The Beta (β) Lactamase Test is the procedure that is used to detect the presence of β-lactamase enzyme produced by some bacteria. It is the enzyme that hydrolyses the β-lactam ring of β-lactam antibiotics like penicillin and ampicillin. When this ring is broken the antibiotic becomes inactive, so the organism becomes resistant. It is the rapid test applied in microbiology laboratories for identifying such resistance.
It is the process where the enzyme acts on a chromogenic or non-chromogenic substrate and the reaction gives a visible change. Among the important method, the chromogenic (Nitrocefin) test is widely used. In this test a paper disk impregnated with Nitrocefin is taken and the organism is applied on the disk. If the enzyme is present the β-lactam ring of Nitrocefin is hydrolysed and the colour changes from yellow to red within a few minutes. This is referred to as the positive reaction. These are used mainly for organisms showing penicillin resistance.
Other methods are also used. The Acidimetric method is the process where hydrolysis of penicillin produces penicilloic acid and this lowers the pH, so the indicator changes its colour. The Iodometric method is based on hydrolysis of penicillin which removes iodine from the starch-iodine complex, and the blue colour disappears. In each case the reaction is simple and the visible change indicates presence of the enzyme.
Some of the main uses are in detecting resistance among Neisseria gonorrhoeae, Haemophilus influenzae, and Staphylococcus aureus. It is the rapid way of identifying narrow-spectrum penicillin resistance. However these test is not useful for detecting complex β-lactam resistance like ESBL producing Gram-negative bacilli because the mechanism is different and requires other techniques for confirmation.
Objectives of Beta (β) Lactamase Test
- To detect the presence of beta-lactamase enzyme produced by bacteria.
- To confirm resistance of bacteria to beta-lactam antibiotics such as penicillin ampicillin and amoxicillin.
- To provide rapid diagnostic result within few minutes as compared to routine susceptibility tests.
- To screen clinically important organisms like Neisseria gonorrhoeae, Haemophilus influenzae, Staphylococcus spp., Enterococcus spp. and Moraxella catarrhalis.
- To predict the effectiveness of beta-lactam antibiotics before starting treatment.
- To help in selecting proper antibiotic therapy and avoiding use of ineffective drugs.
- To assist in infection control by early detection of resistant bacterial strains.
Principle of Beta (β)-Lactamase Test
The principle of the β-lactamase test is the process where the β-lactam ring of β-lactam antibiotics is hydrolysed by the enzyme produced by the bacteria. It is the amide bond of the ring that is broken, and once the ring is opened the antibiotic becomes inactive. The reaction forms penicilloic acid or similar products, and these products bring a chemical change that is easily detected in different test methods.
In the chromogenic (Nitrocefin) method the substrate changes its colour because the structure of the molecule is altered after hydrolysis. It is the process where the colour shifts from yellow to red indicating the presence of the enzyme. In the acidimetric method the penicilloic acid lowers the pH, and the indicator (phenol red) changes from red or violet to yellow. The iodometric method depends on the reducing nature of penicilloic acid, and this reduces iodine so the blue colour of the starch-iodine complex disappears. These are the reactions that show the hydrolysis of the β-lactam ring, and the visible colour change is taken as the positive result.
Requirement for Beta (β) Lactamase Test
General Requirement
- Test organism grown overnight (18–24 hours) on non-selective culture media.
Chromogenic (Nitrocefin) Test
- Nitrocefin disc stored at 2–8°C.
- Sterile distilled water for rehydration.
- Clean glass slide or empty sterile petri dish.
- Sterile forceps for handling disc.
- Sterile Pasteur pipette for adding water.
- Sterile wooden applicator stick or inoculating loop for smearing colonies.
Acidimetric Test
Disk or Strip Method
- Acidimetric discs stored at 2–8°C.
- Sterile distilled water.
- Glass slide or empty sterile petri dish.
- Sterile Pasteur pipettes.
- Sterile wooden sticks or inoculating loops.
Tube Method
- Penicillin substrate (crystalline potassium penicillin G).
- pH indicator phenol red solution.
- 1 N sodium hydroxide and sterile distilled water.
- Sterile capped polystyrene tubes.
- Sterile 1 ml and 10 ml pipettes with bulbs.
