The Leifson technique is the staining method used to visualize these very thin structures with the help of a mordant–dye mixture. It is the process in which tannic acid together with the dye forms a colloidal precipitate that is deposited on the flagellar surface and thus makes the filament thick enough to be seen under the light microscope. This technique was introduced as a simple method and it revealed that changes from flagellated to non-flagellated forms may occur, as well as changes in motility. It is used in laboratories because it reveals both presence and arrangement of flagella around the cell perimeter, and it provides a quick way to study motility in bacteria that cannot be examined by electron microscope.
Flagella are long helical appendages that arise from the bacterial surface and these are responsible for most forms of motility in bacteria. It is known that the filament is made of flagellin protein and it is anchored in the cell envelope by a basal body, and because its diameter is extremely small it cannot be directly resolved under the bright-field microscope. The number and arrangement of these structures vary among organisms, and some of the common forms are monotrichous, amphitrichous, lophotrichous and peritrichous types. The presence of flagella helps the cell to move in response to chemical or light gradients and it can rotate at very high speed which is powered by either proton motive force or sometimes sodium gradient. These structures therefore have importance in motility and survival, and their observation is used for phenotypic identification of bacteria.
What is Leifson Technique?
The Leifson Technique is the staining method that is used for demonstrating the delicate bacterial flagella under the bright-field microscope. It is based on the use of tannic acid together with basic fuchsin which acts as a mordant–dye mixture. It is the process by which the dye and tannic acid form a colloidal precipitate that becomes deposited on the thin flagellar filament, so the filament becomes thickened and visible as red-colored structures under oil immersion.
It is very sensitive to the condition of the slide and the preparation is not heat-fixed because the proteinaceous flagella is easily destroyed. This technique is important because it helps in observing the number and arrangement of flagella like monotrichous, amphitrichous, lophotrichous, or peritrichous and thus it is used for identification of motile bacteria including some pathogenic forms.
Aim
The main purpose of flagella staining is to stain bacterial flagella and observe the presence or absence of bacterial flagella as well as their arrangement on bacterial cell.
Principle of Flagella Staining
The principle of flagella staining is based on increasing the visible diameter of the very thin flagellar filament so that it can be observed under the light microscope. It is known that bacterial flagella are made of the protein flagellin and their width is extremely small, usually 10–50 nm, which is below the resolution limit of bright-field microscopy.
The stain uses a mordant that is deposited on the flagellum surface, and this coating makes the filament thick enough to be seen. It is the process in which tannic acid or other mordant reacts together with the dye to form a colloidal precipitate, and this precipitate is absorbed by the flagellum causing an artificial enlargement.
When the stain dries slowly, the evaporation of alcohol increases the concentration of mordant–dye complex and precipitation occurs on the flagellar surface. The reaction is sensitive because the pH, salt content, age of culture and staining solution, thickness of smear and temperature all influence how the precipitate forms. This method allows the helical appendage to be visualized and helps in identifying the arrangement of flagella in the bacteria.
Requirements for Leifson Technique
- New microscope slides are required for preparing the smear. It is cleaned with 95% ethanol and Kimwipes. It is important because any grease can interfere with proper stain deposition.
- Heat is needed during the procedure and it is provided by a Bunsen burner, alcohol lamp, ceramic heater or any suitable heat source.
- Micropipettors is used to deliver about 5 to 200 ml volume and sterile disposable tips is used with these pipettes.
- Distilled water is required for washing, and bibulous paper is used for drying the slide.
Solutions
For Leifson flagella stain three solutions is prepared.
- Solution A– Sodium chloride (1.5 g) is dissolved in distilled water (100 ml).
- Solution B– Tannic acid (3.0 g) is dissolved in distilled water (100 ml).
- Solution C– Pararosaniline acetate (0.9 g), Pararosaniline hydrochloride (0.3 g) is dissolved in ethanol 95% (100 ml).
Mix equal volumes of solution A and solution B. In this step the mixture act as a mordant. Then two volumes of this mixture is added to one volume of solution C. The resulting stain solution is kept refrigerated for about 1 to 2 months.
Flagella Staining Procedure
The stain solutions are supplied directly from Presque Isle Cultures and the exact components is proprietary. Two solutions is used for this method. These are the Flagella mordant (Solution I) and the Silver stain (Solution II). Solutions I and II can be kept at room temperature for several weeks without losing activity.
