What is Benedict’s Test?
It is one of the simplest and most widely used chemical tests for the presence of reducing sugars in a solution. Benedict’s test therefore gives a simple but useful means of studying how the carbohydrates are classified into two: reducing and non-reducing.
Its a characteristic of reducing sugars to contain free aldehyde or ketone groups. These groups can donate electrons and oxidation reactions are possible. Most simple sugars are in the list, including glucose, fructose, as well as several disaccharides and some complex sugars. This means that it is useful for use in both basic and applied sciences as a reducing agent.
How does it work? Benedict’s reagent reacts differently with reducing sugars. The reagent contains copper(II) sulfate, which is reduced to copper(I) oxide in the test. This reaction produces a characteristic brick-red solid. The appearance of this reddish solid indicates the presence of reducing sugars.
The test does not show exact amounts, but it can give a general idea of sugar levels by looking at how much the color changes. The stronger the color change, the more sugar there is.
Benedict’s test is used in so many applications: teaching and even medical purposes. For instance, it is very common in checking for glucose in urine; it serves as an early test for diabetes. Although the new methods may be more accurate, this test can still be of great use in simple screenings.
This was developed by Stanley Rossiter Benedict, an American chemist who made a significant contribution to biochemistry. His work on the methods for the detection of carbohydrates is still used today.
This test can be done in schools, labs, and medical institutions. Its simplicity and utility have made it a reliable tool in chemical and biological research.
Benedict’s Test Definition
Benedict’s Test is a qualitative chemical assay used to detect the presence of reducing sugars in a solution, characterized by the formation of a brick-red precipitate upon reaction with Benedict’s reagent.
Objectives of Benedict’s Test
Below are its main objectives, explained in different ways:
- Finding Reducing Sugars– The primary aim of this test is to find reducing sugars. These sugars, like glucose and fructose, have parts that can change copper(II) ions in Benedict’s reagent. If a brick-red solid appears, it shows these sugars are in the solution.
- Use in Diagnosing Diabetes- In medicine, this test is used to find glucose in urine samples. If glucose is found in urine, it may mean that a person has diabetes mellitus. This makes Benedict’s test a useful first step in screening for diabetes.
- Estimating Sugar Concentration– The test shows whether sugar is present, but the strength of the reaction gives hints about how much sugar is there. A darker red color means higher sugar levels, but this method cannot measure the exact amount.
- Carbohydrate Differentiation – Another objective is the differentiation of various types of carbohydrates. Benedict’s test helps in the differentiation of unknown carbohydrate samples obtained from food or living organisms by categorizing sugars into two categories: reducing and non-reducing.
Principle of Benedict’s Test
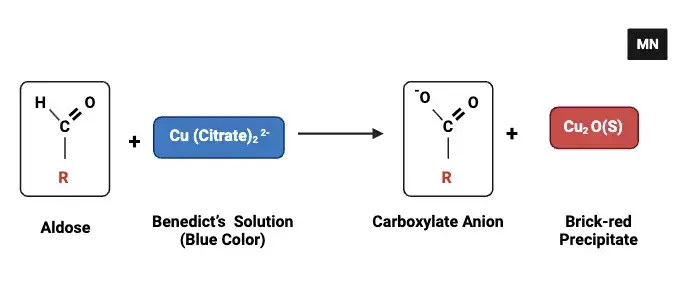
Benedict’s test is based upon the chemical reaction of reducing sugars with the reagent. Sodium carbonate in the reagent elevates the pH of the solution and it turns alkaline. In this environment, the reducing sugars undergo tautomerism that later leads to enediols formation. Eendiols are effective reducing agents.
These compounds react with cupric ions (Cu²⁺) dissolved in copper sulfate; the latter forms the main component of the reagent. It is reduced to cuprous ions (Cu⁺). In this reaction, insoluble red colored cuprous oxide (Cu₂O) is produced that deposits a red-coloured residue in the test-tube. A red-coloured residue becomes red in colour because of the presence of reducing sugars.
The colour of the reaction mixture changes with increasing concentration of reducing sugars. Firstly, it is greenish and then yellowish, orange, and then brick red. When the amount of sugar taken is very low, a faint greenish colour could be obtained whereas when large amounts of sugar are used it gives an intense brick red color.
