
- Biosafety Level 4 (BSL-4)
- Agents that are both dangerous and exotic, presenting a significant individual risk of aerosol-transmitted laboratory infections and potentially life-threatening diseases.
- Access is strictly controlled; personnel are required to change clothing before entry, shower upon exit, and decontaminate all materials prior to leaving.
- Class III BSCs or full-body, air-supplied, positive pressure suits for personnel.
- A designated area equipped with its own supply and exhaust air systems, vacuum capabilities, and decontamination processes.
- E.g: Ebola virus, Marburg virus.

1. Biosafety level 1 (BSL 1)
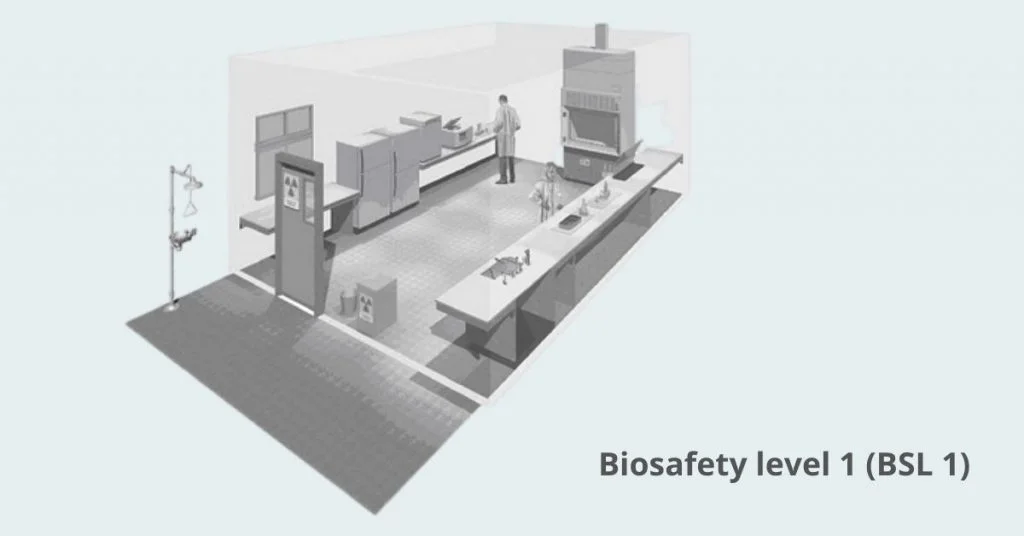
Biosafety level 1 means working with well characterized agents that do not cause disease in healthy adults
It relies on standard microbiological practices such as hand washing, no eating or drinking in the lab, and restricted access
No special containment equipment is required beyond a sink and a door that can close to limit entry
Personal protective equipment is lab coats, gloves and eye protection as needed
Waste is decontaminated with autoclaving or approved chemical disinfectants before disposal
Common organisms at BSL-1 are non-pathogenic strains of Escherichia coli, Bacillus subtilis, and Saccharomyces cerevisiae
Requirements for Biosafety level 1 (BSL 1)
- Standard microbiological practices: hand washing before and after work, no eating, drinking, smoking or applying cosmetics in the lab; facility specific biosafety manual and hazard signage must be available
- Lab training and hazard communication: personnel must be trained on BSL-1 agents, risks and safe practices
- Laboratory access control: must have a door that can close to limit entry; full isolation from the general building is not required
- Personal protective equipment: lab coat, gloves and eye protection as needed; long hair should be restrained to prevent contamination
- Mechanical pipetting devices: mouth pipetting is prohibited; use mechanical devices to prevent ingestion or splash exposure
- Sharp safety practices: policies for handling needles, scalpels and broken glassware; use puncture resistant containers and autoclave or chemically disinfect sharps before disposal
- Decontamination: all work surfaces, cultures, stocks and waste must be decontaminated before disposal using autoclaving or approved chemical disinfectants
- Splash and aerosol minimization: perform procedures to reduce creation of splashes or aerosols during manipulations
- Pest management and facility maintenance: integrated pest control plan in place; laboratory must have adequate illumination for safe work
- Waste management: segregate infectious materials and contaminated disposables; decontaminate before final disposal
Standard microbiological practices for Biosafety level 1 (BSL 1)
- Authorized personnel only
- No eating, drinking, smoking, applying cosmetics or handling contact lenses in the lab
- Hand washing after handling infectious materials and before leaving the lab
- Daily and after spills decontaminate work surfaces with approved disinfectants
- Personal protective equipment: lab coats, gloves and eye protection as needed
- Mechanical pipetting devices must be used to prevent mouth pipetting
- Proper handling and disposal of sharps in puncture-resistant containers and established protocols
- Biohazardous materials must be labeled with identity and hazard warnings
- Remove protective clothing before leaving the lab
- Biosafety manual and visible signage must be available to communicate lab hazards
Safety Equipment (Primary Barriers) of Biosafety level 1 (BSL 1)
- General lab coats are required
- Gloves must be worn when handling cultures and materials
- Protective eyewear (goggles or face shield) is required for procedures that may generate splashes or aerosols
- Mechanical pipetting devices must be used to eliminate mouth pipetting risks
- Centrifuge safety cups or sealed rotors are used for aerosol-generating centrifugation steps
- A sink and eyewash station must be nearby for immediate decontamination
- Autoclave or approved chemical disinfectants are required to decontaminate waste and reusable equipment
Laboratory Facilities (Secondary Barriers) of Biosafety level 1 (BSL 1)
- Lab surfaces and design allow for easy cleaning and decontamination
- Bench tops are water resistant and resistant to acids, alkalis, organic solvents and moderate heat
- Lab furniture is sturdy and arranged so spaces between benches, cabinets and equipment are accessible for cleaning
- Each lab has a dedicated handwashing sink for immediate decontamination
- Any windows that open to the outside are screened to prevent insect entry
Uses of Biosafety level 1 (BSL 1)
- Undergraduate and secondary school teaching labs use BSL-1 to introduce students to microbiology
- Basic research with non-pathogenic, well-characterized microorganisms that are low risk
- Quality control testing in food, beverage and cosmetic industries for indicator organisms
- Environmental monitoring and water quality testing for non-pathogenic microbial indicators
- Production of recombinant proteins, enzymes and other bioproducts with GRAS microorganisms
- Hands-on biosafety training and protocol validation for lab personnel
- Pilot scale and method development before scaling up to higher containment levels
- Small scale fermentation and standard cell culture applications in industrial microbiology