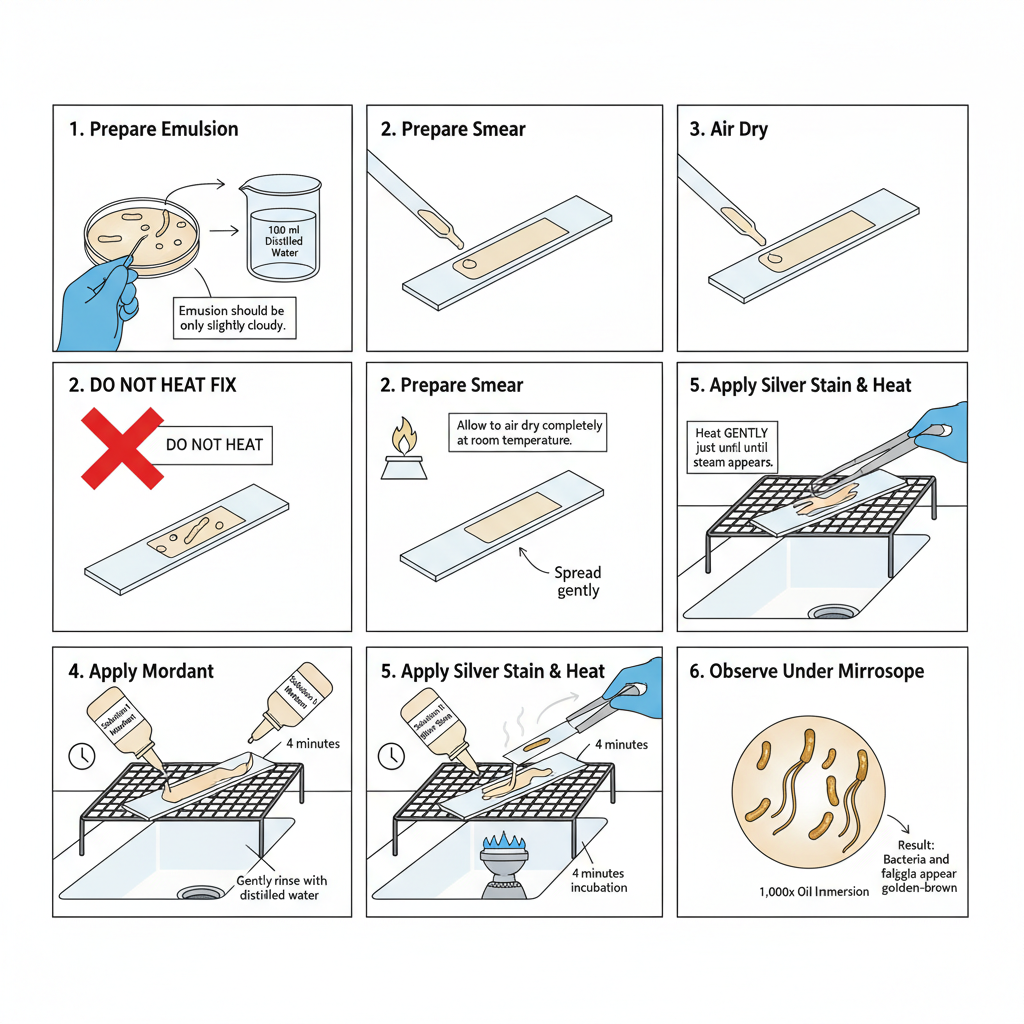
A. Preparation of Cultures
It is possible to use solid or liquid media cultures for this staining. Cultures should incubate for about 16 to 20 hours because older cultures lose flagella. This is important for organisms like Bacillus spp. that form spores and lose flagella during this process. If the culture is clumped and vortexing is needed, it is done gently since flagella is easily removed from the bacterium.
Method
From an agar plate or slant a small amount of growth is removed with an inoculating loop and emulsified in 100 ml distilled water. The emulsion should look only slightly cloudy. Using too much inoculum will make visualization of flagella difficult.
For liquid cultures Leifson recommends two rounds of centrifugation. About 100 ml is taken in a microcentrifuge tube, centrifuged and medium removed. It is resuspended in 100 ml distilled water with gentle vortexing. Again it is centrifuged and suspension is made in about 200 ml distilled water to form a slightly cloudy emulsion. Washing may need optimization because any medium component interfere in proper flagella staining.
B. Preparation of Slides
- A new microscope slide is cleaned with 95% ethanol and Kimwipe. It is flamed to dry and used immediately.
- After cooling the slide is labelled with the organism name.
- About 5 to 10 ml of the emulsion is placed at one end of the slide and spread with the pipette tip held parallel to slide surface.
- The smear is dried at room temperature. Heat fixing is avoided because it destroy the protein flagella.
C. Flagella Staining (Presque Isle Cultures Method)
- The prepared slide is placed on staining rack.
- Presque Isle Cultures Solution I (mordant) is flooded on the smear and incubated at room temperature for 4 minutes.
- It is gently rinsed with distilled water and excess water is shaken off.
- The slide is then flooded with Presque Isle Cultures Solution II, which is the silver stain.
