It is a biochemical test used in microbiology for the identification of Streptococcus pneumoniae from other alpha hemolytic streptococci. It is mainly based on the ability of pneumococcal cells to undergo lysis in presence of bile salts. This test is commonly performed in diagnostic laboratories for confirmation of pneumococcal isolates.
It is the process in which bile salts such as sodium deoxycholate act on the bacterial cell wall. Streptococcus pneumoniae contains an intracellular autolytic enzyme (amidase) which is responsible for self digestion of the cell wall. When bile salts are added, the surface tension of the cell membrane is reduced and this activates the autolytic enzyme. As a result, the peptidoglycan layer is broken down and the bacterial cells are lysed.
In this test, sodium deoxycholate solution (2% or 10%) is added either to a suspension of the organism or directly over a colony grown on agar medium. In case of positive reaction, the turbidity of the suspension disappears or the colony dissolves completely within 15 to 30 minutes. Other alpha hemolytic streptococci do not possess this active autolytic enzyme and therefore they remain unchanged and are referred to as bile insoluble.
This test should be performed using young cultures (18–24 hours old) because older cultures may lose enzymatic activity and may give false negative results. Thus bile solubility test is considered as a reliable confirmatory test for identification of pneumococci in clinical microbiology.
Objectives of Bile Solubility Test
- To differentiate Streptococcus pneumoniae from other alpha hemolytic streptococci.
- To study the lytic action of bile salts such as sodium deoxycholate on bacterial cells.
- To distinguish Streptococcus pneumoniae from closely related organisms like Streptococcus pseudopneumoniae.
- To confirm pneumococcal isolates showing typical morphology such as lancet shaped diplococci or draughtsman colonies.
- To classify organisms as bile soluble or bile insoluble based on the test reaction.
Principle of Bile Solubility Test
It is based on the susceptibility of Streptococcus pneumoniae to bile salts such as sodium deoxycholate. This organism possesses a specific intracellular autolytic enzyme which is an amidase (N-acetylmuramoyl-L-alanine amidase). This enzyme normally takes part in cell wall metabolism and separation of cells during growth.
When bile salts are added, they act by reducing the surface tension at the cell membrane interface. This physical change activates the autolytic enzyme or allows it to act effectively on the cell wall. As a result, the enzyme hydrolyses the peptidoglycan layer of the bacterial cell wall and causes lysis of the pneumococcal cells. This leads to clearing of turbidity in a bacterial suspension or dissolution of colonies on solid media.
Other alpha hemolytic streptococci do not possess this active autolytic system. Therefore, they are not affected by bile salts and remain intact. This difference forms the basic principle of bile solubility test and is used for identification of Streptococcus pneumoniae.
Media, Reagents, and Supplies Used for Bile Solubility Test
Reagents
- Sodium deoxycholate solution (2% or 10%).
- Sterile normal saline (0.85% NaCl) for preparation of bacterial suspension.
- Sterile distilled water used as control or for reagent preparation.
- Phenol red indicator for monitoring pH of the solution.
- Sodium hydroxide (0.1 N NaOH) for adjustment of pH to neutral.
Media
- 5% sheep blood agar used for growth of test organism.
- Chocolate agar used as an alternative culture medium.
- Todd Hewitt broth for preparation of broth culture.
- Brain heart infusion broth used as an alternative liquid medium.
Supplies and Equipment
- Test tubes for tube method of the test.
- Glass slides for direct testing methods.
- Inoculating loop or wire for picking bacterial colonies.
- Pipettes or droppers for adding reagents.
- Incubator maintained at 35°C to 37°C.
- Heat block which may be used instead of incubator.
- Marker or wax pencil for marking colonies on agar plate.
Procedure of Bile Solubility Test
The bile solubility test is performed by the following methods.
1. Test Tube Method
- Prepare a heavy suspension of the test organism from a fresh culture (18–24 hours old) in sterile saline or suitable broth.
- Shake the suspension properly to make it uniform.
- Adjust the pH of the suspension to neutral (7.0) to avoid false negative results.
- Divide the suspension equally into two clean test tubes and label them as TEST and CONTROL.