- Routine diagnostic or analytical testing on low risk samples
- Validation of molecular biology assays (e.g. PCR with non-infectious templates) and genetic constructs
Waste Management and Decontamination Process of Biosafety level 1
- Segregate waste into biohazardous and nonhazardous streams before treatment
- Decontaminate biohazardous solids and reusable materials by autoclaving at 121 °C for at least 15 minutes prior to disposal
- Treat liquid infectious waste by adding a validated chemical disinfectant (e.g., 10 % bleach) with proper contact time before disposal down the sanitary sewer
- Perform immediate surface decontamination after spills using an EPA-registered disinfectant and clean work surfaces daily
- Use leak-proof, labeled biohazard bags or containers for holding decontaminated waste until final disposal
- Employ secondary containment (e.g., a tray or cart) when transporting infectious materials outside the laboratory
- Ensure an accessible autoclave or other decontamination equipment is available nearby
- Use mechanical pipetting devices and sealed containers (e.g., safety cups) to minimize aerosol generation during waste handling
- Dispose of disposable sharps in puncture-resistant, labeled sharps containers that are autoclaved or incinerated when full
- Remove and decontaminate personal protective equipment before leaving the laboratory to prevent carrying contaminants outside
Examples of BSL-1 Organisms:
- nonpathogenic strains of Escherichia coli used in teaching and basic research
- Bacillus subtilis commonly employed for enzyme and protein production studies
- Saccharomyces cerevisiae yeast used in genetics, cell biology, and fermentation research
- Lactobacillus species applied in food microbiology and probiotic investigations
- Bacteriophage lambda as a model viral system for molecular biology experiments
2. Biosafety level 2 (BSL 2) – What is Biosafety level 2 (BSL 2)?
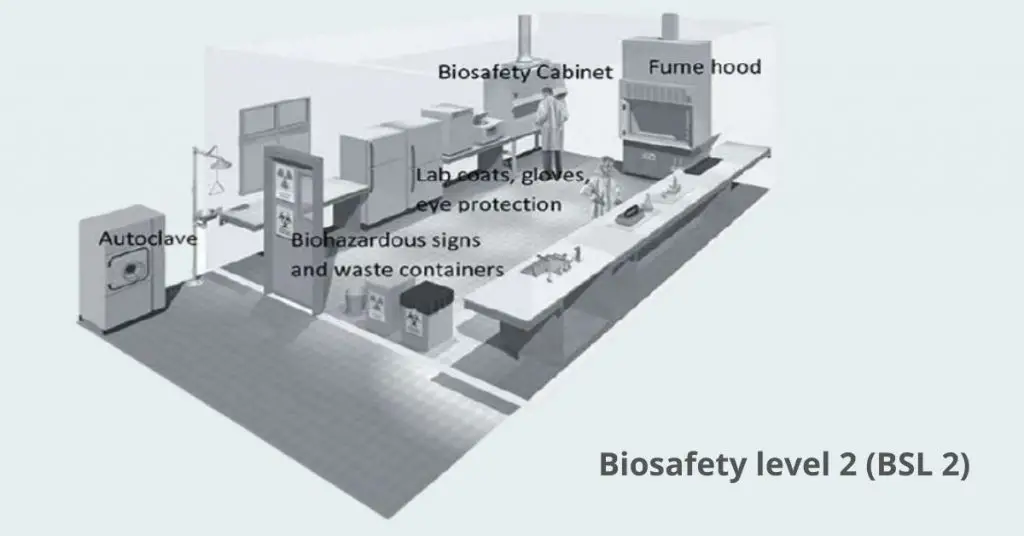
BSL-2 applies to work with agents of moderate risk to personnel and environment
All BSL-1 practices remain in force, plus personnel must have specific training in handling pathogenic agents and work under supervision
Laboratory access is restricted when infectious materials are in use, doors can be locked or secured
Procedures that may generate aerosols or splashes must be performed in a Class II biological safety cabinet or other physical containment device
Extreme care with sharps; mechanical pipetting devices are required and contaminated sharps are decontaminated before disposal
Personal protective equipment: lab coats, gloves, eye/face protection as needed
Laboratory must have an autoclave or approved chemical disinfectants for decontamination of waste and reusable materials
Handwashing sink and eyewash station must be available at all times
For agents such as seasonal flu virus, HIV, hepatitis B and C viruses, Salmonella spp., Toxoplasma gondii
Requirements for Biosafety level 2 (BSL 2)
- personnel must complete specific training in handling moderate-risk pathogens and follow a laboratory-specific biosafety manual
- laboratory access restricted and secured with self-closing, lockable doors during work with infectious materials
- all manipulations that may generate aerosols or splashes performed within a certified Class II biological safety cabinet
- personal protective equipment including lab coat, gloves and eye/face protection worn as needed, with clothing removed before exit
- mechanical pipetting devices used; mouth pipetting strictly prohibited
- decontamination of all waste and reusable materials by autoclaving or approved chemical disinfectants before disposal or reuse
- readily accessible handwashing sink and eyewash station located near exit of laboratory
- strict sharps management using puncture-resistant containers and decontamination of sharps before disposal
- clear labeling of biohazardous materials with identity and associated risk warnings
- availability of medical surveillance programs for personnel and procedures for exposure incident reporting and response
Standard microbiological practices for Biosafety level 2 (BSL 2)
- Follow all BSL-1 practices (no eating, drinking, cosmetic use; hand washing; surface decontamination)
- Complete lab-specific training on BSL-2 agents, risks, and safe work practices
- Restrict access: close and lock doors when infectious materials are in use; post biohazard signage at entry points
- Use certified Class II biosafety cabinet or other primary containment for procedures that may generate aerosols or splashes
- Wear personal protective equipment: lab coat, gloves, eye/face protection; remove before exiting the lab
- Prohibit mouth pipetting; use mechanical pipetting devices for all liquid transfers
- Minimize sharps use; handle needles and other sharps with extreme care and dispose in puncture-resistant containers
- Decontaminate all work surfaces daily and after spills with an appropriate disinfectant (e.g., 10% bleach)
- Autoclave or chemically disinfect all infectious waste and reusable materials before removal from the lab
- Maintain readily accessible handwashing sink and eyewash station near the exit
- Keep a laboratory-specific biosafety manual and emergency procedures readily available for all personnel
Safety Equipment (Primary Barriers) of Biosafety level 2 (BSL 2)
- Class II biosafety cabinet or other physical containment device for all aerosol-generating procedures.