- The slide is heated over a Bunsen burner by moving it back and forth just until steam is seen. Alternate heat sources can be used but require optimization. Excess heat should not be applied because it destroys flagella. The slide is incubated at room temperature for about 4 minutes.
- Rinse with distilled water and blot dry using bibulous paper.
- Observation is done under bright-field microscope at 1,000x oil immersion. Bacteria and flagella appear golden brown. Excess stain is often present and it show that the slide must be thoroughly cleaned before starting.
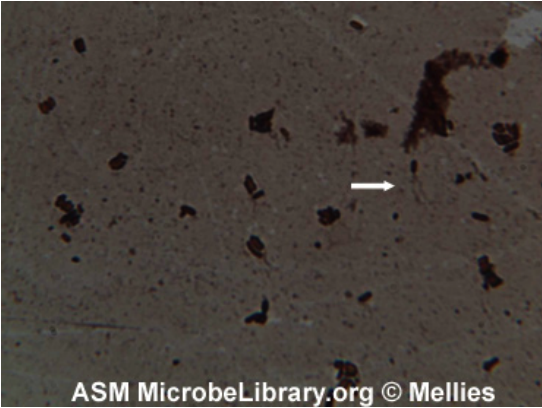
Uses of Leifson Technique
- It is used for clear visualization of bacterial flagella which is very thin structures and not visible normally under bright-field microscope.
- It is used to determine the motility of bacteria as the presence or absence of flagella is easily seen.
- It is the method for providing phenotypic characters that help in taxonomic classification of different bacteria.
- It is used for identifying motile bacterial species. These include non-fermentative aerobic gram-negative bacilli, anaerobic forms like Anaerobiospirillum, Campylobacter, Selenomonas, Wolinella and common motile species like Escherichia coli, Salmonella spp., Pseudomonas aeruginosa and Vibrio cholerae.
- It helps to determine the arrangement of flagella on cell surface. These arrangements include monotrichous, amphitrichous, lophotrichous, peritrichous and also whether the flagella is polar or lateral.
- It is the process used in clinical diagnosis of Helicobacter pylori in gastric biopsy touch smears. It helps in visualizing the typical lophotrichous sheathed flagella and differentiating it from contaminants like Proteus spp. or non-flagellated Streptococcus spp.
- It is used for retrospective study of bacterial flagella when stained slides are stored properly at room temperature.
- It is used in research of flagellar genetics to observe mutants that lose motility or lose flagella formation and to study structural variations.
Advantages of Leifson Technique
- It is useful for clear visualization of very thin bacterial flagella as the stain thickens the filament and makes it visible under bright-field microscope.
- It is important for identification because the presence or absence of flagella and their number is easily seen.
- It helps to determine the exact flagellar arrangement like monotrichous, amphitrichous, lophotrichous and peritrichous which support in identifying motile species such as E. coli, Salmonella, Pseudomonas aeruginosa and Vibrio cholerae.
- It differentiates motile organisms when biochemical reactions give weak or uncertain results. It also helps to distinguish H. pylori from contaminants like Proteus spp. or non-flagellated Streptococcus spp.
- It is the method that helps to relate bacterial morphology with function because it shows mutants with loss of flagella or mutants with non-motile characters even when flagella is present.
- It has high diagnostic sensitivity for Helicobacter pylori infection and is comparable to histology.
- It is more sensitive than rapid urease test which is commonly used in laboratories.
- It is rapid and economical because the whole staining process can be finished within a short time and results are obtained quickly.
- It is cost-effective as it can be used as an alternative test due to its sensitivity and simple procedure.
- It allows retrospective study of stained slides when they are stored at room temperature.
- It is easily adapted in routine laboratory work and simplified forms of Leifson stain is also available.
Limitations of Leifson Technique
- It is difficult to prepare because the working solution is unstable and can be used only for 2 to 3 days.
- It precipitates quickly at room temperature and the stain must be kept for some time so that large aggregates settle down.
- It is affected by chemical degradation mainly due to tannic acid which becomes unstable in presence of alcohol and this causes weak staining.
- It gives inconsistent results and the procedure is considered complex to perform.
- It requires very clean slides as any grease or contaminants interfere with the precipitation reaction needed for staining.
- It is highly sensitive to different factors like pH, temperature, alcohol concentration, dye amount and airflow which affect the staining.
- It is difficult to standardise because of these variations and this led to the development of alternative simple techniques.
- It does not allow heat fixation because heat will damage the delicate flagella and the smear must dry slowly at room temperature.
- It has risk of flagella breaking because the flagella is very fragile and is easily removed by strong washing or mechanical shaking.
- It has risk of sample wash-off since the smear is unfixed and can be removed easily by strong water stream.
- It forms background artefacts when the stain is old or when slides are not washed properly which hides the bacterial cell and flagella.
- It needs proper inoculum density because too much culture causes overlapping cells and flagella cannot be seen clearly.