- Add sodium deoxycholate solution (2% or 10%) to the TEST tube.
- Add an equal volume of sterile saline or distilled water to the CONTROL tube.
- Mix both tubes gently and incubate at 35–37°C.
- Observe after 10–15 minutes for clearing of turbidity.
- If no change is seen, continue incubation and observe up to 3 hours.
2. Direct Plate (Spot) Method
- Select a young isolated colony from blood agar plate (18–24 hours old).
- Place one drop of sodium deoxycholate solution (usually 10%) directly on the colony.
- Tilt the plate gently so that the reagent spreads over the colony without removing it.
- Incubate the plate in upright position at 35–37°C for 15–30 minutes.
- Observe for flattening or complete dissolution of the colony.
3. Direct Slide Blood Culture Method
- Place one drop of blood culture broth on a clean glass slide.
- Add one drop of bile salt reagent and mix properly.
- On a separate area of the slide mix one drop of broth with one drop of water as control.
- Allow the slide to air dry completely.
- Perform Gram staining of the slide.
- Observe under microscope for presence or absence of cocci.
Result interpretation of Bile Solubility Test
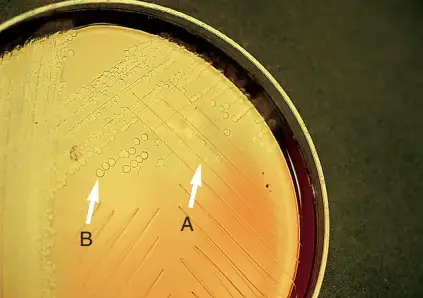
Positive Result (Bile Soluble)
- In tube method, the bacterial suspension becomes clear in the test tube containing bile salts while the control tube remains turbid.
- In direct plate method, the colony flattens, disintegrates, or completely disappears within 15–30 minutes.
- In slide blood culture method, no cocci are seen in the bile treated smear whereas intact bacteria are seen in control smear.
- The organism is identified as Streptococcus pneumoniae.
Negative Result (Bile Insoluble)
- In tube method, the suspension remains turbid and shows no difference from control tube.
- In direct plate method, the colony remains intact with no visible change.
- In slide blood culture method, cocci are present in both test and control smears.
- The organism is other alpha hemolytic streptococci such as viridans group or Streptococcus pseudopneumoniae.
Inconclusive Result
- Partial clearing or incomplete lysis is observed.
- Such result is not considered positive and further confirmatory tests are required.
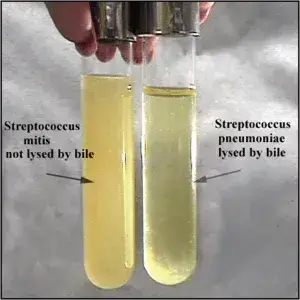
List of Organisms Showing Positive and Negative Result in Bile Solubility Test
Organisms Giving Positive Result (Bile Soluble)
- Streptococcus pneumoniae.
- Haemophilus influenzae (alpha hemolytic strains).
- Haemophilus aegypticus (alpha hemolytic strains).
Organisms Giving Negative Result (Bile Insoluble)
- Streptococcus mitis.
- Streptococcus pseudopneumoniae.
- Enterococcus faecalis.
- Streptococcus oralis.
- Streptococcus sanguinis.
- Streptococcus mutans.
- Streptococcus anginosus.
- Streptococcus salivarius.
- Staphylococcus aureus.
- Viridans group streptococci (other than Streptococcus pneumoniae).
Quality Control Organisms of Bile Solubility Test
Positive Control (Bile Soluble)
- Streptococcus pneumoniae ATCC 49619.
- Streptococcus pneumoniae ATCC 6305.
- Streptococcus pneumoniae NCTC 12977.
- Streptococcus pneumoniae ATCC 12344.
- Streptococcus pneumoniae ATCC 33400T.
Negative Control (Bile Insoluble)
- Streptococcus mitis ATCC 49456.
- Streptococcus mitis NCTC 10712.
- Streptococcus mitis ATCC 15909.
- Enterococcus faecalis ATCC 29212.