- Solid-front gown, lab coat, scrubs, or coverall worn only inside the laboratory and removed before exit
- Gloves (single or double layer) selected by risk assessment, changed when contaminated, and removed before leaving the lab.
- Eye and face protection (goggles, mask, or face shield) for splash or aerosol hazards.
- Respiratory protection (eg, N95 respirator or PAPR) when required by agent risk and procedures.
- Sealed centrifuge rotors or safety cups for aerosol-generating centrifugation steps.
- Mechanical pipetting devices to eliminate mouth pipetting risks.
Laboratory Facilities (Secondary Barriers) of Biosafety level 2 (BSL 2)
- Self closing lockable doors restrict entry when infectious materials are handled
- Sealed windows and penetrations prevent air leaks and simplify decontamination
- Walls floors and ceilings constructed of seamless nonporous materials for easy cleaning
- Bench tops made of impervious surfaces resistant to chemicals heat and solvents
- Laboratory layout separates clean areas from work areas and service spaces
- Directional airflow maintains negative pressure relative to adjacent areas
- Ventilation system provides single pass air exhausted outside without recirculation
- HEPA filtered exhaust for biosafety cabinets and critical containment equipment
- Dedicated autoclave or decontamination facility available within or close to the lab
- Convenient handwashing sink and eyewash station installed near laboratory exit
- Adequate illumination and ergonomic furniture arrangement for safe work
- Visible biohazard signage at laboratory entrance identifying risks
Waste Management and Decontamination Process of Biosafety level 2 (BSL 2)
- Segregate biohazardous waste into solid, liquid, sharps, and non‐infectious categories before treatment
- Autoclave all solid infectious waste at 121 °C for a minimum of 15 minutes prior to disposal
- Chemically disinfect liquid waste in containers using validated biocidal solutions (e.g., 10 % bleach, phenolics) with appropriate contact time
- Use incineration where available as an alternative for high‐risk or bulk infectious waste
- Employ effluent decontamination systems (EDS) to sterilize liquid effluents before release into sewer lines
- Decontaminate work surfaces, biosafety cabinet interiors, and equipment after each procedure or spill using EPA-registered disinfectants
- Validate autoclave performance routinely with biological indicators and maintain logs of each sterilization cycle
- Dispose of sharps in puncture-resistant, leak-proof, clearly labeled containers and autoclave or incinerate when full
- Pre-clean reusable glassware and labware, then autoclave or apply high-level chemical disinfection before reuse
- Keep detailed records of waste treatment methods, dates, personnel involved, and disposal certificates to meet institutional and regulatory mandates
Uses of BSL-2 Laboratories
- Clinical diagnostic testing of human specimens to detect moderate‐risk pathogens such as Salmonella and HIV
- Basic and applied research into pathogenesis, host–pathogen interactions, and molecular biology of Risk Group 2 organisms
- Vaccine development and preclinical evaluation using viral antigens or attenuated strains under controlled BSL-2 conditions
- Serological assays (e.g., ELISA, Western blot) and immunological studies involving moderately hazardous agents
- Training laboratory personnel and students in biosafety procedures, aseptic techniques, and handling of pathogenic microorganisms
- Quality control testing and safety assessment in pharmaceutical, food, and beverage industries for microbial contaminants
- Environmental microbiology and water quality monitoring for indicator organisms and contamination surveys
- Handling and production of recombinant DNA constructs and viral vectors for gene therapy and research applications
- Small‐scale fermentation and bioprocess development using genetically modified microorganisms posing moderate risk
- Preclinical toxicological evaluations of microbial toxins and bioproducts under contained BSL-2 conditions
Agents Managed in BSL-2 Laboratories
- Bacillus anthracis*
- Bordetella pertussis
- Salmonella sp
- Escherichia coli (pathogenic strains)
- Staphylococcus aureus
- Streptococcus pyogenes
- Listeria sp.
- Blastomyces dermatitidis
- Cryptococcus neoformans
- Toxoplasma sp.
- Giardia sp.
