What are B cells or B lymphocytes?
- B cells, also known as B lymphocytes, are a crucial component of the immune system, specifically the adaptive immune response. These specialized white blood cells play a vital role in humoral immunity, which involves the production of antibodies to defend against pathogens.
- The development of B cells begins during fetal life in the liver and continues in the bone marrow after birth. They originate from hematopoietic stem cells and undergo various stages of differentiation. Throughout this process, B cells acquire two important characteristics of adaptive immunity: the ability to discriminate between self and non-self antigens and the capacity for memory.
- The name “B cell” originated from experiments conducted on chickens in the 1960s. Max Cooper discovered that the production of antibodies in chickens required a specific organ called the bursa of Fabricius. When the bursa was surgically removed, antibody production was inhibited. The cells responsible for antibody production were named bursa-derived or B cells. In humans, B-cell development predominantly occurs in the bone marrow. Differentiation pathways of B cells can be identified through specific surface markers known as CD markers and immunoglobulin gene arrangements. Throughout the development process, there are checkpoints to ensure the cells follow the normal pathway and prevent alternative pathways that would result in cell death.
- B cells possess B cell receptors (BCRs) on their cell membranes, distinguishing them from other lymphocytes such as T cells and natural killer cells. BCRs enable B cells to bind to foreign antigens, initiating an immune response. The specificity of BCRs is remarkable, as all BCRs on a single B cell recognize the same epitope.
- When a naïve or memory B cell is activated by an antigen, it undergoes proliferation and differentiation. It transforms into an antibody-secreting effector cell called a plasmablast or plasma cell. These plasma cells produce and release antibodies, which can be secreted into the bloodstream or inserted into the plasma membrane as part of the B-cell receptors.
- In addition to antibody production, B cells also act as professional antigen-presenting cells (APCs). They can present antigens and secrete cytokines, further contributing to the immune response. B cells play a crucial role in the successful removal of antigens from the body, making them essential for effective immune function.
- While B cells mature in the bone marrow in mammals, in birds, they mature in a specific organ called the bursa of Fabricius. This is where they were initially discovered by Chang and Glick, explaining the origin of the name “B” for bursa.
- Overall, B cells are vital players in the immune system, involved in humoral immunity, antibody production, antigen presentation, and memory response. Their ability to recognize foreign antigens, produce antibodies, and contribute to the immune response makes them indispensable for the body’s defense against pathogens.
Definition of B cell (B lymphocyte)
A B cell, or B lymphocyte, is a type of white blood cell that produces antibodies and plays a crucial role in the immune response against infections.
Morphology of B cell (B lymphocyte)
- The morphology of B cells, or B lymphocytes, can be observed using light microscopy, transmission electron microscopy (TEM), and scanning electron microscopy (SEM).
- Under light microscopy, B lymphocytes appear as spherical or ovoid cells with sizes ranging from 6 to 15 μm. Staining techniques such as Giemsa or Wright stains are commonly used to enhance the visibility of lymphocytes on glass slides. There are two main groups of lymphocytes observed: giant cells with diameters of 9 to 15 μm and smaller lymphocytes with diameters of 6 to 9 μm. Lymphocytes appear as dark purple cells with a deep blue nucleus and pale azure cytoplasm. The nucleus occupies a significant portion of the cellular interior due to the presence of condensed chromatin.
- Transmission electron microscopy provides a closer examination of the internal structures of B cells. The nucleus of B lymphocytes under TEM displays electron-dense heterochromatin, characteristic of non-dividing cells. The nucleoli within the nucleus appear round in section. Lymphocytes are organized into three concentric zones: the central region (agranular zone), the intermediate region (fibrillar region), and the outer region (granular zone) consisting of intranuclear chromatin. The cytoplasmic organelles of B cells are typical of eukaryotic cells.
- Scanning electron microscopy allows for a three-dimensional view of B cells, although the resolution is lower compared to TEM. B cells prepared for SEM observation are cleaned, collected on silver membranes, and preserved with glutaraldehyde. B cells observed under SEM have diameters ranging from 5.1 to 6.4 μm. They are characterized by an intricate surface architecture, with numerous finger-like microvilli covering the entire surface.
- In summary, the morphology of B cells can be observed through different microscopy techniques. Light microscopy provides an overview of their size and general appearance, while TEM and SEM allow for closer examination of the internal structures and surface features of B lymphocytes, respectively.
Structure of B cell (B lymphocyte)
Stage-specific Markers
- B cells, throughout their development, maturation, and activation, exhibit stage-specific markers that can be used to identify and characterize different stages of the B cell lineage.
- CD10, also known as common acute lymphoblastic leukemia antigen (CALLA), is a stage-specific marker expressed on early B cells, including pro-B cells, pre-B cells, and germinal center cells. Its expression helps identify these early stages of B cell development.
- CD19 and CD20 are markers that are expressed on all cells of the B cell lineage, excluding plasma cells. CD19 is a reliable marker for B cells and is widely used in immunophenotyping to identify B cell populations. CD20 is another B cell-specific marker commonly used for diagnostic purposes and therapeutic targeting.
- CD27 is an important marker that is exclusively expressed on memory B cells and plasma cells. It is often used to identify and distinguish these specialized B cell populations involved in long-term immune memory and antibody production.
- B-1 cells, a subset of B cells with distinct characteristics, are characterized by the expression of CD5. CD5 is a marker commonly associated with T cells but is also present on B-1 cells, helping to distinguish them from conventional B cells.
- By analyzing the expression of these stage-specific markers, researchers and clinicians can gain insights into the development, maturation, and activation status of B cells. These markers provide valuable information about the differentiation and functional properties of B cell populations, aiding in the understanding and diagnosis of various immune-related disorders and diseases.
Antigen-binding Molecules (membrane immunoglobulin)
- Antigen-binding molecules, also known as membrane immunoglobulins, are an essential component of the B cell antigen receptor complex. The B cell receptor (BCR) is a multimolecular protein complex located on the surface of B cells and is responsible for recognizing and binding to specific antigens.
- The BCR complex consists of several proteins that work together to transmit signals and activate B cells. The core components of the BCR are the membrane-bound immunoglobulin receptor (mIg), Ig-alpha (CD79a), and Ig-beta (CD79b). The mIg serves as the antigen-binding molecule and is a transmembrane form of immunoglobulin (antibody) that extends from the cell surface into the cytoplasm. It is responsible for directly binding to antigens and initiating the signaling cascade.
- Ig-alpha and Ig-beta are signal transduction molecules that are noncovalently bound to mIg as a disulfide-linked heterodimeric complex. They contain a sequence called the immune receptor tyrosine-based activation motif (ITAM), which is crucial for transmitting signals from the cell surface to the cytoplasm. During B cell activation, the ITAMs in Ig-alpha and Ig-beta become phosphorylated by protein tyrosine kinases (PTKs), enabling them to interact with cytoplasmic signaling proteins and initiate intracellular signaling pathways.