Using this color change, the amount of reducing sugar in any sample can be estimated.
Materials Required of Benedict’s Test
A few simple tools and supplies are needed to carry out Benedict’s test. Each one plays an essential role in delivering a proper conclusion.
- Sample Solution– The requirement for this test is the sample with the suspected reducing sugar. The sample can either be an unknown carbohydrate solution or even urine sample. The sample determines the applied use of the test in real life.
- Test-Tubes – This mixture is held in glass test-tubes for the reaction to take place. These test-tubes must be clean because whatever left behind will interfere with the chemical reaction. A test-tube holder might come in handy when handling the tubes while heating.
- Pipette – The pipette is used to collect the sample and the reagent in a measured amount. This avoids improper results because proper amounts must be taken for similar results.
A heat source, such as a Bunsen burner, provides the heat necessary for the reaction. Heating is involved in converting the cupric ions into cuprous oxide, thus creating the colored solid. - Benedict’s Reagent– This chemical solution consists of copper sulfate, sodium carbonate, and sodium citrate. This is the main reagent applied to the sample to identify the presence of reducing sugars.
Benedict’s Reagent Preparation
Benedict’s reagent is prepared in the right mixture and concentration of chemicals. The steps involved are relatively simple and are to be carried out so that the reagent is ready for the test of reducing sugars. This is how it is prepared:
- Chemicals Measurement
Weigh 17.3 grams of copper sulfate (CuSO₄), 173 grams of sodium citrate (Na₃C₆H₅O₇), and 100 grams of anhydrous sodium carbonate (Na₂CO₃). If using the decahydrate form of sodium carbonate, that is Na₂CO₃·10H₂O, weigh 270 grams. Weighing in this manner will guarantee the reagent works correctly. - Mix the Ingredients
Place all the weighed ingredients in a 1-litre volumetric flask. This type of flask will be used to make easy mixing and volume adjustment possible. - Add Distilled Water
Fill the flask with distilled water up to the 1000 mL line. Do not spill over. - Dissolve Mixture
Swirl the flask gently to ensure that everything dissolves well. The chemical mixture should completely dissolve and make for a homogeneous solution. If this is not so, it will affect how the reagent works out in test sessions.
Procedure of Benedict’s Test
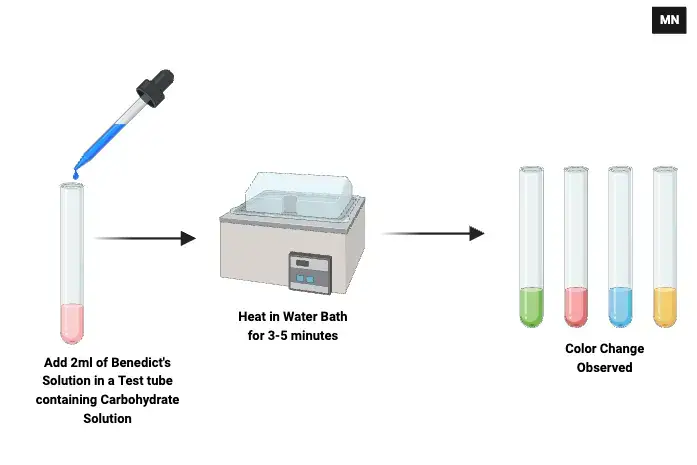
Benedict’s test involves some quick steps. Each step is crucial, since otherwise, the amount of reduced sugars might not be detected accurately.
- Prepare Sample– Use a clean test-tube. Add 1 mL of the sample solution-this can be urine or any carbohydrate solution being tested. Cleanliness of the test-tube prevents contamination.
- Add Benedict’s Reagent– Pour 2 mL of Benedict’s reagent carefully into the test-tube. The reagent must completely mix with the sample for proper reaction.
- Heating of the Mixture – Place the test-tube in a boiling water bath and heat it up for 3–5 minutes. Direct heating over a flame can be used but take care to avoid burning or shattering.
- Colour Change – Upon heating, note the colour change of the solution. It is greenish and may turn yellow, orange, then eventually brick-red as the quantity of reducing sugar varies.