- Coxiella burnetii*
- Adenoviruses
- Influenza virus
- Hepatitis B virus
- Retroviruses including HIV & SIV
- Shigella sp.
- Campylobacter fetus var. jejuni
3. Biosafety level 3 (BSL 3)
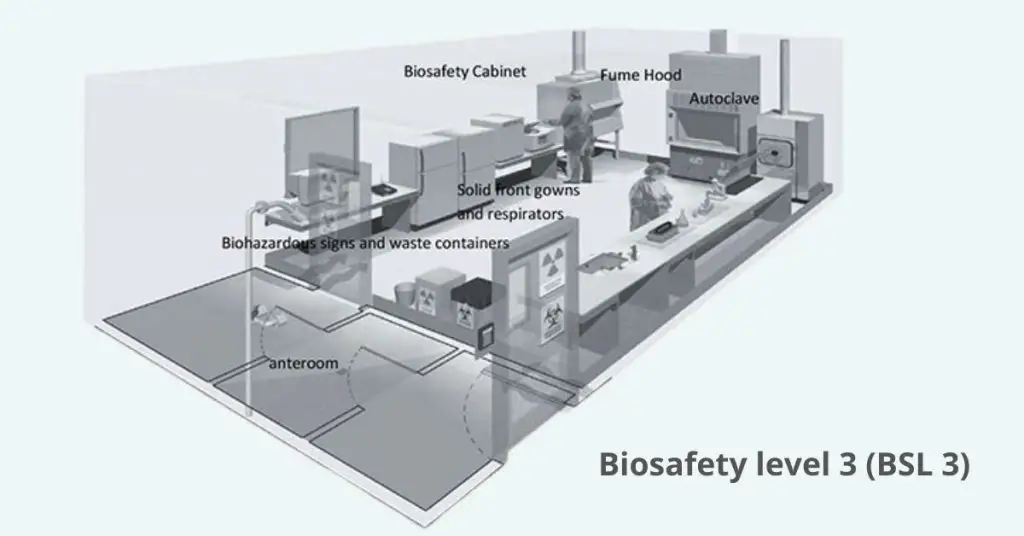
Biosafety Level 3 (BSL-3) means the containment protocols for work with indigenous or exotic agents that can cause serious or life threatening disease by inhalation.
- Early biosafety concerns in the 1950s after research on airborne pathogens like tuberculosis
- Formal standards introduced by the US Centers for Disease Control and Prevention (CDC) and National Institutes of Health (NIH) in the 1970s
- International guidelines refined by the World Health Organization (WHO) in the 1980s to standardize lab practices worldwide
- Special ventilation systems to direct airflow away from clean areas
- Mandatory use of personal protective equipment including respirators or powered air-purifying respirators (PAPRs)
- All manipulations of infectious materials done in certified biosafety cabinets to prevent aerosol release
- Study pathogens like Mycobacterium tuberculosis, SARS-CoV-2 and West Nile virus
- Support vaccine development, diagnostic testing and advanced infectious disease research under strict containment
Requirements for Biosafety level 3 (BSL 3)
- Follow all BSL-1 and BSL-2 lab guidelines
- Write and keep a lab specific biosafety manual with procedures, responsibilities and compliance requirements
- Enroll all personnel in medical surveillance and offer relevant vaccinations to prevent accidental or undiagnosed infections
- Restrict access at all times to prevent unauthorized entry
- Have a hands-free sink and eyewash station near the lab exit for routine decon
- Do all operations involving infectious material inside a certified Class II (or higher) biosafety cabinet
- Require PPE, including solid front gowns that tie in the back and respirators when needed
- Equip aerosol generating instruments with primary barrier devices that exhaust through HEPA filtration before discharge
- Decon work surfaces after each procedure and immediately after any spill with an effective disinfectant
- Have an in-lab decon method (e.g. autoclave or chemical decon) for all cultures, stocks and wastes before disposal
- House the lab behind two sets of self-closing and lockable doors to contain aerosols
- Have all surfaces with sealed seams in floors, walls and ceilings; no carpeting to allow for easy cleaning and decon
- Seal all windows and maintain directional airflow from “clean” to “dirty” zones with exhaust air HEPA filtered before release
Standard Microbiological Practices for Biosafety level 3 (BSL 3)
- Restrict laboratory access to authorized, trained personnel only
- Prohibit eating, drinking, smoking, applying cosmetics, and handling contact lenses in the laboratory
- Wash hands after handling infectious materials and before leaving the laboratory
- Routinely decontaminate work surfaces after each procedure and immediately after spills using an EPA-registered disinfectant
- Forbid mouth pipetting; use mechanical pipetting devices for all liquid handling
- Place all cultures, stocks, and potentially infectious materials in durable, leak-proof containers for transport within the facility
- Use a certified Class II (or higher) biosafety cabinet for all procedures with a risk of aerosol or splash generation
- Prove proficiency in BSL-3 practices and techniques before independent work; supervised training under an experienced BSL-3 investigator is required
- Wear solid-front, wrap-around gowns or coveralls, gloves, and eye/face protection; don respiratory protection when indicated by risk assessment
- Decontaminate all biological waste, including lab coats and reusable equipment, by autoclaving or validated chemical methods before removal from the laboratory
- Maintain a laboratory-specific biosafety manual outlining standard practices, emergency procedures, and roles/responsibilities
Safety Practices for Biosafety level 3 (BSL 3)
- Restrict entry to individuals who have received comprehensive BSL-3 training and demonstrated proficiency in required practices.
- Conduct a formal risk assessment for each agent and procedure, updating it whenever protocols or agents change.