- The genetic arrangement and diversity of B cell receptors allow for the recognition of a wide range of antigens. B cell receptors are inherited as gene fragments, and during B cell development, these fragments are joined differently in each cell, resulting in a diverse array of receptors. It is estimated that an individual can generate up to 10^11 different antibodies, theoretically providing enough B cell receptor diversity to recognize virtually any microbe. Additionally, B cell receptors undergo a process called somatic hypermutation, which introduces further variations and creates unique receptors with enhanced affinity for antigens.
- In summary, antigen-binding molecules, or membrane immunoglobulins, are an integral part of the B cell antigen receptor complex. The complex consists of mIg, Ig-alpha, and Ig-beta, which work together to recognize antigens and initiate signaling pathways for B cell activation. The genetic diversity and somatic hypermutation of B cell receptors contribute to the vast repertoire of antigen recognition by B cells.
Co-receptor Molecules of B Cells
- Co-receptor molecules play a crucial role in enhancing the signaling and activation of B cells in response to antigens. These co-receptors, which are clusters of proteins, work in conjunction with the B cell receptor (BCR) to amplify the signaling process by up to a thousand-fold.
- The co-receptor molecules of B cells include CD21 (complement receptor 2), CD19, CD81, and CD225. These proteins are located in close proximity to the BCR on the cell membrane but are not part of the BCR itself. When activated, the co-receptor proteins become phosphorylated along with the Ig-alpha and Ig-beta subunits of the BCR, leading to the amplification of activation signals from the cell surface to the cytoplasm.
- One of the key functions of co-receptors is to reduce the stimulation threshold of the BCR. This means that fewer antigens are required to initiate BCR stimulation and subsequent B cell activation. The co-receptors effectively lower the activation threshold, allowing for a more sensitive and efficient response to antigens.
- The significance of co-receptor function is particularly evident when microbial pathogens activate the complement system. When antigens bind to complement protein C3d, this complex can bind simultaneously to CD21 and the BCR. This interaction enables the co-receptor complex to cluster and cross-link with the BCR, leading to the phosphorylation of the CD19 tail. This clustering and phosphorylation process results in an increased concentration of signaling molecules around the BCR, further enhancing the activation signals and promoting a robust B cell response.
- In summary, co-receptor molecules such as CD21, CD19, CD81, and CD225 function alongside the BCR to enhance B cell signaling and activation. They lower the threshold for BCR stimulation and amplify activation signals from the cell surface to the cytoplasm. Co-receptors play a critical role in optimizing B cell responses to antigens and are particularly effective when antigens activate the complement system, leading to enhanced clustering and phosphorylation of the co-receptor complex.
Signal Transduction Molecules (Molecule involved in T-B cells interaction)
Signal transduction molecules play a vital role in the interaction between T cells and B cells during T-cell-dependent B cell activation. Several molecules are involved in this process:
- Major histocompatibility complex class II (MHC II) molecules: These molecules are expressed on the surface of B cells (excluding pro-B cells) and play a crucial role in presenting peptides derived from T-cell-dependent protein antigens to helper T cells (CD4+). MHC II molecules are essential for antigen recognition and activation of T-helper cells, which in turn provide signals for B cell activation.
- Co-stimulatory molecules: Co-stimulatory molecules provide a second signal alongside antigen binding to facilitate effective T-B cell interaction and activation. These molecules are expressed in high quantities on activated cells compared to naive B cells. Two well-known co-stimulatory molecules are B7 and CD40.
- B7: B7 is a family of molecules that interact with CD28 on the surface of T cells. This interaction provides a co-stimulatory signal necessary for T cell activation and optimal B cell response.
- CD40: CD40 is a molecule expressed on the surface of B cells, and it interacts with CD40 ligand (CD40L or CD154) on activated T cells. This interaction is crucial for processes such as somatic hypermutation and class switch, which are important for generating diverse antibody responses. CD40-CD40L interaction is also involved in germinal center formation, a specialized microenvironment where B cell maturation and antibody affinity maturation occur.
- Inducible co-stimulatory ligand (ICOSL): ICOSL is expressed on B cells and interacts with ICOS (inducible co-stimulator) on activated T cells. This interaction is critical for germinal center formation and the development of effective B cell responses. Deficiencies in ICOS or ICOSL result in impaired production of IgG, IgA, and IgE antibodies.
- Cytokine receptors: Cytokines produced by activated CD4+ T lymphocytes play a crucial role in mediating B cell responses to protein antigens. The CD40 ligand on the surface of T-helper cells interacts with the CD40 molecule on B cells, facilitating B cell development into antibody-secreting plasma cells. This interaction, along with cytokine signaling, promotes B cell proliferation, differentiation, and antibody production.
In summary, signal transduction molecules, including MHC II, co-stimulatory molecules (such as B7, CD40, and ICOSL), and cytokine receptors, are involved in T-B cell interactions during T-cell-dependent B cell activation. These interactions are necessary for effective antigen recognition, T cell help, and the development of antibody-secreting plasma cells.
Origin of B cells
- The genesis of antibody production is explained by the hypothesis of clonal selection. According to this hypothesis, each immunologically competent B cell includes a receptor for either IgM or IgD that can combine with a single antigen or antigens that are closely related.
- Following antigen binding, the B cell is stimulated to grow and generate a clone. Certain B cells are converted into plasma cells that produce antigen-specific antibodies.
- Plasma cells produce immunoglobulins with the same antigenic specificity as B cells that have been activated. T cells experience the same clonal selection.
- During development, B cell precursors first multiply and grow in the foetal liver. From there, they travel to the bone marrow, the primary maturation destination for adult B-cells.
- They do not require the thymus for maturation, unlike T cells. Only heavy chains are present in the cytoplasm of Pre-B cells, which lack surface immunoglobulins and light chains.
- Pre-B cells are located in the bone marrow, whereas B cells are located in the bloodstream. B cells mature in two phases:
- The antigen-independent phase, which comprises of stem cells and pre-B cells, and the antigen-dependent phase.
- Antigen-dependent phase, which consists of activated B cells and plasma cells that proliferate in response to antigen-B cell interactions.
- B cells have surface IgM, which functions as an antigen receptor. Some B lymphocytes may also carry IgD on their surface as an antigen receptor.
- On the surface of B cells are expressed numerous other molecules with various roles. Among them are B220, molecules of class II MHC, CR1 and CR2, CD40, and others.
Effector functions of B cells
- Activation of B cells results in the production of many plasma cells. The plasma cells then create an abundance of immunoglobulins specific to the antigen’s epitope.
- Some activated B cells generate memory cells that persist in a quiescent state for months or years.
- Surface IgG serves as the antigen receptor on the majority of memory B cells, but some also express surface IgM.
- These latent memory cells are rapidly triggered upon antigen reexposure. Memory T cells generate interleukins that enhance memory B cell antibody synthesis.
- These cells are responsible for the fast emergence of antibodies during secondary immune reactions.
Types of B cell or B lymphocyte
1. Transitional B cells
- Transitional B cells serve as an intermediate stage in the development of B lymphocytes, bridging the gap between immature B cells in the bone marrow and mature B cells in the lymphoid organs. These cells are derived from myeloid progenitor cells in the bone marrow but have not reached full maturation.