- Sterile applicator sticks or inoculating loops.
Iodometric Method
- Penicillin solution prepared in phosphate buffer (pH 6.0).
- Starch reagent.
- Iodine reagent with potassium iodide.
- Sterile microdilution tray or small test tubes.
- Sterile 1 ml pipettes with bulbs.
- Sterile wooden applicator sticks or inoculating loops.
Procedure of Beta (β)-Lactamase Test
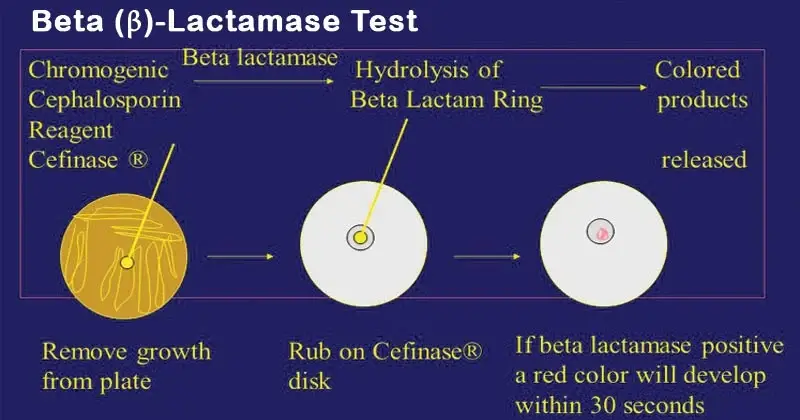
A. Chromogenic Cephalosporin (Nitrocefin) Disk Method
- Place one Nitrocefin disc on a clean glass slide or empty sterile petri dish using sterile forceps.
- Allow the disc to attain room temperature before use.
- Add one drop of sterile distilled water to moisten the disc (do not over saturate).
- Smear few well isolated colonies of the test organism on the disc using sterile loop or wooden stick.
- Observe the disc for colour change from yellow to red within 15 seconds to 5 minutes.
- If no colour change is seen after 5 minutes the result is considered negative.
B. Acidimetric Method
Disk Method
- Place required number of acidimetric discs on a clean slide or petri dish.
- Moisten each disc with one drop of sterile distilled water.
- Smear few colonies of test organism on the disc surface using sterile loop or stick.
- Observe for colour change from violet or red to yellow within 10 minutes.
Tube Method
- Allow penicillin phenol red reagent tube to reach room temperature.
- Add four to five colonies of test organism to the tube to form a milky suspension.
- Mix gently and observe for colour change.
- Change of colour from violet to yellow within 15 minutes indicates positive result.
C. Iodometric Method
- Add 0.1 ml of penicillin solution into a small sterile test tube or microdilution well.
- Add test organism to prepare an opaque suspension.
- Add two drops of starch solution and mix properly.
- Incubate at room temperature for 30 to 60 minutes.
- Add one drop of iodine reagent and mix for one minute.
- Observe immediately for disappearance of blue colour.
- Rapid decolorization indicates positive test while persistence of blue colour indicates negative result.
Result Interpretation of Beta Lactamase Test
Chromogenic (Nitrocefin) Test
- Positive result – Colour of the disc changes from yellow to red or deep pink indicating presence of beta-lactamase enzyme.
- Negative result – Disc remains yellow showing absence of beta-lactamase enzyme.
Acidimetric Test
- Positive result – Colour changes from violet or red to yellow due to formation of penicilloic acid.
- Negative result – No colour change and colour remains violet or red.
Iodometric Test
- Positive result – Blue or purple colour disappears and solution becomes colourless.
- Negative result – Blue or purple colour persists indicating absence of enzyme.
Uses of Beta Lactamase Test
- To detect production of beta-lactamase enzyme by bacteria.
- To identify resistance to penicillinase labile antibiotics such as penicillin ampicillin and amoxicillin.
- To screen clinically important organisms like Neisseria gonorrhoeae, Haemophilus influenzae, Staphylococcus aureus and Moraxella catarrhalis.
- To detect beta-lactamase production in Enterococcus species.
- To provide rapid results for early laboratory diagnosis.
- To help in selection of appropriate antimicrobial therapy.
