What is Bacitracin Susceptibility Test?
The Bacitracin Susceptibility Test is the simple microbiological test that is used for the presumptive identification of Group A β-hemolytic streptococci. It is the process where a bacitracin disk (0.04 units) is placed on the blood agar plate that has been streaked with the suspected organism. The antibiotic acts on the cell wall synthesis pathway and it prevents the formation of peptidoglycan by interfering with the lipid carrier molecules.
After incubation for 18–24 hours at about 35–37°C, the plate is examined. In this step, if a clear inhibition zone is formed around the disk, then the organism is considered susceptible. This is referred to as the positive test and it is mainly shown by Streptococcus pyogenes. When the growth is present very near to the disk, it indicates resistance and these are usually other β-hemolytic streptococci.
This test is also used for differentiating Micrococcus and Staphylococcus. Micrococcus species is susceptible while Staphylococcus species is resistant. These are the basic characters that help in the primary differentiation of cocci in routine culture plates, and it is the process used because it is simple and does not require complicated steps.
Objectives of Bacitracin Susceptibility Test
- To identify Group A β-haemolytic streptococci, mainly Streptococcus pyogenes.
- To differentiate Group A streptococci from other β-haemolytic groups that is resistant to bacitracin.
- To distinguish Micrococcus species from Staphylococcus species based on their susceptibility pattern.
- To provide a simple screening method before doing serological grouping.
- To determine the basic susceptibility or resistance of the organism toward bacitracin.
Principle of Bacitracin Susceptibility Test
The principle of the Bacitracin Susceptibility Test is based on the action of bacitracin on the bacterial cell wall synthesis. It is the process where the antibiotic binds with the lipid carrier molecule (undecaprenyl pyrophosphate) that is required for transporting the peptidoglycan precursors across the membrane. When this carrier is blocked, the movement of the cell wall building units is stopped and the cell wall formation cannot proceed. It is the step that results in inhibition of growth of the susceptible organisms.
In the test procedure, a disk containing a very low concentration of bacitracin is placed on the inoculated agar plate. The antibiotic diffuses into the medium and acts on the organism surrounding the disk. If the organism is sensitive to this amount, then a clear zone of inhibition is formed. This is referred to as the positive reaction. Among the important organisms showing this reaction, Streptococcus pyogenes (Group A) is the main one. Other β-hemolytic streptococci do not show this sensitivity at such low concentration.
This principle is also applied for differentiating Micrococcus and Staphylococcus. Micrococcus species is inhibited by the antibiotic while Staphylococcus grows close to the disk. It is the basic method used in routine bacteriology labs because the reaction is simple and easily visible on the culture plate.
Requirements
- Bacitracin disk (0.04 units) for differentiating susceptible and resistant organisms.
- Blood agar plate prepared on suitable nutrient base for streaking the test organism.
- Mueller Hinton agar may be used when differentiating Micrococcus and Staphylococcus.
- Broth medium for preparing the inoculum suspension when required.
- Pure culture of the test organism, generally 18–24 hours old.
- Standard inoculum density prepared using McFarland turbidity standard.
- Known quality-control strains to check the activity of the disk and media.
- Sterile forceps for placing the disk on the agar surface.
- Inoculating loop or sterile swab for spreading the organism to get confluent growth.
- Incinerator or flame source for sterilizing metal instruments.
- Incubator maintained at about 35–37°C for proper growth.
- Suitable atmospheric condition depending on the organism tested.
- Incubation time of about 18–24 hours for observing the inhibition pattern.