- Transitional B cells can be found in various locations, including the bone marrow, peripheral blood, and spleen. However, only a small fraction of immature B cells successfully navigate the transitional phase and progress to become matured B cells.
- Upon leaving the bone marrow, transitional B cells undergo several checkpoints to ensure that they do not produce autoantibodies, which could potentially lead to autoimmune reactions. These checks help maintain immune tolerance and prevent the development of harmful self-reactive antibodies.
- Transitional B cells can be further categorized into two stages: T1 and T2. The T1 stage represents the period from the migration of cells from the bone marrow to their entry into the spleen. Once within the spleen, the cells enter the T2 stage, where they undergo further development and maturation into mature B cells.
- It is worth noting that only a small percentage of immature B cells successfully complete the transitional phase and mature into functional B cells. The exact mechanisms regulating this process and the factors influencing the survival and maturation of transitional B cells are still being studied. However, it is believed that an excessive number of transitional B cells may contribute to the development of autoimmune disorders such as lupus erythematosus and rheumatoid arthritis, although further research is needed to fully understand these associations.
2. Naïve B cells
- Naïve B cells refer to mature B cells that have not yet encountered specific antigens. They are at a stage of differentiation where they have the potential to develop into plasma cells or memory B cells upon exposure to a specific antigen.
- These naïve B cells can be found primarily in the secondary lymphoid organs, such as the lymph nodes and spleen, after they have completed the transitional stage of B cell development. During the transitional stage, B cells undergo maturation and selection processes to ensure their functionality and tolerance.
- Once naïve B cells encounter an antigen that matches their specific B cell receptor (BCR), they can be activated and differentiate into plasma cells. Plasma cells are responsible for producing large quantities of antibodies that target and neutralize the specific antigen. Alternatively, naïve B cells can differentiate into memory B cells, which are long-lived cells that retain the ability to respond rapidly and robustly upon re-exposure to the same antigen in the future.
- In addition to their role in antibody production, naïve B cells have recently been recognized as a distinct subcategory called regulatory B cells or Breg cells. These cells work in coordination with naïve T cells to regulate the immune response. Breg cells play a role in suppressing excessive immune activation and promoting immune tolerance. They have been shown to have immunosuppressive functions and can modulate the activities of other immune cells, including T cells.
- Overall, naïve B cells are an essential component of the adaptive immune system, representing a pool of mature B cells ready to respond to specific antigens. Their differentiation into plasma cells or memory cells, as well as their newly recognized role as regulatory B cells, contribute to the immune response and maintenance of immune homeostasis.
3. Plasma Cells
- Plasma cells, also known as plasma B cells or effector B cells, are a type of white blood cell that plays a crucial role in the immune response. They are derived from activated naïve B cells and are responsible for the production and secretion of large quantities of antibodies, also known as immunoglobulins (Igs), in response to the presence of antigens.
- The differentiation of naïve B cells into plasma cells requires interaction with helper T cells. When a naïve B cell encounters an antigen that matches its specific B cell receptor (BCR), the antigen is internalized and presented to a helper T cell. This interaction leads to the activation of the helper T cell, which, in turn, activates the B cell. The activated B cell undergoes a series of changes, including differentiation into plasma cells.
- Plasma cells can also be generated through a process called T-cell independent antigen stimulation. In this case, the B cell can be directly activated by certain antigens without the need for T cell involvement. However, the antibodies produced by plasma cells generated through this pathway are predominantly of the IgM class.
- Each plasma cell is dedicated to producing antibodies specific to a particular antigen encountered during the activation process. This specificity is determined by the initial antigen presentation to the naïve B cell by the helper T cell. Therefore, a single plasma cell can only secrete antibodies of one specific type.
- Plasma cells have a relatively short lifespan compared to memory B cells. They circulate through the body, guided by the distribution of cytokines, to produce antibodies at sites of infection or inflammation. Their abundance of cytoplasm and characteristic eccentric nucleus, along with their larger size, distinguish them morphologically from other B cell types.
- Surface antigen expression on plasma cells differs from that of naïve B cells. They express fewer surface antigens such as CD27++ but do not express CD19 and CD20, which are present on naïve B cells. This altered antigen profile reflects their differentiation and specialization as antibody-secreting cells.
- In summary, plasma cells are specialized effector B cells that produce and secrete antibodies in response to antigen activation. They play a vital role in the immune response by providing immediate protection against pathogens and aiding in the elimination of infections.
4. Memory cells
- Memory cells are a specialized type of lymphocytes, specifically B cells, that play a crucial role in the immune system’s long-term defense against pathogens. They are formed during the maturation process and are derived from naïve B cells, which have been exposed to antigens.
- Unlike naïve B cells, which circulate through the bloodstream in a stationary phase, memory B cells have the ability to move freely throughout the bloodstream for extended periods, sometimes lasting for years. They are distributed throughout the body, including lymphoid organs, mucosal tissues, and peripheral blood.
- The exact factors that trigger the differentiation of naïve B cells into memory cells are not fully understood. However, it is known that memory B cells are generated during the immune response to an infection or vaccination. These cells possess an affinity for the specific antigen encountered by their parent B cell.
- The primary function of memory B cells is to “remember” the characteristics of the antigen that activated their parent B cell. This immunological memory allows memory B cells to mount a rapid and robust secondary immune response if they encounter the same antigen again. The secondary response is quicker and more potent compared to the primary response, resulting in the production of a higher quantity of specific antibodies.
- The development of memory B cells occurs primarily within the germinal centers of lymphoid organs. This differentiation can be driven by either a T cell-dependent mechanism or a T cell-independent mechanism, depending on the nature of the antigen and the immune response it elicits.
- After differentiation, memory B cells may remain concentrated in specific areas of the body where they are more likely to encounter the antigens to which they have memory. This localization enhances their ability to detect and respond to re-infection or re-exposure to the specific antigen.
- In summary, memory B cells are a specialized population of B lymphocytes that possess a long lifespan and circulate throughout the body. They play a critical role in the secondary immune response by “remembering” previous encounters with specific antigens and mounting a faster and stronger immune response upon re-exposure. Memory B cells contribute to the long-term immunity and protection against pathogens.
5. Plasmablast
- Plasmablasts are a type of short-lived, proliferating cell that plays a crucial role in the early stages of an immune response. They are generated from the differentiation of B cells and are responsible for producing and secreting antibodies.
- Plasmablasts arise early in the course of an infection or immune response. They are often formed in large numbers and rapidly proliferate to amplify the immune response. Unlike long-lived plasma cells, which are derived from plasmablasts and reside in the bone marrow or other specialized niches, plasmablasts have a shorter lifespan.
- The antibodies produced by plasmablasts generally have a weaker affinity towards their target antigen compared to the antibodies produced by fully differentiated plasma cells. This is because plasmablasts are generated early in the immune response when the immune system is still encountering and adapting to the specific antigen.