- Enroll all personnel in ongoing medical surveillance and provide recommended immunizations for targeted pathogens
- Maintain a detailed, laboratory-specific biosafety manual that outlines approved practices, emergency procedures, and roles.
- Display biohazard signage at all access points and on equipment where infectious materials are used or stored.
- Prohibit all food, drink, smoking, cosmetic application, and contact lens handling inside the laboratory
- Require hand washing upon entry and exit, and immediately after handling infectious materials.
- Perform all manipulations of infectious agents that may generate aerosols or splashes within a certified Class II (or higher) biosafety cabinet.
- Use sealed containers and safety cups for centrifugation; open rotors only within a biosafety cabinet after decontamination .
- Wear appropriate personal protective equipment—solid-front gowns or coveralls, gloves, eye/face protection, and respiratory protection as indicated by the risk assessment.
- Decontaminate work surfaces before and after each procedure and immediately after any spill with an EPA-registered disinfectant.
- Autoclave or chemically disinfect all biological waste and reusable sharps before removal from the laboratory.
- Maintain directional airflow from “clean” to “contaminated” areas, with exhaust air HEPA-filtered before discharge.
- Keep the laboratory behind two sets of self-closing, lockable doors to contain aerosols.
- Establish and practice spill response and emergency decontamination protocols routinely.
- Report any laboratory-associated incidents or exposures promptly and review them to improve safety measures
Safety Equipment (Primary Barriers) of Biosafety level 3 (BSL 3)
- certified Class II biosafety cabinet with HEPA-filtered airflow to contain aerosols and protect personnel and environment
- sealed centrifuge rotors or safety cups for aerosol-generating equipment to prevent release of infectious particles.
- HEPA-filtered exhaust systems on containment devices tested at least annually
- solid-front wraparound gowns or coveralls as physical barrier against splashes and droplets.
- gloves worn in single or double layers depending on risk assessment to protect hands.
- eye and face protection such as safety goggles or face shields to guard against splashes.
- respiratory protection including N95 respirators or powered air-purifying respirators based on agent risk.
- leak-proof primary sample containers for safe handling and intra-facility transport of infectious materials
Laboratory Facilities (Secondary Barriers) of Biosafety level 3 (BSL 3)
- The laboratory is distinct from areas that allow for unrestricted movement throughout the building.
- The laboratory can be accessed via two pairs of self-closing, locking doors.
- The laboratory features a ventilation system designed to create directional airflow, effectively pulling air from clean zones into areas that may be contaminated.
- Exhaust air undergoes HEPA filtration and is not recirculated.
- A hands-free sink is conveniently located near the exit for your handwashing needs.
Waste Management and Decontamination of Biosafety level 3 (BSL 3)
- Segregate waste at point of generation into sharps, solid, and liquid categories in clearly labeled, leak-proof containers.
- Place all solid waste in autoclavable bags or containers and decontaminate within the BSL-3 suite using a validated autoclave cycle before removal.
- Treat liquid waste by chemical disinfection (e.g., 10% sodium hypochlorite fresh daily with ≥30 min contact time) or autoclaving based on pathogen risk and waste composition.
- Use puncture-resistant sharps containers for needles, glass pipettes, and scalpels; autoclave or incinerate sharps as per institutional or select agent guidelines.
- Transport waste within the facility in sealed, biohazard-labeled carts or carriers along approved routes to minimize exposure.
- Validate autoclave performance regularly with biological indicators and document sterilization logs for each waste load.
- Incinerate or use alternative high-temperature disposal (e.g., rendering or tissue digesters) for select agents or where autoclaving is not feasible.
- Decontaminate reusable equipment, work surfaces, and spill areas immediately after waste handling or spills using an EPA-registered disinfectant.
- Maintain on-site decontamination methods (autoclave, chemical, incineration) within the laboratory to avoid off-site transport of untreated infectious waste.
- Keep detailed records of waste generation, treatment, and disposal activities in the laboratory biosafety manual.
Uses of BSL-3 Laboratories
Here are the uses of Biosafety Level 3 (BSL-3) laboratories:
- Research on serious or lethal human pathogens-Study agents causing severe or fatal diseases (e.g., tuberculosis, anthrax, COVID-19) transmitted via inhalation.
- Diagnostic testing of high-risk infectious diseases– Identify and confirm infections from hazardous pathogens in clinical or environmental samples
- Work with zoonotic agents– Handle pathogens transmissible from animals to humans (e.g., avian influenza, Rift Valley fever, prion diseases).
- Vaccine and therapeutic development -Conduct preclinical research and testing of treatments for airborne or highly infectious diseases.
- Characterization of emerging or re-emerging pathogens -Investigate novel or poorly understood agents with high morbidity/mortality.
- Animal infection studies– Perform research involving animals exposed to BSL-3 pathogens to model human disease or transmission.
- Handling select agents -Manage pathogens classified as potential bioterror threats under national security regulations
Agents Managed in BSL-3 Laboratories
Here are the agents managed in Biosafety Level 3 (BSL-3) laboratories:
- Anthrax (Bacillus anthracis).
- West Nile virus.
- Venezuelan equine encephalitis virus.
- SARS coronavirus (Severe Acute Respiratory Syndrome coronavirus).
- MERS coronavirus (Middle East Respiratory Syndrome coronavirus).