Procedure of Bacitracin test
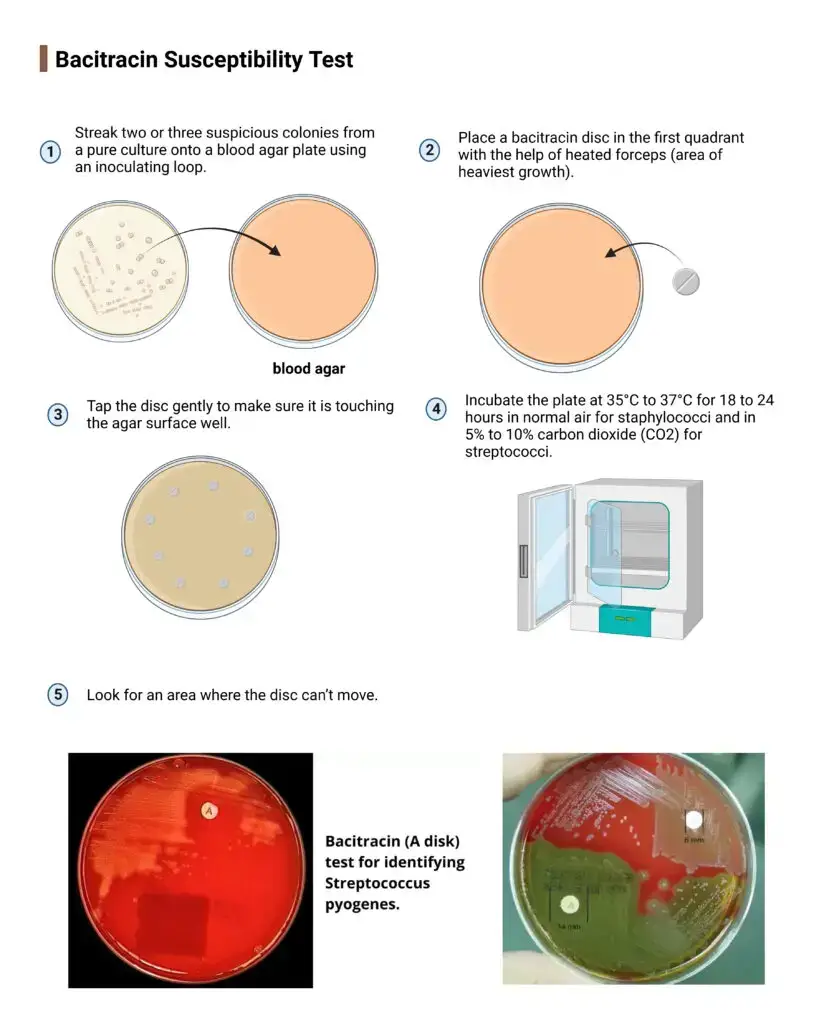
1. Inoculum Preparation
It is started by selecting 2 to 4 well-isolated colonies from a pure culture plate. A sterile loop or swab is used to pick the colonies. It is the process done from an 18–24 hours old culture. Testing from primary culture plates is sometimes possible but not preferred because mixed colonies may be present.
2. Inoculation on Plate
The organism is streaked on the Blood Agar Plate. It is the process of preparing a heavy lawn of growth. The streaking is usually done in three directions so that the organisms cover the plate uniformly. A light inoculum may produce false zones.
3. Disk Placement
The bacitracin disk is placed aseptically at the center of the plate. Sterile forceps is used for transferring the disk. It is placed on the region of maximum growth so that proper diffusion occurs.
4. Adherence of Disk
The disk is pressed gently with the help of forceps. This helps in proper contact of the disk with the agar surface.
5. Incubation
The plate is inverted and incubated at 35°C–37°C for 18–24 hours.
- Streptococci is incubated in 5–10% CO₂.
- Staphylococci and Micrococci are incubated in normal air conditions.

Result of Bacitracin Susceptibility Test
Positive Result (Susceptible)
Observation– A clear zone of inhibition is seen around the bacitracin disk. It is the area where no growth is present.
Criteria
- For Streptococci, any visible zone extending beyond the disk edge is taken as positive. In some guidelines, a zone measuring 14 mm or more is considered.
- For Micrococcus, the zone more than 10 mm indicates susceptibility.
Interpretation
- It is the presumptive identification of Group A β-hemolytic Streptococci (Streptococcus pyogenes).