- Plasmablasts can be generated through different mechanisms. They can result from T cell-independent activation of B cells, which occurs when B cells directly recognize certain antigens without the need for T cell help. Alternatively, plasmablasts can be generated through the extrafollicular response from T cell-dependent activation of B cells. In this case, B cells receive signals from T cells and undergo rapid proliferation and differentiation into plasmablasts.
- Overall, plasmablasts play a critical role in the initial stages of an immune response, rapidly producing antibodies to combat invading pathogens. While their antibody affinity may be weaker compared to fully differentiated plasma cells, their early response helps to limit the spread of infection and provide a bridge until more specialized and long-lived plasma cells can develop.
6. Lymphoplasmacytoid cell
- A lymphoplasmacytoid cell is a unique cell type that exhibits morphological features of both B lymphocytes and plasma cells. It is considered to be closely related to or a subtype of plasma cells. These cells are commonly found in pre-malignant and malignant plasma cell dyscrasias, specifically those associated with the secretion of IgM monoclonal proteins.
- Two notable conditions where lymphoplasmacytoid cells are observed are IgM monoclonal gammopathy of undetermined significance (MGUS) and Waldenström’s macroglobulinemia (WM). MGUS is a benign condition characterized by the presence of abnormal IgM monoclonal proteins in the blood without any associated symptoms or organ damage. WM, on the other hand, is a type of non-Hodgkin lymphoma that involves the proliferation of lymphoplasmacytoid cells and the production of excessive amounts of IgM monoclonal protein.
- Lymphoplasmacytoid cells in these dyscrasias typically secrete IgM monoclonal proteins, which contribute to the symptoms and complications associated with the conditions. The presence of these abnormal cells can be detected through laboratory tests and specialized examinations, such as bone marrow biopsy and immunohistochemistry.
- Overall, lymphoplasmacytoid cells represent a distinct cell population with both B lymphocyte and plasma cell characteristics. Their presence in plasma cell dyscrasias, particularly those associated with IgM monoclonal protein secretion, highlights their involvement in the pathogenesis of these diseases. Studying and understanding the behavior of lymphoplasmacytoid cells is crucial for the diagnosis, classification, and management of conditions such as IgM MGUS and Waldenström’s macroglobulinemia.
7. B-2 cell
- B-2 cells, also known as Follicular (FO) B cells, are the most common type of B cells in the body. They are primarily found in the lymphoid follicles of secondary lymphoid organs (SLOs) such as lymph nodes and the spleen. B-2 cells play a crucial role in generating the majority of high-affinity antibodies during an infection.
- These B-2 cells are responsible for the adaptive immune response, where they recognize specific antigens and produce antibodies to neutralize pathogens. Within the lymphoid follicles, B-2 cells undergo clonal expansion and differentiation into plasma cells or memory B cells upon encountering an antigen. Plasma cells secrete large quantities of antibodies, while memory B cells provide long-term immunological memory for future encounters with the same antigen.
- In addition to B-2 cells, there are also Marginal-zone (MZ) B cells. MZ B cells are mainly located in the marginal zone of the spleen, which acts as a first line of defense against blood-borne pathogens. The marginal zone receives a high volume of blood from the general circulation, allowing MZ B cells to quickly respond to pathogens present in the bloodstream.
- MZ B cells can be activated through both T cell-independent and T cell-dependent pathways. T cell-independent activation occurs when the B cell directly recognizes certain antigens, such as those present on the surface of bacteria or viruses. T cell-dependent activation involves the interaction between MZ B cells and helper T cells, which provide additional signals for B cell activation and antibody production.
- Overall, B-2 cells (FO B cells) are the predominant type of B cells responsible for generating high-affinity antibodies during infections. On the other hand, MZ B cells serve as a frontline defense against blood-borne pathogens and can be activated through both T cell-independent and T cell-dependent pathways. Together, these B cell subsets contribute to the diverse and effective immune response against various pathogens.
8. B-1 cell
- B-1 cells are a unique subset of B cells that arise from a developmental pathway distinct from follicular (FO) B cells and marginal-zone (MZ) B cells. In mice, B-1 cells are primarily found in the peritoneal cavity and pleural cavity. They have distinct characteristics and functions compared to other B cell subsets.
- One of the notable features of B-1 cells is their ability to produce natural antibodies, which are antibodies produced without prior infection or immunization. These natural antibodies provide immediate protection against a wide range of pathogens. B-1 cells contribute significantly to the first line of defense against mucosal pathogens.
- B-1 cells exhibit a preference for T cell-independent activation, meaning they can be stimulated to produce antibodies without the need for helper T cells. This activation pathway involves direct recognition of antigens by the B-1 cell receptors. This is in contrast to T cell-dependent activation, which relies on the interaction between B cells and helper T cells to produce a robust immune response.
- It is important to note that a true homologue of mouse B-1 cells has not yet been identified in humans. However, several cell populations with similar characteristics to B-1 cells have been described in humans. These populations share some functional similarities, such as natural antibody production and the ability to respond to certain antigens in a T cell-independent manner.
- Overall, B-1 cells are a distinct subset of B cells that primarily reside in the peritoneal and pleural cavities in mice. They play a critical role in generating natural antibodies, defending against mucosal pathogens, and exhibiting T cell-independent activation. While the direct equivalent of mouse B-1 cells has not been identified in humans, related cell populations with similar characteristics have been observed. Further research is needed to fully understand the functional counterparts of B-1 cells in humans.
9. Regulatory B (Breg) cell
- Regulatory B cells, also known as Breg cells, are a specialized subset of B cells that play a crucial role in immune regulation and maintaining immune tolerance. They possess immunosuppressive functions that help modulate immune responses and prevent excessive inflammation. Breg cells achieve their regulatory effects through various mechanisms.
- One of the key functions of Breg cells is their ability to suppress the expansion and activity of pathogenic and pro-inflammatory lymphocytes. They accomplish this by secreting anti-inflammatory cytokines such as interleukin-10 (IL-10), interleukin-35 (IL-35), and transforming growth factor-beta (TGF-β). These cytokines act locally to inhibit the activation and proliferation of other immune cells, dampening immune responses.
- Additionally, Breg cells promote the generation and function of regulatory T cells (Tregs). They directly interact with T cells, leading to the induction and expansion of Tregs. Tregs are an important subset of immune cells that help maintain immune homeostasis and suppress excessive immune reactions. Breg cells play a role in skewing the differentiation of T cells towards a regulatory phenotype, contributing to immune tolerance.
- It is important to note that Breg cells do not have a single defined identity. Instead, multiple subsets of Breg cells with regulatory functions have been identified in both mice and humans. These subsets may differ in their phenotypic markers and functional characteristics. The exact developmental relationship among these subsets and how B cells differentiate into Breg cells are still not fully understood. Research is ongoing to elucidate the specific mechanisms involved in Breg cell differentiation.
- Furthermore, it has been observed that nearly all types of B cells have the potential to differentiate into Breg cells under certain conditions. Inflammatory signals and the recognition of antigens by the B cell receptor (BCR) are implicated in the differentiation of B cells into Breg cells. This suggests that Breg cell differentiation can occur in response to inflammatory stimuli and immune activation.