- Streptococcus sanguinis ATCC 10556.
- Streptococcus mutans.
Precautions of Bile Solubility Test
- Use young and actively growing cultures (18–24 hours old) for performing the test.
- Ensure the pH of saline or broth suspension is neutral (around 7.0) especially in tube method.
- Do not use acidic suspension as it may cause precipitation of bile salts and give false negative result.
- While performing direct plate method, avoid dislodging or washing away the colony with reagent.
- Keep the agar plate in upright position and do not invert during incubation.
- Use bile reagent only if it is clear and light amber in colour.
- If bile reagent has thickened due to cold storage, warm it to 37°C before use.
- Do not touch the agar surface with dropper tip while adding the reagent.
- Confirm that the organism is alpha hemolytic, Gram positive and catalase negative before performing the test.
- Partial clearing of turbidity should not be considered as positive result.
- Perform procedures carefully to avoid aerosol formation and follow laboratory safety measures.
Uses of Bile Solubility Test
- To differentiate Streptococcus pneumoniae from other alpha hemolytic streptococci.
- To distinguish Streptococcus pneumoniae from Streptococcus pseudopneumoniae.
- To confirm pneumococcal isolates showing doubtful or atypical reactions in other tests.
- To determine the autolytic activity of organisms in presence of bile salts such as sodium deoxycholate.
- To identify Streptococcus pneumoniae directly from positive blood culture samples by rapid methods.
- To help in differentiation of alpha hemolytic Haemophilus species which are bile soluble from bile insoluble species.
Advantages of Bile Solubility Test
- It is highly specific for identification of Streptococcus pneumoniae.
- It helps in clear differentiation of Streptococcus pneumoniae from other alpha hemolytic streptococci.
- It distinguishes Streptococcus pneumoniae from Streptococcus pseudopneumoniae.
- It is more reliable and specific than optochin susceptibility test.
- It is simple to perform and does not require special equipment.
- It is a cost effective method used in routine laboratories.
- It gives rapid results especially by direct plate or slide method.
- It is considered as a definitive phenotypic test for identification of pneumococci.
Limitations of Bile Solubility Test
- The test depends on the activity of autolytic enzyme and old cultures (more than 18–24 hours) may give false negative result.
- Some strains of Streptococcus pneumoniae do not undergo complete lysis and show only partial clearing which is not taken as positive result.
- Atypical pneumococcal strains with altered amidase enzyme may remain bile insoluble and can be misidentified.
- The test requires neutral pH and acidic conditions may cause precipitation of bile salts leading to false negative reaction.
- High concentration of bile salts may inhibit autolysis and affect accuracy of the test.
- Interpretation of clearing of turbidity is subjective and may vary between observers.
- In plate method, accidental removal of colony may be mistaken as lysis and give false positive result.
- Rare bile soluble strains of other streptococci and bile soluble Haemophilus species may cause confusion if morphology is not confirmed.
- Improper storage of bile reagent may lead to precipitation and reduced reliability of the test.
FAQ
Q1. What is the bile solubility test?
A. It is a biochemical test used in microbiology for identification of Streptococcus pneumoniae. It helps in differentiating pneumococci from other alpha hemolytic streptococci based on their ability to undergo lysis in presence of bile salts.
Q2. What is the principle of the bile solubility test?
A. It is based on the presence of an intracellular autolytic enzyme (amidase) in Streptococcus pneumoniae. When bile salts are added, they reduce surface tension and activate this enzyme which digests the peptidoglycan layer of cell wall resulting in lysis of the organism.
Q3. How is the bile solubility test performed?
A. The test is performed by tube method or direct plate method. In tube method, bile salt reagent is added to a bacterial suspension and clearing of turbidity is observed. In plate method, bile reagent is added directly over a colony and dissolution of colony is noted.
Q4. What is the purpose of the bile solubility test?
A. The main purpose is to differentiate Streptococcus pneumoniae from other alpha hemolytic streptococci and to confirm the identity of pneumococcal isolates.
Q5. How are the results of the bile solubility test interpreted?
A. Clearing of turbidity in tube method or dissolution of colony in plate method indicates positive result. No change in turbidity or intact colony indicates negative result.