- To detect beta-lactamase producing strains in veterinary infections.
- To support epidemiological surveillance of penicillin resistant strains.
- To detect beta-lactamase enzyme in Gram negative bacteria using chromogenic method.
Advantages of Beta (β) Lactamase Test
- It gives rapid result within few minutes to one hour.
- The test is simple to perform and easy to interpret.
- It does not require complex instruments or equipment.
- It helps in early detection of beta-lactamase enzyme production.
- It guides in proper selection of antibiotic therapy.
- It helps in avoiding use of ineffective beta-lactam antibiotics.
- It is highly sensitive for organisms like Neisseria gonorrhoeae, Haemophilus influenzae and Staphylococcus spp.
- It is cost effective and suitable for routine laboratory use.
- Chromogenic method can detect wide range of beta-lactamase enzymes.
Limitations of Beta (β) Lactamase Test
- A negative test result does not rule out resistance due to other mechanisms.
- It cannot detect resistance caused by altered penicillin binding proteins or efflux pumps.
- The test is not suitable for Enterobacteriaceae and other aerobic Gram negative bacilli.
- It cannot properly detect complex resistance mechanisms like ESBLs and AmpC enzymes.
- It is not useful for organisms such as Streptococcus pneumoniae where resistance is not enzyme mediated.
- Beta-lactamase detection in some Staphylococcus species may be delayed.
- Weak or unclear colour reactions may lead to false interpretation.
- Acidimetric method cannot differentiate beta-lactamase from acylase activity.
- Iodometric method is less sensitive and reagents are unstable.
- Chromogenic method may give error if disc is over dried or over moistened.
- Rapid tests are insufficient for detailed characterization of complex beta-lactam resistance.
FAQ
Q1. What is a Beta (β) Lactamase Test?
A. Beta (β) Lactamase Test is a rapid laboratory test used to detect the production of beta-lactamase enzyme by bacteria. It is the enzyme which breaks the beta-lactam ring of antibiotics like penicillin and ampicillin. Due to this action the antibiotics becomes ineffective.
Q2. Why is the Beta (β) Lactamase Test important?
A. It is important because it helps in early detection of antibiotic resistance. The test guides the selection of proper antibiotics and avoids treatment failure caused by resistant bacteria.
Q3. What is the principle of the Beta (β) Lactamase Test?
A. It is based on the hydrolysis of beta-lactam ring by beta-lactamase enzyme. When the enzyme acts on a specific substrate it produces a visible colour change which indicates presence of enzyme.
Q4. What are the different types of Beta (β) Lactamase Tests?
A. The different types are chromogenic (Nitrocefin) test, Acidimetric test and Iodometric test. These tests differ in substrate and indicator used.
Q5. How is the Nitrocefin test performed for beta-lactamase detection?
A. In this test a Nitrocefin disc is moistened with distilled water and bacterial colonies are smeared on it. If beta-lactamase is present the disc changes colour from yellow to red within few minutes.
Q6. How are Beta (β) Lactamase Test results interpreted?
A. Results are interpreted based on colour change. Colour change indicates positive result while absence of colour change indicates negative result.
Q7. What color indicates a positive beta-lactamase test?
A. Red or deep pink colour in Nitrocefin test yellow colour in Acidimetric test and loss of blue colour in Iodometric test indicates positive result.
Q8. What bacteria are typically tested for beta-lactamase production?
A. Common bacteria tested are Staphylococcus aureus, Neisseria gonorrhoeae, Haemophilus influenzae, Moraxella catarrhalis and Enterococcus species.
Q9. What is the Acidimetric method for beta-lactamase detection?
A. Acidimetric method detects fall in pH due to formation of penicilloic acid. This causes colour change of indicator from violet or red to yellow.
Q10. What is the Iodometric method for beta-lactamase detection?
A. Iodometric method is based on decolorization of starch iodine complex. The beta-lactamase activity prevents iodine from binding with starch resulting in loss of blue colour.
Q11. How does beta-lactamase contribute to antibiotic resistance?
A. Beta-lactamase destroys the beta-lactam ring of antibiotics. This inactivation prevents the drug from acting on bacterial cell wall synthesis.
Q12. What are the applications of the Beta (β) Lactamase Test?