Observation and Results of Benedict’s Test
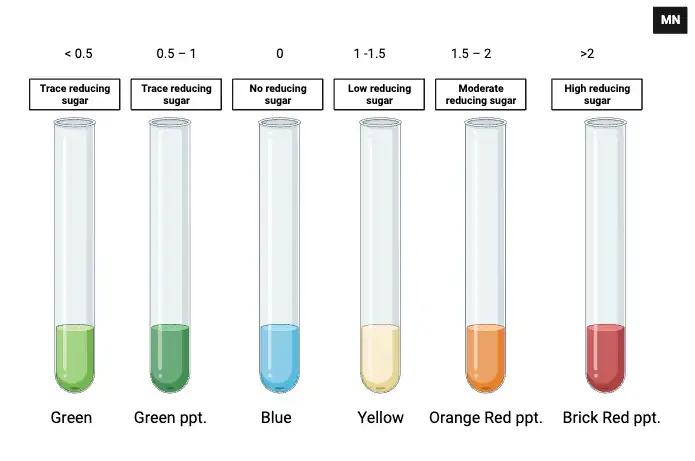
When performing Benedict’s test, changes in colour and precipitate formation are crucial indicators. A shift from the reagent’s blue hue to another shade signals the presence of reducing sugar. This transformation typically occurs within three minutes of heating.
- If no colour change is observed, it suggests the absence of reducing sugars in the sample. The solution remains blue.
- A green colouration indicates a trace amount of reducing sugar—less than 0.5 g%. A green precipitate may also form in slightly higher concentrations.
For concentrations between 1 and 1.5 g%, a yellow precipitate is formed. This corresponds to a low level of reducing sugar in the solution.
As the sugar concentration increases, the colour deepens. An orange-red precipitate forms when the reducing sugar concentration lies between 1.5 and 2 g%.
Finally, at greater than 2 g%, the sample develops a brick-red precipitate, confirming a high concentration of reducing sugar.
These observations can also be used for semiquantitative analysis, offering a practical way to estimate the sugar concentration based on the shade of the colour. This information is essential in clinical and biochemical assessments.
| Shade of Color | Approx. Concentration of Reducing Sugar (in g%) | Indication |
|---|---|---|
| Blue | 0 | No reducing sugar |
| Green (Solution) | < 0.5 | Trace reducing sugar |
| Green (Precipitate) | 0.5 – 1 | Trace reducing sugar |
| Yellow Precipitate | 1 – 1.5 | Low reducing sugar |
| Orange-Red Precipitate | 1.5 – 2 | Moderate reducing sugar |
| Brick-Red Precipitate | >2 | High reducing sugar |
Advantages of Benedict’s Test
Its merits are many: it is of great utility as well as of low cost; these qualities suit every use, however miscellaneous, in school and clinic alike.
- Ease in Preparation. The test is simple and readily performed. There are only a few pieces of apparatus needed for it, and the operations may be completed without much time-consuming effort. So little training is required.
- Benedict’s reagent is non-toxic. This minimizes the risk to the user. It is safer than some other tests.
- Cost-Effectiveness. The materials and reagents used in the test are inexpensive. This characteristic makes it available in low-budget laboratories.
- Versatile Evaluation. The test gives both qualitative and semi-quantitative information. The approximate concentration of reducing sugars in a sample can be estimated by observing colour changes.
Limitation of Benedict’s Test
Although Benedict’s test is highly popular, it has a number of drawbacks in its results.
- False-Positive Results. There are drugs that interfere with the test. Such drugs include penicillin, isoniazid, and streptomycin. These chemicals can react with Benedict’s reagent, thus causing false-positive results. This complicates the interpretation of the presence of reducing sugars.
- Interference from Other Chemicals. Such chemicals include creatinine, ascorbic acid, and urate, which are common in urine. These slow down the Benedict reaction, so the color change might not be what is expected, and results can be wrong.
- It is hard to measure the exact concentration of a substance. Benedict’s test cannot give an exact measure of the levels of reducing sugars. It only gives a sort of rough estimate, which means it can show that there is sugar, but it cannot state how much is present. Only the colors seen—green, yellow, or red—hint at the amount.