- In summary, regulatory B cells (Breg cells) are a diverse group of B cells with immunosuppressive functions. They secrete anti-inflammatory cytokines, suppress the expansion of pro-inflammatory lymphocytes, and promote the generation of regulatory T cells. Breg cells play an important role in maintaining immune tolerance and modulating immune responses. However, further research is needed to fully understand the developmental pathways and functional characteristics of different Breg cell subsets.
B cell (B lymphocyte) Development
The initial stages of B cell development occur in niches, which are complex microenvironments comprised of stromal cells of the bone marrow. The process is triggered by stimuli and factors that initiate a series of cell signals that regulate cell survival, proliferation, and development by modulating the expression of specific target genes.
The development of B cells begins with hematopoietic stem cells, which differentiate into an early lymphoid progenitor and then a common lymphoid progenitor. The absence or suppression of Notch-1 protein signaling in the bone marrow is a prerequisite for the development of B cells. Following the stages of maturation, activation, differentiation, and memory formation, B cells develop globally.
1. Maturation of B cell
- The maturation of B cells is a crucial process that occurs within the bone marrow and leads to the development of mature B cells capable of participating in immune responses. This maturation process involves several stages and is characterized by specific gene expression patterns and rearrangements of immunoglobulin H chain and L chain genes.
- The maturation of B cells begins in the bone marrow, where immature B cells undergo a series of developmental stages. Throughout these stages, B cells generate diverse B cell receptors (BCRs) as part of the selection process. The BCRs are essential for antigen recognition and subsequent immune responses.
- The selection of B cells occurs through two mechanisms: positive selection and negative selection. Positive selection is an antigen-independent signaling process, where B cells that fail to bind to their ligands do not receive the necessary signals for further development. On the other hand, negative selection occurs when self-antigens bind strongly to the BCR. In such cases, the development of B cells is halted to prevent the production of autoreactive cells.
- Once the maturation process is complete, immature B cells migrate from the bone marrow to the spleen as transitional B cells. This migration occurs in two stages known as T1 and T2. Initially, the cells are referred to as T1 B cells during their migration to the spleen. After entering the spleen, they mature into T2 B cells.
- The fate of T2 B cells is determined by the signals received through their receptors. Depending on these signals, T2 B cells can differentiate into two main subsets: follicular B cells and marginal zone B cells. Follicular B cells mainly reside in the lymphoid follicles of secondary lymphoid organs, while marginal zone B cells are found in the marginal zone of the spleen and serve as a first line of defense against blood-borne pathogens.
- Following their differentiation in the spleen, B cells are considered mature B cells or naïve B cells. These mature B cells have completed their maturation process and are capable of recognizing antigens and initiating immune responses upon encountering a specific antigen.
- In summary, the maturation of B cells occurs within the bone marrow and involves distinct stages characterized by gene expression patterns and immunoglobulin gene rearrangements. Positive and negative selection mechanisms ensure the development of functional B cells while preventing the production of autoreactive cells. After migration to the spleen, the B cells undergo further maturation and differentiation, ultimately becoming mature B cells ready to participate in immune responses.
2. Activation of B cell
- The activation of B cells is a crucial step in initiating an immune response and occurs in the secondary lymphoid organs, such as the spleen and lymph nodes. B cells, after maturing in the bone marrow, migrate through the blood to these organs, where they encounter antigens through circulating lymph.
- B cell activation begins when the B cell binds to an antigen via its B cell receptor (BCR). This binding triggers a series of events that lead to B cell activation and the initiation of an immune response. The exact sequence of events immediately following B cell activation is still being studied, but it is believed to follow the kinetic segregation model, initially described in T lymphocytes.
- According to this model, before antigen stimulation, receptors on the B cell membrane diffuse and come into contact with proteins called Lck and CD45 in equal frequency, resulting in a balance between phosphorylation and non-phosphorylation. When the B cell encounters an antigen-presenting cell, such as a dendritic cell, the larger CD45 protein is displaced due to the close proximity between the two cell membranes. This displacement allows for a net phosphorylation of the BCR and the initiation of the signal transduction pathway, which leads to B cell activation.
- B cell subsets differ in their preference for T cell-dependent or T cell-independent activation. Follicular (FO) B cells preferentially undergo T cell-dependent activation, whereas marginal zone (MZ) B cells and B1 B cells preferentially undergo T cell-independent activation.
- The activation of B cells is enhanced through the activity of CD21, a surface receptor that forms a complex with CD19 and CD81, collectively known as the B cell coreceptor complex. When a BCR binds to an antigen tagged with a fragment of the C3 complement protein, CD21 binds to the C3 fragment. This co-ligation of CD21 with the bound BCR leads to the transduction of signals through CD19 and CD81. This signaling cascade lowers the activation threshold of the B cell, enhancing its responsiveness to antigen stimulation.
- In summary, B cell activation occurs in secondary lymphoid organs when B cells encounter antigens through their BCRs. The binding of antigens to the BCR triggers a series of events that lead to B cell activation. The process is further enhanced through the activity of the B cell coreceptor complex, which lowers the activation threshold of the cell. The type of B cell activation, either T cell-dependent or T cell-independent, depends on the B cell subset involved.
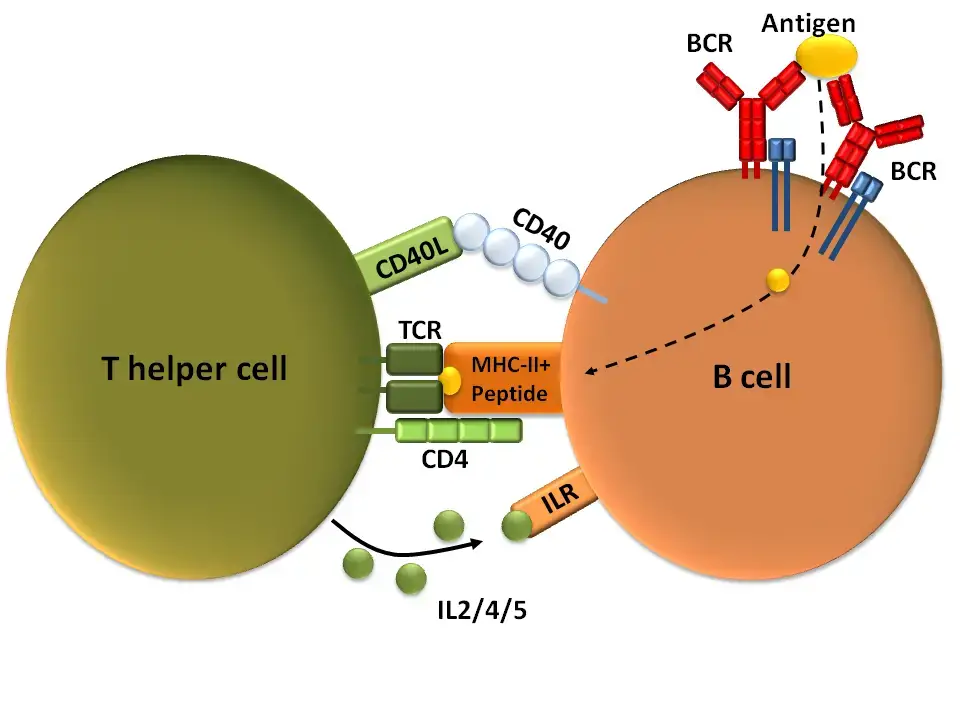
a. T cell-dependent activation
T cell-dependent activation is a process by which B cells are activated in response to antigens with the help of T cells. This type of activation is important for generating a robust and highly specific immune response. Here are the key steps involved in T cell-dependent B cell activation:
- B cell recognition: B cells initially bind to the antigen through their immunoglobulin (Ig) receptors. Some of the antigens are internalized by the B cells into specialized vesicles.