A. It is used for rapid detection of beta-lactamase enzyme guiding antibiotic therapy. It is also used in epidemiological surveillance and routine microbiology laboratories.
Q13. What are the limitations of the Beta (β) Lactamase Test?
A. The test cannot detect resistance due to non enzymatic mechanisms. It is not useful for detecting complex resistance like ESBLs and carbapenemases.
Q14. What are Extended-Spectrum Beta-Lactamase (ESBL) detection methods?
A. ESBL detection methods include double disc synergy test combination disc test and molecular methods. These are required as routine beta-lactamase tests are insufficient.
Q15. What is a Beta-Lactamase Activity Assay Kit used for?
A. It is used to quantitatively or qualitatively measure beta-lactamase enzyme activity in bacterial isolates. It is mainly used in research and advanced diagnostic laboratories.
- Bidya, S., & Suman, R. S. (2014). Comparative study of three β lactamase test methods in Staphylococcus aureus isolated from two Nepalese hospitals. Open Journal of Clinical
- Diagnostics, 4(1), 47–52. https://doi.org/10.4236/ojcd.2014.41009
- Bush, K. (2010). Bench-to-bedside review: The role of β-lactamases in antibiotic-resistant Gram-negative infections. Critical Care, 14(3), 224. https://doi.org/10.1186/cc8892
- Bush, K., & Bradford, P. A. (2016). β-Lactams and β-lactamase inhibitors: An overview. Cold Spring Harbor Perspectives in Medicine, 6(8), a025247. https://doi.org/10.1101/cshperspect.a025247
- Bush, K., & Bradford, P. A. (2020). Epidemiology of β-lactamase-producing pathogens. Clinical Microbiology Reviews, 33(2), e00047-19. https://doi.org/10.1128/CMR.00047-19
- Bush, K., & Jacoby, G. A. (2010). Updated functional classification of beta-lactamases. Antimicrobial Agents and Chemotherapy, 54(3), 969–976. https://doi.org/10.1128/AAC.01009-09
- Centers for Disease Control and Prevention. (2025, June 12). About ESBL-producing Enterobacterales. https://www.cdc.gov/esbl-producing-enterobacterales/about/index.html
- Gajic, I., Kabic, J., Kekic, D., Jovicevic, M., Milenkovic, M., Mitic Culafic, D., Trudic, A., Ranin, L., & Opavski, N. (2022). Antimicrobial susceptibility testing: A comprehensive review of currently used methods. Antibiotics, 11(4), 427. https://doi.org/10.3390/antibiotics11040427
- Jorgensen, J. H., & Ferraro, M. J. (2000). Antimicrobial susceptibility testing: Special needs for fastidious organisms and difficult-to-detect resistance mechanisms. Clinical Infectious Diseases, 30(5), 799–808. https://doi.org/10.1086/313788
- Llanes, R., González, M., Martínez, I., Sosa, J., Guzmán, D., Gutiérrez, O., Llop, A., & Sánchez, L. (2003). Evaluation of four methods for detecting the beta-lactamase activity in Neisseria gonorrhoeae isolated in Cuba. Memórias do Instituto Oswaldo Cruz, 98(8). https://doi.org/10.1590/S0074-02762003000800020
- Mohammed, T. (2023). Beta (β) lactamase test [Lecture notes]. Department of Microbiology, College of Veterinary Medicine, University of Basrah.
- Pitkälä, A., Salmikivi, L., Bredbacka, P., Myllyniemi, A.-L., & Koskinen, M. T. (2007). Comparison of tests for detection of β-lactamase-producing staphylococci. Journal of Clinical Microbiology, 45(6), 2031–2033. https://doi.org/10.1128/JCM.00621-07
- Stathopoulos, M., Stathopoulos, P., Vourli, C., Tzvetanova, I. D., & Falagas, M. E. (2025). New β-lactam/β-lactamase inhibitor combination antibiotics. Pathogens, 14(4), 307. https://doi.org/10.3390/pathogens14040307
- The β-lactamase test: A critical pillar in the detection, classification, and management of contemporary antimicrobial resistance. (n.d.). [Unpublished manuscript/Internal document].