- Need for Extra Tests. The test does not indicate the type of carbohydrate present. Further test is required to determine precisely which carbohydrate exists because the reaction does not narrow down to just one kind of reducing sugar.
Applications/Uses of Benedict’s Test
From clinical diagnosis to biochemistry research, Benedict’s test is a vital instrument with many uses in several disciplines. Some noteworthy applications for it are:
- Benedict’s test is most used in clinical diagnostics to find glucose in urine. Commonly found in diabetes mellitus, a positive reaction denotes higher levels of lowering sugars. Usually functioning as an early indication for the ailment, this test provides fast detection. This easy exam offers important information in healthcare environments that can direct next medical investigations.
- Benedict’s test is quite important in the food sector in guaranteeing the quality and safety of goods. For fruit juices, honey, and other sweeteners, for instance, it is used to gauge the declining sugar count. This ensures that their products satisfy customer expectations and helps businesses with food labelling regulations. Consistency in product quality depends on such tests absolutely.
- Turning now to biochemistry research, this assay is often used to examine unidentified carbohydrate extracts taken from biological sources. Revealing the presence of reducing sugars helps scientists investigate how various sugars behave in living systems and examine metabolic processes. The test helps greatly to clarify difficult metabolic pathways.
- Furthermore, Benedict’s test has educational value especially for teaching about chemical reactions. For students studying about redox processes, it provides a practical model. This is a good teaching tool since students can visually grasp how reducing sugars interact with copper ions by seeing the color change from blue to green, yellow, or brick-red.
- Especially in food and pharmaceutical sectors, Benedict’s test is quite helpful in quality control. Manufacturers verify the sugar level of their goods using it. Maintaining the standard of manufacturing and following regulations depend on proper amounts of reducing sugars, so this is crucial. This is especially true with medications since product consistency depends on exact amounts.
- At last, the identification of unidentified carbohydrates depends much on Benedict’s test. It separates reducing from non-reducing sugars, a procedure essential in the laboratory when dealing with unidentified materials. Scientific analysis depends on this capacity to differentiate, which has been generally embraced in both academic and commercial research environments.
Precautions of Benedict’s Test
- Measuring accurately is very important. Precision ensures that the results are reliable, especially when preparing the Benedict’s solution. Use a pipette or graduated cylinder to measure the solution carefully.
- The heating rate is also very important. Do not hurry the heating process. It is essential to heat the mixture slowly using a water bath. Heating too fast will result in an incorrect result.
- Use the right tools. When heating, always use a test-tube holder. This keeps the tube steady and helps avoid accidents. It’s easy to forget, but safety is very important!
- Test-tube safety. Never point the test tube at yourself or anyone else while heating it. Gases or liquid may escape suddenly, which can hurt you. Hold the tube in the right way.
- Heat thoroughly. It is not enough to heat once. Always heat the solution three times before finishing the test. Only after heating it several times can you confidently say the result is negative.
- Be careful with chemical reactions. As the solution heats up, you might see clear colour changes. Make sure you are ready to watch these changes closely, as they show the presence of reducing sugars.
- Remember the safety rules. Wear goggles and gloves because chemicals and heat are quite dangerous and can cause many problems if one takes a little bit of care.
Quiz
- Hernández-López A, Sánchez Félix DA, Zuñiga Sierra Z, García Bravo I, Dinkova TD, Avila-Alejandre AX. Quantification of Reducing Sugars Based on the Qualitative Technique of Benedict. ACS Omega. 2020 Dec 10;5(50):32403-32410. doi: 10.1021/acsomega.0c04467. PMID: 33376877; PMCID: PMC7758970.
(2022). Retrieved 4 May 2022, from https://www.vedantu.com/chemistry/benedicts-test. - Vodopich, D., & Moore, R. (1996). Biology (9th ed., pp. 57-59). WCB/McGraw-Hill.
- https://www.abdn.ac.uk/rowett/documents/Sourcing_Sugars_Teacher_BENEDICTS_2.pdf
- https://www.jbc.org/article/S0021-9258(19)61050-1/fulltext
- https://theory.labster.com/benedicts_procedure/
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.