- SARS-CoV-2 (COVID-19 virus)
- Influenza A H5N1 (avian flu)
- Hantaviruses (e.g., Hantaan virus)
- Cholera (Vibrio cholerae)
- Tuberculosis (Mycobacterium tuberculosis)
- Typhus (Rickettsia prowazekii and related species)
- Rift Valley fever virus
- Rocky Mountain spotted fever (Rickettsia rickettsii)
- Yellow fever virus
- Malaria parasites (Plasmodium falciparum and related species)
4. Biosafety level 4 (BSL 4)
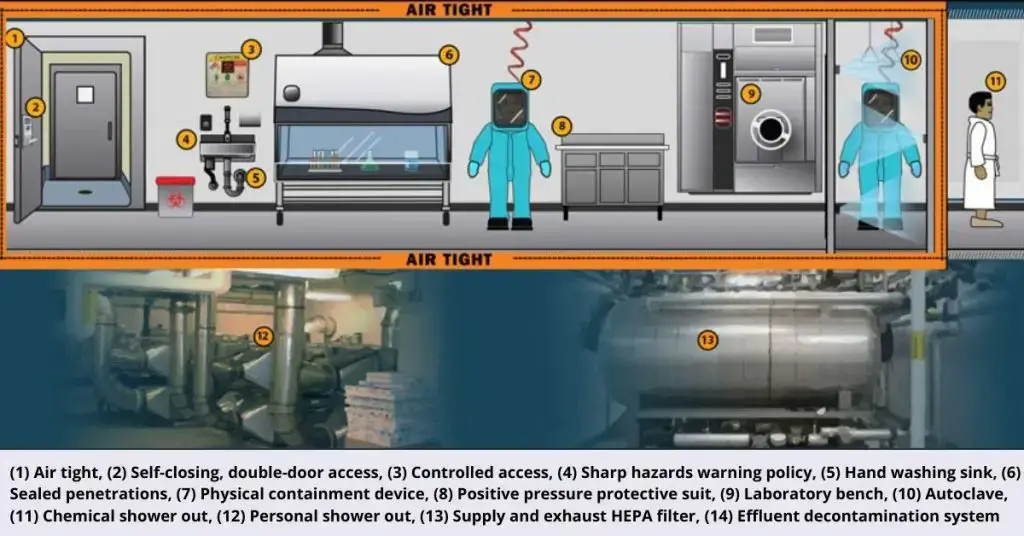
Biosafety level 4 (BSL-4) is the highest containment tier, reserved for labs working with pathogens that cause life-threatening disease and lack known vaccines or effective treatments
Definition: BSL-4 laboratories are specialized facilities designed to safely study and contain dangerous, easily aerosol-transmitted agents for which no prophylaxis or therapy exists.
Purpose: protect laboratory personnel, the environment, and the public when handling high-consequence pathogens.
Common agents: Ebola virus, Marburg virus, Lassa fever virus, Crimean-Congo hemorrhagic fever virus, Nipah virus.
- Mid-1970s: formal designation of four biosafety levels introduced.
- 1980s: emergence of full-body positive-pressure “space” suits as standard practice.
- 1990s: construction of first purpose-built BSL-4 suites at the U.S. CDC and international centers.
- 2000s–2010s: widespread adoption of advanced HVAC systems, multi-layered security, and automated decontamination features
Requirements for Biosafety level 4 (BSL 4)
- Physical Isolation: Must be in a separate building or a strictly controlled, isolated zone.
- Controlled Access: Entry restricted via secured, double-door airlocks.
- Negative Pressure: Maintain constant negative air pressure relative to surroundings.
- Directional Airflow: Airflow must always move from clean areas towards potentially contaminated areas.
- Exhaust Air Filtration: All exhaust air must pass through two HEPA filters in series or be incinerated.
- Sealed Facility: All penetrations (walls, floors, ceilings, utilities) must be sealed to be airtight and impermeable.
- Decontamination Procedures: Require chemical showers for personnel exit and autoclaves/effluent decontamination systems for all waste and materials exiting the lab.
- Personnel Protective Equipment (PPE): Personnel must wear full-body, positive pressure air-supplied suits with life support systems OR conduct all work within Class III biological safety cabinets (glove boxes).
- Personnel Training: Mandatory specialized, rigorous training for all personnel handling agents or entering the lab.
- Medical Surveillance: Mandatory medical surveillance program for all personnel (e.g., immunizations where available, baseline serum collection).
- Emergency Protocols: Strict, validated emergency procedures for containment failures, accidents, or loss of power.
- System Validation: Annual verification and certification of containment systems (HVAC, HEPA, decontamination).
- Regulatory Compliance: Adherence to national and international BSL-4 standards and regulations (e.g., CDC BMBL, WHO LBM).
Standard microbiological practices for Biosafety level 4 (BSL 4)
- Restrict lab access to authorized, trained personnel only.
- Implement a two-person rule for all work with infectious materials; no solo work permitted.
- Decontaminate all materials (including waste, equipment, and PPE) before removal from the lab via validated methods (e.g., autoclaving, chemical decontamination).
- Perform all procedures within Class III biological safety cabinets (BSCs) or while wearing a full-body, air-supplied positive pressure suit.
- Inspect PPE integrity (e.g., suit, gloves, respirators) before entry and after any potential compromise.
- Decontaminate gloves with appropriate disinfectant immediately after handling infectious materials or before touching clean surfaces.
- Prohibit sharps unless absolutely essential; use engineered sharps injury protection devices when unavoidable.
- Minimize aerosol generation through strict procedural controls (e.g., no open centrifugation, vortexing only in BSCs).
- Decontaminate work surfaces with effective disinfectant:
- After spills of infectious material.
- After work completion.
- At the end of each shift.
- Report all accidents, exposures, or breaches in containment immediately to lab management.
- Maintain accurate, real-time records of pathogen inventories, procedures, and personnel entry/exit.
- Undergo medical surveillance before working with agents and after any exposure incident.
- Exit the lab only after completing full decontamination protocols (e.g., chemical shower for suit labs, sequential decon for glove boxes).