- It depends on culture age as older cultures lose flagella or show damaged structures.
- It is limited only for morphological purpose to observe presence and arrangement of flagella.
- https://asm.org/asm/media/protocol-images/bacterial-flagella-stain-protocol.pdf?%20ext=.pdf
- https://hereditybio.in/blog/flagella-staining-with-leifson-method-a-step-by-step-guide/
- Alfa Chemistry. (n.d.). Flagella Stain.
- Bianco, M. I., Ponso, M. A., Garita-Cambronero, J., & Yaryura, P. M. (2023). Genomic and phenotypic insight into Xanthomonas vesicatoria strains with different aggressiveness on tomato.
- Carolina Biological Supply Company. (2018, August 21). Safety Data Sheet Flagella Stain, Leifson.
- Clark, W. A. (1976). A Simplified Leifson Flagella Stain. J Clin Microbiol, 3(6), 632–634. https://doi.org/10.1128/jcm.3.6.632-634.1976
- Dowell, V., Jr., & Hawkins, T. (1976). Laboratory methods in anaerobic bacteriology.
- ENG Scientific. (n.d.). ENG Scientific Leifson Bacterial Flagella Stain Kit 4 x 250 mL. Fisher Scientific.
- Fiveable Inc. (2025). Flagella staining.
- Flagella staining [Presentation slides]. (2021, February 4).
- Flagellum. (n.d.). In Wikipedia. Retrieved [Date], from https://en.wikipedia.org/w/index.php?title=Flagellum&oldid=1322034136
- Heimbrook, M. E., Wang, W. L. L., & Campbell, G. (1989). Staining bacterial flagella easily.
- Heredity Bioscience. (2024, January 30). Flagella Staining with Leifson Method: A Step-by-Step Guide.
- Khan, M. A. R., Cervellera-Dominguez, A., Sales, M. G. F., & Riu, J. (2025). Exploration of a polymer responsive potentiometric biosensor for the detection of flagella and whole cell of Proteus mirabilis: proof-of-concept.
- Kodaka, H., Armfield, A. Y., Lombard, G. L., & Dowell, V. R., Jr. (1982). Practical procedure for demonstrating bacterial flagella. Journal of Clinical Microbiology, 16(5), 948–952. https://doi.org/10.1128/JCM.16.5.948-952.1982
- Leifson, E. (1961). An Atlas of Bacterial Flagellation. AM J MED SCI.
- Leifson, E., & Hugh, R. (1953). Variation in shape and arrangement of bacterial flagella.
- Leonard, H., Jiang, X., Arshavsky-Graham, S., & Segal, E. (2022). Shining Light in Blind Alleys: Deciphering Bacterial Attachment in Silicon Microstructures.
- Lopez, N. L., Heredia, J. B., Hernández, C. S. M., & García-Estrada, R. S. (2021). TINCIÓN MODIFICADA PARA OBSERVACIÓN DE FLAGELOS EN Bacillus amyloliquefaciens POR MICROSCOPÍA ÓPTICA.
- MBBS NAIJA. (n.d.). Types of Flagella Arrangement in Bacteria ; Monotrichous, Amphitrichous, Lophotrichous, Peritrichous [Video transcript]. YouTube.
- Mellies, J. (2008, September 8). Bacterial Flagella Stain Protocol. American Society for Microbiology.
- Piccolomini, R., Di Bonaventura, G., Neri, M., Di Girolamo, A., Catamo, G., & Pizzigallo, E. (1999). Usefulness of Leifson Staining Method in Diagnosis of Helicobacter pylori Infection. Journal of Clinical Microbiology, 37(1), 199–201. https://doi.org/10.1128/jcm.37.1.199-201.1999
- Rekha, P. D., Shastry, R. P., Hameed, A., & Nagaraj, A. (2022). Genomic potential for exopolysaccharide production and differential polysaccharide degradation in closely related Alteromonas sp. PRIM-21 and Alteromonas fortis 1T.
- Ren, Q., Chen, H., Zhanbin, S., & Zhang, W. (2021). Umezawaea beigongshangensis sp. nov., Isolated From the Mash of Baijiu. CURR MICROBIOL.
- Study.com. (n.d.). What are some potential problems with the Flagella Stain?
- The Leifson Tannic Acid-Fuchsin Technique: A Comprehensive Guide to Bacterial Flagella Staining, Phenotypic Analysis, and Diagnostic Application. (n.d.).
- Yap, Z. L., Rahman, Z., Hogan, A. M., & Cardona, S. T. (2024). A CRISPR-Cas-associated transposon system for genome editing in Burkholderia cepacia complex species.
- Zhanbin, S., Guo, L., Yan, Y., & Ren, Q. (2022). Sporosarcina beigongshangi sp. nov., isolated from pit mud of Baijiu. ARCH MICROBIOL.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.