- Antigen presentation: The internalized antigens are processed within the B cells, and the resulting antigenic peptides are presented on the surface of the B cell bound to class II major histocompatibility complex (MHC) molecules.
- T cell recognition: T cells that have encountered antigen-presenting cells, such as dendritic cells, can now recognize and bind to the antigenic peptides presented by the B cells. This interaction is mediated by the T cell receptor (TCR) on the T cell surface.
- Co-stimulation and cytokine signaling: The binding of T cells to the antigen-presenting B cells is further enhanced by interaction between accessory molecules on the surfaces of the T and B cells. This interaction includes the engagement of CD40L on T cells with CD40 on B cells. T cells also release cytokines, such as interleukin-4 (IL-4) and interleukin-21 (IL-21), which play important roles in B cell activation and differentiation.
- B cell activation: The engagement of CD40 by CD40L provides co-stimulatory signals that promote B cell proliferation, immunoglobulin class switching, and somatic hypermutation. Additionally, the cytokines released by T cells bind to cytokine receptors on B cells, further stimulating their proliferation and differentiation.
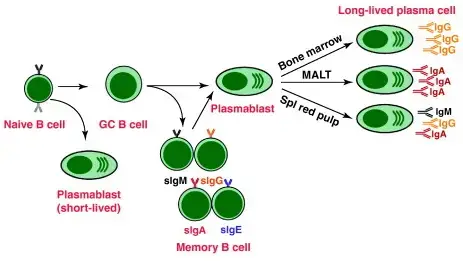
- Differentiation and antibody production: Activated B cells can follow two pathways. In the extrafollicular response, activated B cells proliferate and differentiate into plasmablasts, which produce early, weak antibodies, primarily of the IgM class. In the second step, activated B cells enter lymphoid follicles and form specialized microenvironments called germinal centers (GCs). Within GCs, B cells undergo extensive proliferation, immunoglobulin class switching, and affinity maturation through somatic hypermutation. This process is facilitated by follicular T helper (TFH) cells. The result is the generation of high-affinity memory B cells and long-lived plasma cells.
- Plasma cell differentiation and migration: Plasma cells, which are terminally differentiated B cells, secrete large quantities of antibodies. They can either remain within the secondary lymphoid organs or preferentially migrate to the bone marrow, where they continue to produce antibodies.
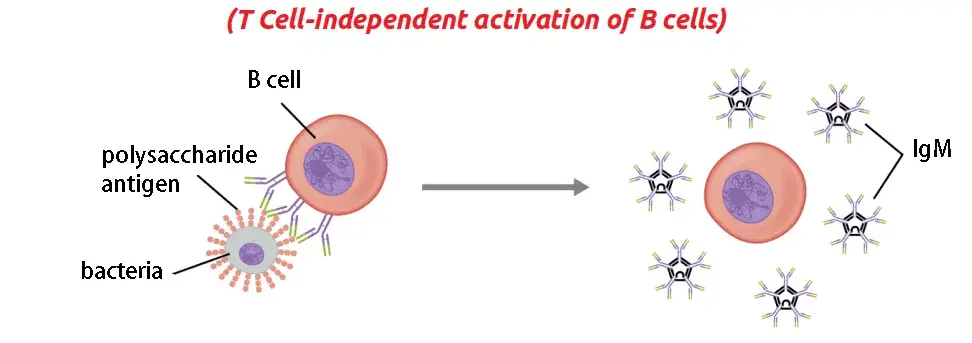
b. T cell-independent activation
T cell-independent activation refers to the activation of B cells without the direct involvement of T cells. This pathway leads to the generation of a specific subset of B cells that can respond with antibody production to certain classes of antigens. Here are the key points about T cell-independent activation:
- Antigen characteristics: T cell-independent antigens, also known as TI antigens, tend to be polyvalent with repeating determinants shared among many microbial species. They include foreign polysaccharides and unmethylated CpG DNA. These antigens can elicit a humoral response even in organisms lacking functional T cells.
- Signal requirements: B cells activated by T cell-independent antigens require additional signals for complete activation. These signals can be provided through two mechanisms. First, recognition and binding of a common microbial constituent to toll-like receptors (TLRs) on B cells can trigger activation. Second, extensive cross-linking of B cell receptor (BCR) epitopes to the surfaces of bacterial or viral particles can also induce activation.
- B cell response: Upon activation by T cell-independent antigens, B cells proliferate outside the lymphoid follicles but still within the secondary lymphoid organs (SLOs). Unlike in T cell-dependent activation, germinal centers (GCs) do not form in this pathway. However, B cells may still undergo immunoglobulin class switching, leading to the production of antibodies of different classes. The activated B cells differentiate into short-lived plasmablasts, which predominantly produce early, weak antibodies of the IgM class. Some populations of long-lived plasma cells may also be generated.
- Rapid but less versatile response: The response to T cell-independent antigens is usually rapid compared to T cell-dependent activation. However, the antibodies generated in this process have lower affinity and are less functionally versatile than those generated through T cell-dependent activation.
c. Memory B cell activation
Memory B cell activation is a crucial process that occurs when memory B cells encounter their target antigen. Here are the key points about memory B cell activation:
- Antigen recognition: Memory B cell activation begins with the detection and binding of their specific target antigen, which is shared by their parent B cell. The memory B cells retain the ability to recognize and bind the antigen that they encountered during the primary immune response.
- T cell-independent and T cell-dependent activation: Some memory B cells can be activated without the assistance of T cells, particularly certain virus-specific memory B cells. However, other memory B cells require T cell help for activation.
- Antigen processing and presentation: Upon binding to the antigen, the memory B cell internalizes the antigen through receptor-mediated endocytosis. The antigen is then degraded, and peptide fragments are presented on the cell membrane in complex with MHC-II molecules.
- Interaction with memory T cells: Memory T helper (TH) cells, typically memory follicular T helper (TFH) cells, that were derived from T cells previously activated with the same antigen recognize and bind the MHC-II-peptide complexes presented by the memory B cell. This interaction occurs through the T cell receptor (TCR) on the memory TFH cells.