- Thomson, K. S. (2010). Extended-spectrum-β-lactamase, AmpC, and carbapenemase issues. Journal of Clinical Microbiology, 48(4), 1019–1025. https://doi.org/10.1128/JCM.00219-10
- Wikipedia. (n.d.). Beta-lactamase. In Wikipedia. Retrieved from https://en.wikipedia.org/w/index.php?title=Beta-lactamase&oldid=1319381916
- Wilson, H., & Török, M. E. (2018). Extended-spectrum β-lactamase-producing and carbapenemase-producing Enterobacteriaceae. Microbial Genomics, 4(7), e000197. https://doi.org/10.1099/mgen.0.000197
Gli evoluzionisti, che non conoscono la genetica, continuano ad attribuire la resistenza agli antibiotici dei batteri a loro mutazioni attive di cui non dispongono, perché procarioti e quindi forniti di un solo filamento di DNA, che si trasmette immutato alle cellule figlie. Tale scoperta risale al 1943, stranamente sottaciuta, dovuta a S. Lauria e Max Delbruck, ai quali fu conferito il Premio Nobel; confermata di recente da Jules Hoffmann dell'Institute d'Etudes Avancées dell'Università di Strasburgo, Premio Nobel 2011 per la Medicina. (Da Giovanni Lo Presti: Darwinismo e Genetica, Albatros 2019. Pagine 63 e 160-164).
L'affermazione secondo cui "gli evoluzionisti, che non conoscono la genetica, continuano ad attribuire la resistenza dei batteri agli antibiotici a mutazioni attive che essi non hanno" non solo è errata ma anche fuorviante. I biologi evoluzionisti e i genetisti hanno da tempo riconosciuto il ruolo cruciale del trasferimento genico orizzontale (HGT) nell’acquisizione della resistenza agli antibiotici da parte dei batteri. Questo processo, che comporta il trasferimento di materiale genetico tra organismi non imparentati, può effettivamente dotare i batteri dei geni necessari per combattere gli antibiotici. L'HGT è prevalente anche nei procarioti, organismi che possiedono un singolo cromosoma circolare. In effetti, l’HGT è considerato un meccanismo più comune di resistenza agli antibiotici rispetto alla mutazione.
L'affermazione secondo cui S. Lauria e Max Delbrück sarebbero stati "insigniti del Premio Nobel per la loro scoperta" dell'HGT è di fatto inesatta. Sebbene i loro contributi alla genetica batterica siano stati significativi, i loro sforzi non sono stati riconosciuti con il Premio Nobel.
Inoltre è errata l'affermazione secondo cui Jules Hoffmann avrebbe "confermato" che i batteri non acquisiscono resistenza agli antibiotici attraverso mutazioni. La ricerca di Hoffmann si è concentrata sul sistema immunitario degli insetti, non sulla genetica batterica.
Per affrontare l’affermazione secondo cui i batteri non presentano mutazioni attive a causa del loro DNA a filamento singolo, è essenziale chiarire che non è così. I batteri possiedono un sofisticato sistema di riparazione del DNA che corregge efficacemente le mutazioni, garantendo l'integrità del loro materiale genetico. Tuttavia, le mutazioni possono ancora verificarsi, anche se a un ritmo inferiore rispetto agli organismi con DNA a doppia elica.
la dichiarazione è piena di inesattezze fattuali e informazioni fuorvianti. È fondamentale fare affidamento su fonti credibili di conoscenza scientifica quando si esplorano argomenti scientifici.
References:
Davies, J. (1994). Horizontal gene transfer in bacteria. Journal of Industrial Microbiology & Biotechnology, 14(5-6), 120-130.
Ochman, H., & Moran, N. A. (2005). Horizontal gene transfer. In The evolution of prokaryotes (pp. 31-50). Oxford University Press.
D'Costa, V. M., King, C. M., Kalanthrofimoorthy, S., & Wright, G. D. (2006). Antibiotic resistance: Is horizontal transfer bad for bacterial fitness?. Journal of molecular biology, 356(5), 923-930.
Zhu, Y.-G., & Ochman, H. (1999). Introduction of bacteriophage immunity genes by horizontal transfer from enteric bacteria to shigellae. Journal of bacteriology, 181(12), 3743-3748.