Safety Equipment (Primary Barriers) of Biosafety level 4 (BSL 4)
Here are the primary barriers (safety equipment) for Biosafety Level 4 (BSL-4):
- Class III Biological Safety Cabinets (BSCs): Fully enclosed, ventilated containment systems; all manipulations performed via sealed gloves attached to ports.
- Positive Pressure Supplied-Air Suits: Full-body, air-tight suits with integrated life support systems and HEPA-filtered air supply.
- HEPA Filters: Installed on exhaust systems of Class III BSCs and suit air supply/return lines.
- Sealed Glove Systems: Integral components of Class III BSCs or positive pressure suits; must withstand decontamination agents.
- Double-HEPA Filtration: Exhaust air from Class III BSCs or suit labs passes through two HEPA filters in series.
- Decontamination Autoclaves: Double-door pass-through autoclaves for sterilizing materials before removal.
- Effluent Decontamination Systems: Treatment of all liquid waste via chemical disinfection or heat sterilization.
- Emergency Air Backup: Redundant breathing air supply for suits during system failure.
- Airlock Pass-Through Chambers: For material transfer, equipped with UV/chemical decontamination.
Laboratory Facilities (Secondary Barriers) of Biosafety level 4 (BSL 4)
Here are the laboratory facilities (secondary barriers) for Biosafety Level 4 (BSL-4):
- Separate building or isolated zone: Dedicated, structurally independent containment area.
- Double-door airlocks: Secured entry/exit points with interlocking doors.
- Negative pressure gradients: Maintained inward airflow from lower to higher risk zones.
- Sealed penetrations: Airtight seals on all utility lines, walls, floors, and ceilings.
- HEPA-filtered exhaust systems: Duplicated HEPA filtration or incineration of all lab exhaust air.
- Chemical shower for personnel decontamination: Mandatory for suit labs prior to final exit.
- Liquid effluent decontamination system: Automated treatment of all lab wastewater.
- Pass-through dunk tanks or fumigation chambers: For material transfer into the lab.
- Double-door autoclave: Validated sterilization of materials prior to removal.
- Backup ventilation systems: Redundant fans and power supplies to maintain containment.
- Continuous pressure monitoring: Alarms for loss of negative pressure or airflow.
- Impervious, cleanable surfaces: Non-porous materials on all interior finishes.
- Visual monitoring systems: Observation capability for all high-containment areas.
Waste Management and Decontamination of Biosafety level 4 (BSL 4)
Here are the waste management and decontamination requirements for Biosafety Level 4 (BSL-4):
- Sterilize all solid waste via double-door autoclaving before removal from containment.
- Decontaminate liquid waste using validated chemical disinfection or heat treatment before release.
- Treat effluent through a facility-wide, automated decontamination system.
- Decontaminate reusable equipment via autoclaving or chemical immersion prior to removal.
- Incinerate animal carcasses and tissues after autoclaving.
- Decontaminate PPE via chemical shower (suit labs) or autoclaving before disposal/removal.
- Decontaminate work surfaces daily and after spills with approved disinfectants.
- Fumigate the laboratory periodically using vaporized hydrogen peroxide or formaldehyde.
- Decontaminate materials via pass-through dunk tanks, fumigation chambers, or double-autoclaving.
- Require personnel showers upon exit in suit laboratories.
- Validate all decontamination processes using biological indicators.
- Document all waste disposal with tracking logs.
Uses of BSL-4 Laboratories
Here are the uses of Biosafety Level 4 (BSL-4) laboratories:
- Research on lethal pathogens– Study highly dangerous viruses (e.g., Ebola, Marburg) causing severe or fatal diseases with no available treatments or vaccines.
- Diagnostic testing -Confirm infections caused by exotic or high-consequence pathogens in human samples.
- Vaccine and therapeutic development – Conduct preclinical research and testing of medical countermeasures for untreatable diseases.
- Characterization of novel pathogens -Investigate newly discovered or poorly understood agents with suspected high mortality or transmissibility risks.
- Animal infection studies -Perform research using animals infected with select agents to model human disease or transmission pathways.
- Pandemic response preparedness– Develop capabilities for rapid identification and containment of emerging global health threats.