- It is also the identification of Micrococcus species when separating it from Staphylococcus.
Negative Result (Resistant)
Observation– The colonies grow up to the disk margin. There is no clear zone of inhibition.
Criteria– The zone is absent or below the required range. It is less than 10 mm or less than 14 mm depending on the organism tested.
Interpretation
- The resistant reaction indicates non-Group A β-hemolytic Streptococci such as Group B (Streptococcus agalactiae), Group C, or Group G.
- It is also used for identifying Staphylococcus species which usually show resistance and have a zone ≤10 mm.
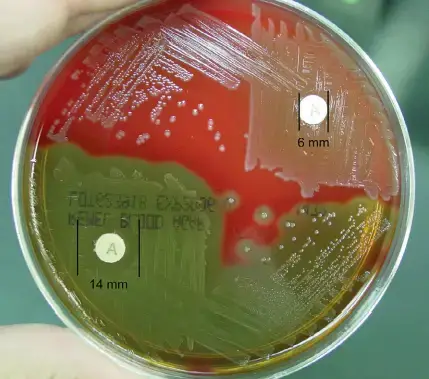
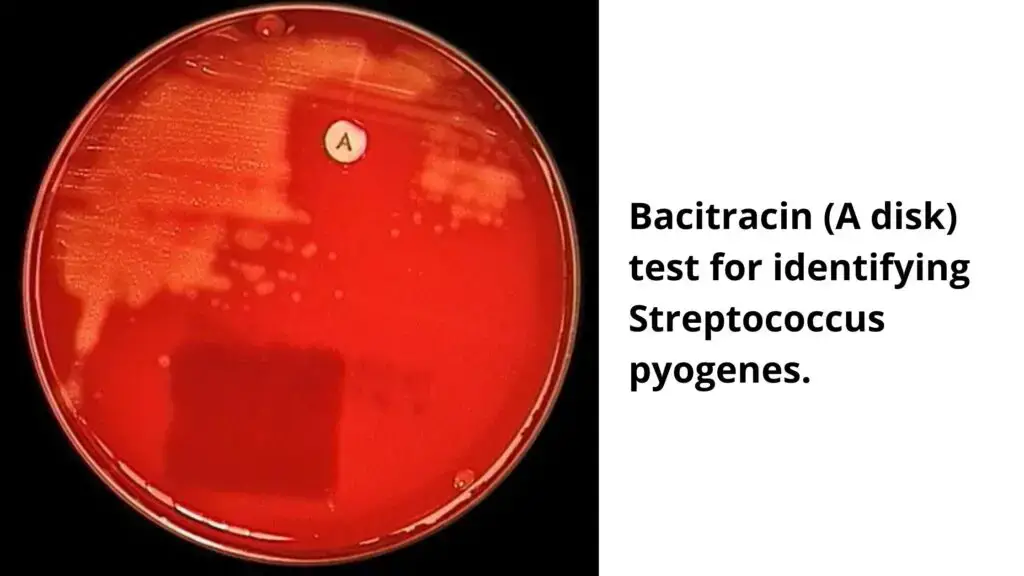
Quality Control
It is the process used to verify that the bacitracin disk and the procedure is functioning properly. It helps in confirming the accuracy of the results by using known organisms.
Control Organisms and Expected Results
Some of the main control organisms used in this test are as follows–
1. Positive Control (Susceptible)
- Organism: Streptococcus pyogenes (Group A), usually ATCC 19615.
- Expected Result: It shows a clear zone of inhibition. The zone is usually 15–20 mm or any proper zone around the disk. It is the indication that the disk potency is correct.
2. Negative Control (Resistant)
- Organism: Streptococcus agalactiae (Group B), such as ATCC 27956 or ATCC 13813.
- Expected Result: No zone of inhibition or a zone less than the required range (for example < 14 mm). It is the confirmation that resistant organisms do not react to the bacitracin disk.