Q6. What reagents are used in the bile solubility test?
A. Sodium deoxycholate (2% or 10%) is the main reagent used. Sterile saline or distilled water is used as control and for preparing suspension.
Q7. What are the limitations of the bile solubility test?
A. Old cultures may give false negative results. Some pneumococcal strains may show partial lysis. The test is pH sensitive and interpretation of results can be subjective.
Q8. Which organisms are differentiated by the bile solubility test?
A. It differentiates Streptococcus pneumoniae (bile soluble) from viridans group streptococci and other alpha hemolytic streptococci which are bile insoluble.
Q9. What is the mechanism of action of bile salts in the bile solubility test?
A. Bile salts act by lowering surface tension of cell membrane and activating autolytic amidase enzyme. This enzyme breaks down peptidoglycan of the cell wall causing lysis.
Q10. What indicates a positive result in the bile solubility test?
A. Clearing of bacterial suspension in tube method or flattening and disappearance of colony in plate method indicates positive result.
Q11. What indicates a negative result in the bile solubility test?
A. Persistence of turbidity in tube method or intact colony in plate method indicates negative result.
Q12. What is the difference between the plate method and tube method for the bile solubility test?
A. In plate method, bile reagent is applied directly on colony grown on agar. In tube method, bile reagent is added to a bacterial suspension and turbidity is observed.
Q13. Why is the bile solubility test not reliable with old cultures?
A. Old cultures may lose active autolytic enzyme required for lysis and this can result in false negative reaction.
Q14. What is sodium deoxycholate used for in the bile solubility test?
A. Sodium deoxycholate is used as bile salt reagent which activates autolytic enzyme and causes lysis of pneumococcal cells.
Q15. What are the quality control strains for the bile solubility test?
A. Streptococcus pneumoniae ATCC 49619 is used as positive control and Streptococcus mitis ATCC 49456 or Enterococcus faecalis ATCC 29212 is used as negative control.
- Arana, M. E., Morales, M., Alonso, M., & Ardanuy, C. (2019). Variants of the bile: solubility test to differentiate Streptococcus pneumoniae from other viridans group streptococci. Future Microbiology, 14(7). https://doi.org/10.2217/fmb-2019-0073
- Arbique, J. C., Poyart, C., Trieu-Cuot, P., Quesne, G., Carvalho, M. G. S., Steigerwalt, A. G., Morey, R. E., Jackson, D., Davidson, R. J., & Facklam, R. R. (2004). Accuracy of phenotypic and genotypic testing for identification of Streptococcus pneumoniae and description of Streptococcus pseudopneumoniae sp. nov. Journal of Clinical Microbiology, 42(10), 4686–4696. https://doi.org/10.1128/JCM.42.10.4686-4696.2004
- Aryal, S. (2022, August 10). Bile solubility test- Principle, reagents, procedure and result interpretation. Microbiology Info. https://microbiologyinfo.com/bile-solubility-test-principle-reagents-procedure-and-result-interpretation/
- Basheer, M., Sasic, K., Carlsson, A., Vestberg, N., Henriques-Normark, B., Blomqvist, K., & Garriss, G. (2024). A novel LAMP-based assay for the identification of Streptococcus pneumoniae and Streptococcus pseudopneumoniae in clinical isolates. Journal of Clinical Microbiology, 62(12), e00912-24. https://doi.org/10.1128/jcm.00912-24
- Dalynn Biologicals. (2014). Bile solubility reagent [Catalogue No. RB60]. https://www.dalynn.com/dyn/ck_assets/files/tech/RB60.pdf
- Eichmann, K., & Krause, R. M. (2013). Fred Neufeld and pneumococcal serotypes: foundations for the discovery of the transforming principle. Cellular and Molecular Life Sciences, 70(13), 2225–2236. https://doi.org/10.1007/s00018-013-1351-z
- Ercibengoa, M. (2021). Assessment of the optochin susceptibility test to differentiate Streptococcus pneumoniae from other viridans group streptococci. Clinical Laboratory, 67, 857-861. https://doi.org/10.7754/Clin.Lab.2020.200438
- Institute of Microbiology and Immunology. (n.d.). Special microbiology practical week 3: Streptococci. Jessenius Faculty of Medicine in Martin, Comenius University.