- Activation and differentiation: Upon TCR-MHC-II-peptide binding and the relay of other signals from the memory TFH cell, the memory B cell is activated. The activated memory B cell can undergo two pathways of differentiation. In the extrafollicular response, it differentiates into plasmablasts and plasma cells, leading to the production of antibodies. Alternatively, the memory B cell can enter a germinal center reaction, where it generates plasma cells and additional memory B cells. It is uncertain whether further affinity maturation occurs within these secondary germinal centers.
- In vitro activation: In laboratory settings, memory B cells can be activated through stimulation with various activators such as pokeweed mitogen or anti-CD40 monoclonal antibodies. However, a study found that a combination of R-848 (a synthetic molecule that mimics viral RNA) and recombinant human IL-2 (interleukin-2) is the most efficient activator for memory B cells.
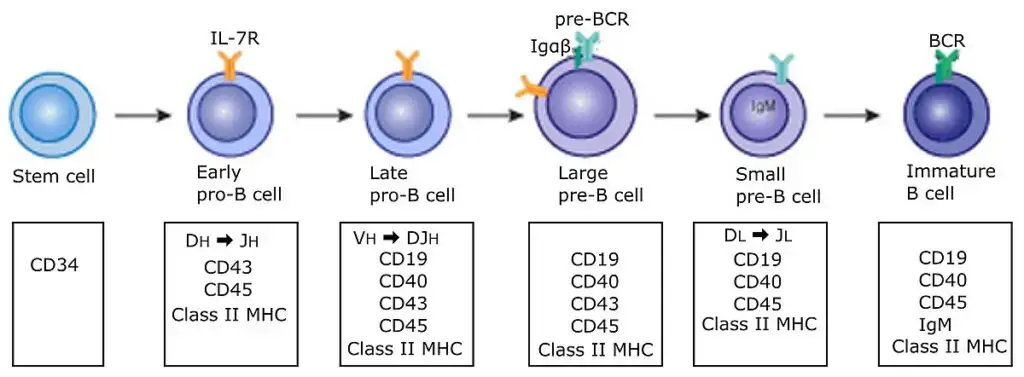
3. Differentiation of B cell
The differentiation of B cells occurs in response to the stimulation of B cell receptors by specific antigens. Here are the key points about the differentiation of B cells:
- Activation and primary foci: Activated B cells, upon encountering their specific antigens, undergo stimulation and start the process of differentiation. Some of these activated cells migrate to regions called primary foci, located at the border of the T cell and B cell areas.
- Differentiation into plasma cells: Within the primary foci, the activated B cells undergo differentiation into plasma cells. This differentiation process typically takes around four days post-stimulation. Plasma cells are specialized cells that produce and secrete large quantities of antibodies.
- Migration and antibody secretion: Once differentiated into plasma cells, these cells migrate to the medullary cord regions of the lymph nodes. In these regions, they continue to secrete significant amounts of antibodies, which are crucial for immune defense against the specific antigens encountered.
- Fate of plasma cells: Following differentiation, some of the plasma cells generated during the primary immune response undergo cell death. However, others can persist in the bone marrow or the gut as long-lived plasma cells, continuously producing antibodies and providing long-term immunity.
- Germinal center formation: In some cases, antigen-stimulated B cells do not enter the primary foci. Instead, they migrate to follicles within lymph nodes or the spleen. As these B cells initiate differentiation, the follicles start to enlarge, forming specialized microenvironments called germinal centers. Germinal centers are characterized by the presence of antigen-specific lymphocytes and play a crucial role in the generation of high-affinity antibodies and the development of immunological memory.
- Memory B cells: At the end of the immune response, memory B cells persist. These memory B cells are the daughter cells of the initially stimulated B cells. They possess an enhanced ability to recognize and respond rapidly to the specific antigen encountered during the primary immune response. Memory B cells contribute to the establishment of immunological memory, providing a faster and more robust secondary immune response upon re-exposure to the antigen.
Regulation of B Cell Development
- Cell-cell interactions and released signals allow bone marrow stromal cells to communicate with progenitor cells. This microenvironment in bone marrow is responsible for B cell development.
- One set of CAMs implicated in the formation of both B and T cells consists of SCF (stem cell factor) on the membrane of stromal cells and kit (CD117) on the membrane of lymphocytes.
- IL-7, which is released by stromal cells and binds to IL-7R on growing lymphocytes, is a crucial cytokine for the development of both B and T cells.
- Signals from these binding events start cytoplasmic cascades that affect the expression of developmentally essential proteins.
- As the B cells mature in the bone marrow, they migrate from the exterior to the interior.
- In the growing B cell, somatic recombination can be either productive (resulting in the synthesis of a functioning H or L chain) or nonproductive due to the inclusion of a stop codon due to frame shift mutations.
- Failure to make productive rearrangements and express Ig at the proper moments during development leads to cell death. B cells have two opportunities to rearrange H chain (maternal and paternal chromosomes) and four opportunities to rearrange L chain (maternal and paternal chromosomes) (paternal and maternal k and l loci).
- Typically, human B cells rearrange DH and JH regions on both chromosomes concurrently. Additionally, DH can be read in any reading frame, making all D-J rearrangements productive.
- Only around half of growing B cells, according to estimates, undergo productive H chain rearrangements. These successful pre-B cells proliferate to produce B cell clones (the big pre-B cell stage), which are capable of L chain recombination.
- During the tiny pre-B cell stage, the V-J joining of the light chain often happens first for the k chain. If the rearrangement is successful, the k chain is produced and the cell develops into an immature B cell that expresses membrane IgM(k) BCR.
- B cells can retry V-J joining multiple times if the initial attempts fail; this mechanism is known as light chain rescue.
- l genes are rearranged if k genes cannot be successfully rearranged on either chromosome. Successful outcomes result in the formation of IgM(l) BCR. If neither k nor l is productively altered, the bone marrow cell undergoes apoptosis. A fraction of human pre-B cells do not develop into B cells.
- During B cell growth, genes producing proteins necessary for somatic recombination and receptor expression are turned on and off at predetermined intervals. RAG-1, RAG-2, and TdT are exclusively expressed during the early and late pro-B cell and tiny pre-B cell stages, when somatic recombination occurs.
- TdT is frequently turned off before recombinases, hence N nucleotide additions to gene segment join in L chain sequences are less frequent than in H chain sequences. The surrogate L chain, as well as the Ig a and Ig b chains, must be expressed in order for the pre-B receptor to emerge on the cell membrane.
- Signal transduction molecules must be expressed at critical moments; inability to express btk results in Bruton’s X-linked agammaglobulinemia, a human B cell immunodeficiency.
- The regulation of gene expression is dependent on soluble transcription factors that bind to DNA regulatory regions. Promoters are DNA sequences that bind RNA polymerase to trigger the synthesis of messenger RNA (mRNA).
- Enhancers are additional non-coding (intron) DNA sequences that boost the activity of promoters. Splicing of gene segments with looping out of intervening DNA brings promoters and enhancers closer together, thereby increasing the manufacture of messenger RNA (mRNA).