3. Differentiation Control (Staphylococcus vs Micrococcus)
- Organism: Staphylococcus epidermidis (ATCC 12228).
- Expected Result: The zone is around 12 mm or less. It is the process used when checking the differentiation between Micrococcus (susceptible) and Staphylococcus (resistant).
Uses of Bacitracin Susceptibility Test
- It is used for the presumptive identification of Group A β-hemolytic Streptococci (Streptococcus pyogenes).
- It is used for separating Group A Streptococci from other β-hemolytic groups like Group B, C and G which usually show resistance.
- It is used for differentiating Staphylococcus from Micrococcus in catalase-positive cocci.
- It is used as a screening method before doing serological grouping where facilities are not available.
- It is used together with the SXT test to improve the accuracy of identification.
- It is used in teaching laboratories for demonstrating basic microbial differentiation.
Limitations of Bacitracin Susceptibility Test
- It gives only presumptive identification and not a confirmatory result, so further biochemical or serological tests is required.
- Some non-Group A Streptococci may show susceptibility which creates false-positive results.
- Light inoculum may form a false zone of inhibition making resistant organisms appear susceptible.
- Heavy inoculum may suppress the antibiotic effect and produce false-negative results.
- Old or dried Blood Agar Plates restrict antibiotic diffusion and may give false-negative reactions.
- It should be used only for β-hemolytic isolates because α-hemolytic species may show misleading sensitivity.
- Using the wrong disk potency (10 units instead of 0.04 units) produces false-positive reactions.
- Improper storage of disks may reduce potency due to light, heat or moisture.
- Primary plating sometimes gives unreliable results because mixed growth may be present.
- In differentiation tests, incomplete incubation may give smaller zones in Micrococcus leading to misinterpretation.
Advantages of Bacitracin Susceptibility Test
- It is used as an effective screening method which saves time, labor and materials before serological grouping.
- It is a low-cost technique and is more economical than serological or molecular methods.
- It is useful in laboratories where serological facilities is not available.
- It provides a presumptive identification of Group A β-hemolytic Streptococci.
- It helps in differentiating Staphylococcus from Micrococcus in routine work.
- It supports clinical diagnosis of streptococcal infections and helps in early treatment decisions.
- It is considered one of the most convenient techniques for routine clinical laboratories.
- When used with SXT disk, it improves diagnostic accuracy by excluding non-Group A or B Streptococci.
- Aryal, S. (2022, August 10). Bacitracin susceptibility test – Principle, procedure, uses and interpretation. Microbiology Info. https://microbiologyinfo.com/bacitracin-susceptibility-test/
- Baker, J. S., Hackett, M. F., & Simard, D. J. (1986). Variations in bacitracin susceptibility observed in Staphylococcus and Micrococcus species. Journal of Clinical Microbiology, 23(5), 963–964. https://doi.org/10.1128/jcm.23.5.963-964.1986
- Bernard, R., El Ghachi, M., Mengin-Lecreulx, D., Chippaux, M., & Denizot, F. (2005). BcrC from Bacillus subtilis acts as an undecaprenyl pyrophosphate phosphatase in bacitracin resistance. Journal of Biological Chemistry, 280(32), 28852–28857. [Source from ResearchGate snippet].
- Buijs, N. P., Vlaming, H. C., Kotsogianni, I., Arts, M., Willemse, J., Duan, Y., Alexander, F. M., Cochrane, S. A., Schneider, T., & Martin, N. I. (2024). A classic antibiotic reimagined: Rationally designed bacitracin variants exhibit potent activity against vancomycin-resistant pathogens. Proceedings of the National Academy of Sciences, 121(29), e2315310121. https://doi.org/10.1073/pnas.2315310121
- Dalynn Biologicals. (2014). Bacitracin disks [Catalogue No. DB10]. https://www.dalynn.com/dyn/ck_assets/files/tech/DB10.pdf
- Hardy Diagnostics. (2013). HardyDisk™ bacitracin differentiation disks [Instructions for Use]. https://hardydiagnostics.com/
- HiMedia Laboratories. (2023). Bacitracin (50 discs/vl) [Technical Data, Code: DD015].