- Keith, E. R., Podmore, R. G., Anderson, T. P., & Murdoch, D. R. (2006). Characteristics of Streptococcus pseudopneumoniae isolated from purulent sputum samples. Journal of Clinical Microbiology, 44(3), 923–927. https://doi.org/10.1128/JCM.44.3.923-927.2006
- Ktari, S., Ben Ayed, N. E. H., Maalej, S., Mnif, B., Rhimi, F., & Hammami, A. (2021). Clinical optochin resistant Streptococcus pneumoniae and Streptococcus pseudopneumoniae strains in Tunisia. The Journal of Infection in Developing Countries, 15(5), 672-677. https://doi.org/10.3855/jidc.13106
- Mellroth, P., Daniels, R., Eberhardt, A., Rönnlund, D., Blom, H., Widengren, J., Normark, S., & Henriques-Normark, B. (2012). LytA, major autolysin of Streptococcus pneumoniae, requires access to nascent peptidoglycan. Journal of Biological Chemistry, 287(14), 11018–11029. https://doi.org/10.1074/jbc.M111.318584
- Mohammadi, J. S., & Dhanashree, B. (2012). Streptococcus pseudopneumoniae: an emerging respiratory tract pathogen. The Indian Journal of Medical Research, 136(5), 877–880.
- Pokhrel, P. (2015, July 3). Bile solubility test- Principle, procedure, result interpretation, examples and limitation. Microbiology Notes. https://microbiologynotes.com/bile-solubility-test-principle-procedure-result-interpretation-examples-and-limitation/
- Public Health England. (2014). Bile solubility test (UK Standards for Microbiology Investigations TP 5 Issue 3).
- Sadowy, E., & Hryniewicz, W. (2020). Identification of Streptococcus pneumoniae and other Mitis streptococci: importance of molecular methods. European Journal of Clinical Microbiology & Infectious Diseases, 39(12), 2247–2256. https://doi.org/10.1007/s10096-020-03991-9
- Sapkota, A. (2022, January 27). Bile solubility test- Principle, procedure, types, results, uses. Microbe Notes. https://microbenotes.com/bile-solubility-test-principle-procedure-and-result-interpretation/
- Sayed, Z. I., Abdel-Ghany, M. F., Ahmed, S. H., Adawy, A. M., & Abd El–Hamid, R. F. (2022). Streptococcus pseudopneumoniae as an emerging respiratory tract pathogen at Assiut University hospitals. Iranian Journal of Microbiology, 14(5), 645-652.
- Tankeshwar, A. (n.d.). Bile solubility test: Principle, procedure, results. Microbe Online. Retrieved from https://microbeonline.com/bile-solubility-test-principle-procedure-expected-result-and-quality-control/
- UK Health Security Agency. (2025). Bile solubility test (UK Standards for Microbiology Investigations TP 5 Issue 4.1).
- Vidal, J. E., Wier, M. N., Angulo-Zamudio, U. A., McDevitt, E., Jop Vidal, A. G., Alibayov, B., Scasny, A., Wong, S. M., Akerley, B. J., & McDaniel, L. S. (2021). Prophylactic inhibition of colonization by Streptococcus pneumoniae with the secondary bile acid metabolite deoxycholic acid. Infection and Immunity, 89(12), e00463-21. https://doi.org/10.1128/IAI.00463-21
- Wessels, E., Schelfaut, J. J. G., Bernards, A. T., & Claas, E. C. J. (2012). Evaluation of several biochemical and molecular techniques for identification of Streptococcus pneumoniae and Streptococcus pseudopneumoniae and their detection in respiratory samples. Journal of Clinical Microbiology, 50(4), 1171–1177. https://doi.org/10.1128/JCM.06609-11
- Wikipedia. (n.d.). Streptococcus pneumoniae. Retrieved from https://en.wikipedia.org/wiki/Streptococcus_pneumoniae
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.