- Biodefense research -Study pathogens classified as potential bioterror threats under national security programs
Agents Managed in BSL-4 Laboratories
Here are the agents managed in Biosafety Level 4 (BSL-4) laboratories:
- Ebola virus
- Marburg virus
- Lassa virus
- Crimean-Congo hemorrhagic fever virus
- Variola virus (smallpox)
- Nipah virus
- Hendra virus
- Junín virus (Argentine hemorrhagic fever)
- Sabia virus (Brazilian hemorrhagic fever)
- Guanarito virus (Venezuelan hemorrhagic fever)
- Machupo virus (Bolivian hemorrhagic fever)
- Kyasanur Forest disease virus
- Herpesvirus B (Cercopithecine herpesvirus 1)
- Severe fever with thrombocytopenia syndrome virus (SFTSV)
- Newly discovered henipaviruses or ebolaviruses
- Andes orthohantavirus
- MERS-CoV
- Agents with unknown transmission risk and severe/fatal outcomes
Secondary Barriers of Different Biosafety levels
| Biosafety Level | Secondary Barriers and Facility Requirements |
|---|---|
| BSL-1 | – Laboratory bench and sink required. – Open bench top sink required. – Laboratory doors; sink for handwashing; laboratory bench; windows fitted with screens; lighting adequate for all activities. – Laboratory doors; sink for handwashing; laboratory bench; windows fitted with screens; lighting adequate for all activities. |
| BSL-2 | – Autoclave available. – Autoclave available. – Self-closing doors; sink located near exit; windows sealed or fitted with screens; autoclave available. |
| BSL-3 | – Physical separation from access corridors. – Self-closing, double-door access. – Exhausted air not recirculated. – Negative airflow into laboratory. – Entry through airlock or anteroom. – Hand washing sink near laboratory exit. – Physical separation from access corridors. – Self-closing, double-door access. – Exhausted air not recirculated. – Negative airflow into laboratory. |
| BSL-4 | – Separate building or isolated zone. – Dedicated supply and exhaust, vacuum, and decontamination systems. – Other requirements outlined in the text. – Entry sequence; entry through airlock with airtight doors; walls, floors, ceilings form sealed internal shell; dedicated, non-recirculating ventilation system required; double-door, pass-through autoclave required. – Separate building or isolated zone. – Dedicated supply and exhaust, vacuum, and decontamination systems. – Other requirements outlined in the text. |
Primary Barriers of Different Biosafety levels
| Biosafety Level | Primary Barriers and Safety Equipment |
|---|---|
| BSL-1 | Standard microbiological practices. – Personal protective equipment (PPE): laboratory coats and gloves; eye and face protection as needed. – No primary barriers required. |
| BSL-2 | BSL-1 practices plus: – Limited access, biohazard warning signs, “sharps” precautions, biosafety manual defining any needed waste decontamination or medical surveillance policies. – Primary barriers: Class I or II Biological Safety Cabinets (BSCs) or other physical containment devices used for all manipulations of agents that cause splashes or aerosols of infectious materials. – PPE: laboratory coats, gloves, face and eye protection as needed. |
| BSL-3 | BSL-2 practices plus: – Controlled access, decontamination of all waste, decontamination of laboratory clothing before laundering, baseline serum. – Primary barriers: Class I or II BSCs or other physical containment devices used for all open manipulations of agents. – PPE: protective laboratory clothing, gloves, face, eye, and respiratory protection as needed. |
| BSL-4 | BSL-3 practices plus: – Clothing change before entering, shower on exit, all material decontaminated on exit from facility. – Primary barriers: all procedures conducted in Class III BSCs, or Class I or II BSCs in combination with full-body, air-supplied, positive pressure personnel suit. |
Uses of Biosafety levels
| Biosafety Level | Agents Handled | Typical Uses | Key Safety Features |
|---|---|---|---|
| BSL-1 | Well-characterized agents not known to cause disease in healthy adults (e.g., E. coli K-12, Bacillus subtilis) | Basic research, teaching, and non-pathogenic microbial studies | Standard microbiological practices; no special containment required; laboratory bench and sink required |
| BSL-2 | Agents associated with human disease; moderate hazard (e.g., HIV, Hepatitis B, Salmonella) | Clinical diagnostics, biomedical research, and teaching with moderate-risk agents | BSL-1 practices plus limited access, biohazard warning signs, sharps precautions; Class I or II Biological Safety Cabinets (BSCs) for procedures that may generate aerosols or splashes; autoclave available |
| BSL-3 | Indigenous or exotic agents with potential for aerosol transmission; serious or lethal consequences (e.g., Mycobacterium tuberculosis, SARS-CoV-2) | Research on airborne pathogens, vaccine development, and diagnostic testing | BSL-2 practices plus controlled access, decontamination of all waste, decontamination of laboratory clothing before laundering; Class I or II BSCs for all open manipulations; negative airflow into laboratory; self-closing, double-door access |
| BSL-4 | Dangerous and exotic agents that pose high individual risk of aerosol-transmitted laboratory infections and life-threatening disease for which there are no vaccines or treatments (e.g., Ebola virus, Marburg virus) | High-security research on highly pathogenic agents, biodefense research, and work with agents lacking effective treatments or vaccines | BSL-3 practices plus change of clothing before entry, daily inspections of essential containment and life support systems, all wastes decontaminated prior to removal from laboratory, shower on exit; full-body, air-supplied, positive pressure suit; entry through airlock with airtight doors; walls, floors, ceilings form sealed internal shell; dedicated, non-recirculating ventilation system required; double-door, pass-through autoclave required |

- National Research Council (US) Committee on Hazardous Biological Substances in the Laboratory. Biosafety In The Laboratory: Prudent Practices for the Handling and Disposal of Infectious Materials. Washington (DC): National Academies Press (US); 1989. Appendix A, Biosafety in Microbiological and Biomedical Laboratories. Available from:
- https://www.ncbi.nlm.nih.gov/books/NBK218631/
- https://www.cdc.gov/training/QuickLearns/biosafetyCE/biosafetyCE.html
- https://www.bu.edu/research/ethics-compliance/safety/biological-safety/ibc/resources/biosafety-manual/chapter-04-biosafety-principles/
- https://orcbs.msu.edu/lab-clinic/bio/biosafety-level-summary-lab.html
- https://www.wired.com/2007/07/cdc-lab-breakdo/
- https://www.cdc.gov/MMWR/preview/mmwrhtml/su6101a1.htm
- https://www.labmanager.com/biological-safety-level-1-2-3-4-19123
- https://orcbs.msu.edu/lab-clinic/bio/biosafety-level-summary-lab.html
- https://www1.udel.edu/ehs/research/biological/biosafety-levels.html
- https://www.bu.edu/research/ethics-compliance/safety/biological-safety/ibc/resources/biosafety-manual/chapter-04-biosafety-principles/
- https://ehs.princeton.edu/laboratory-research/biological-safety/biosafety-manual/biological-safety-levels
- https://essr.umd.edu/about/research-safety/biological-safety/infectious-agents/summary-biosafety-levels-recommended
- https://ehs.stanford.edu/reference/summary-recommended-biosafety-levels-infectious-agents