Kingston, A. W., Zhao, H., Cook, G. M., & Helmann, J. D. (2014). Accumulation of heptaprenyl diphosphate sensitizes Bacillus subtilis to bacitracin: Implications for the mechanism of resistance mediated by the BceAB transporter. Molecular Microbiology, 93(1), 37–49. https://doi.org/10.1111/mmi.12637 - Kurzynski, T., Meise, C., Daggs, R., & Helstad, A. (1979). Improved reliability of the primary plate bacitracin test on throat cultures with sulfamethoxazole-trimethoprim blood agar plates. Journal of Clinical Microbiology, 9(1), 144–146. https://doi.org/10.1128/jcm.9.1.144-146.1979
- Mast Group. (n.d.). MASTDISCS® ID bacitracin discs [Product Instructions].
- McKesson Medical-Surgical. (n.d.). Differentiation disc BBL™ Taxo™ A bacitracin 0.04 U. Retrieved from McKesson Medical-Surgical website.
- Merck KGaA. (2018). 08382 Bacitracin disks [Product Specification Sheet]. Sigma-Aldrich.
Micromaster Laboratories. (2018). Bacitracin (0.04 Units) (ID001) [Product Specification Sheet].
NewYork-Presbyterian. (2017, February 6). It happened here: Bacitracin. Health Matters. https://healthmatters.nyp.org/bacitracin-discovery/ - Nguyen, R., Khanna, N. R., Safadi, A. O., Patel, P., & Sun, Y. (2024, June 8). Bacitracin topical. In StatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK536993/
- Patsnap Synapse. (2024, July 18). What is the mechanism of bacitracin?
- Pokhrel, P. (2015, September 30). Bacitracin test- principle, procedure, result with limitation. Microbiology Notes. https://microbiologynotes.com/bacitracin-test-principle-procedure-result-with-limitation/
- Public Health England. (2021). UK standards for microbiology investigations: Identification of Streptococcus species, Enterococcus species and morphologically similar organisms (Issue 4).
Radeck, J., Lautenschläger, N., & Mascher, T. (2017). The essential UPP phosphatase pair BcrC and UppP connects cell wall homeostasis during growth and sporulation with cell envelope stress response in Bacillus subtilis. Frontiers in Microbiology, 8, 2403. https://doi.org/10.3389/fmicb.2017.02403 - Sapkota, A. (2022, January 27). Bacitracin susceptibility test- principle, procedure, results, uses. Microbe Notes. https://microbenotes.com/bacitracin-susceptibility-test-principle-procedure-and-result-interpretation/
- Schalock, P. C., & Zug, K. A. (2005). Bacitracin. Cutis, 76(2), 105–107.
- Sigma-Aldrich. (n.d.). Inhibition of cell wall biosynthesis by antibiotics. Retrieved from Sigma-Aldrich website.
- Smith, P. A. (2017, June). One girl’s mishap led to the creation of the antibiotic bacitracin. Smithsonian Magazine.
- Spellerberg, B., & Brandt, C. (2016). Laboratory diagnosis of Streptococcus pyogenes (group A streptococci). In J. J. Ferretti, D. L. Stevens, & V. A. Fischetti (Eds.), Streptococcus pyogenes: Basic biology to clinical manifestations [Internet]. University of Oklahoma Health Sciences Center. https://www.ncbi.nlm.nih.gov/books/NBK343617/
- VUMIE. (2022, June 5). Bacitracin susceptibility test. Virtual Microbiology Lab Simulator Software. https://vumicro.com/docs/bacitracin-susceptibility-test/
- Wikipedia. (2024). Bacitracin. Retrieved from Wikipedia. https://en.wikipedia.org/wiki/Bacitracin
- Williams, G. S. (2003). Group C and G streptococci infections: Emerging challenges. Clinical Laboratory Science, 16(4), 209.