- Also required are tissue-specific enhancers, such as RAG-1 and RAG-2, which recombine only Ig gene segments in developing B cells and only TCR gene segments in developing T cells.
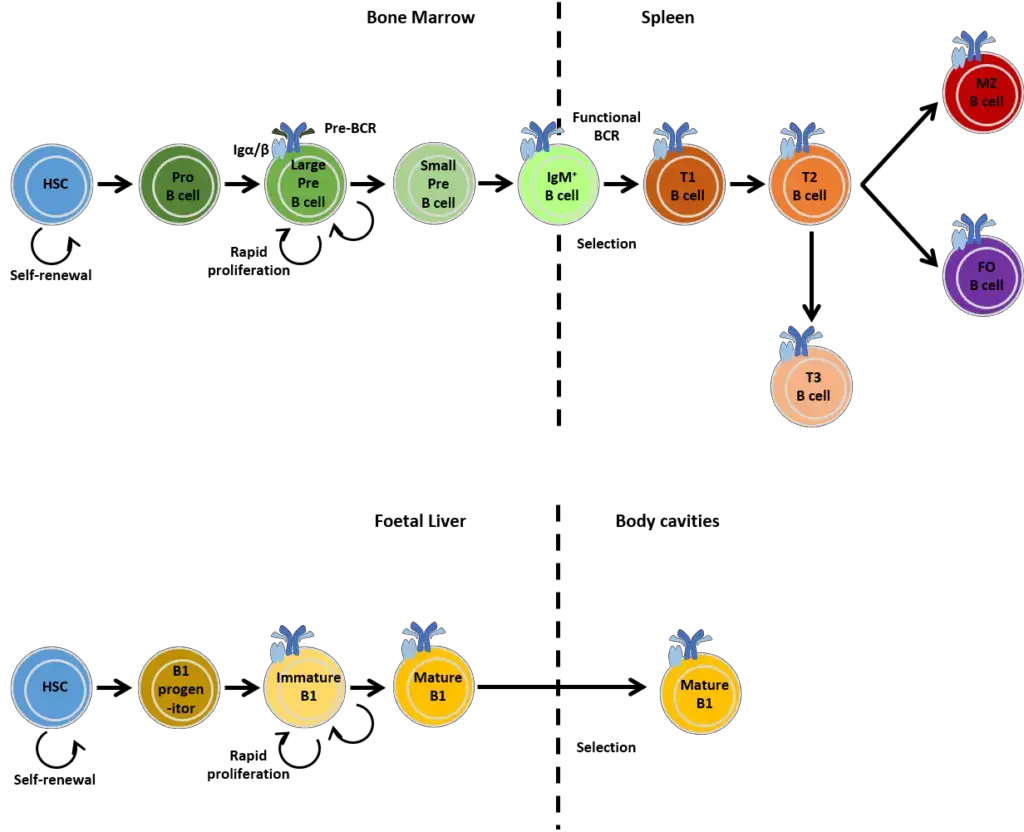
Positive Selection of B Cells
- In the primary lymphoid organs, B and T lymphocytes are both subject to positive and negative selection. Positive selection necessitates antigen receptor-mediated signalling for cell survival.
- The positive selection of developing B cells occurs when the pre-B receptor binds its ligand. (Developing T lymphocytes are favourably chosen on the basis of their ability to bind MHC and peptide.)
- Negative selection indicates that receptor binding results in cell death. If immature B and T cells bind self antigen, they are both negatively selected.
- Membrane pre-B receptor and membrane IgM expression transmit signals necessary for B cell survival and progression through the correct phases of gene expression. Two types of experiments have offered evidence for this claim.
- The H and L chains can be rearranged and then introduced into fertilised mouse eggs to produce transgenic mice. Mice transgenic for both recombined Ig H and L chains do not often recombine other Ig genes; all of their B cells express the transgene H and L chains.
- Transgenic mice for the H chain continue to recombine their genes for the L chain and vice versa. Therefore, the presence of an altered VH or VL gene communicates to the B cell to inhibit further recombination of that gene.
- Knock-out mice are mice in which functional genes (or portions of genes needed for their function) have been removed.
- Experiments demonstrating the importance of membrane expression of the BCR complex for delivering these signals involved creating mice lacking the H chain transmembrane exon (so that the H chain would not be inserted into the membrane), the genes for Iga or Igb (or just their ITAMs), or the genes for the surrogate light chains l5 and VpreB.
- Even if all other proteins can be produced or the complete pre-B receptor can be expressed on the membrane with the IgaIgb missing ITAMs, the elimination of any one of these proteins prevents the formation of B cells.
- Surrogate light chain l5 resembles the constant section of the l chain, but is encoded by a distinct gene. l5 forms a non-covalent association with a VpreB domain that mimics an Ig V domain.
- Since pre-B cells display several VH sections, it is theorised that the VpreB shared by all pre-B cells binds a ligand that tells the pre-B cell to divide and subsequently initiate light chain recombination via the signal transduction protein IgaIgb. Similar signals from unidentified ligands inhibit recombination.
- Somatic recombination results in allelic exclusion for both H and L chains in individual B cells, as only one H chain gene and one L chain gene are productively recombined in each B cell.
- In a heterozygote, each allele (allotype) is present on around fifty percent of B cells and fifty percent of serum Ig molecules. Light chains also exhibit isotypic exclusion, as a single cell or molecule contains either k or l chains. k and l are not equally represented on B cells and serum Igs. k is favoured over l by 65% to 35% in humans.
- In mice, 95% of the serum Ig is k, while in cats it is l. The ratio of k to l represents the proportional quantity of V region segments in each isotype and their recombination efficiency into functional L chain genes.
- Once a B cell leaves the bone marrow, its survival is believed to be dependent on additional signals supplied by lymphoid follicles of secondary lymphoid tissue.
- B cell homeostasis is likely maintained by the competition between newly generated and older B cells for these signals.
- It has been demonstrated that the survival of injected transgenic B cells (whose unique receptor can be recognised by flow cytometry) depends on the irradiation of the host’s regular B cells.
Negative Selection of B Cells
- B cells that express only IgM are destroyed or inactivated (negatively selected) when they bind multivalent ligands, in contrast to mature B cells that are activated by BCR cross-linking.
- In the bone marrow, binding to multivalent (cell-associated) self induces B cell death and clonal deletion. Binding to soluble self does not kill the B cell; the cell is able to migrate to the periphery and express mainly IgD and very little IgM.
- These cells lack the ability to respond to antigen and have a short lifespan. Non-self-binding cells express normal amounts of IgM and IgD; if they reach lymphoid follicles, they can live for a few weeks until they encounter their particular antigen or perish.
- Although many self-specific B cells undergo clonal deletion, others can undergo further somatic recombination to generate non-self-specific VH and VL combinations.
- Mice bearing Ig transgenes expressing self-MHC-specific BCR have showed that receptor editing can change the specificity of some self-specific B cells, thereby rescuing them.
- The few B lymphocytes produced by these mice are not self-specific, as they are capable of producing new (non-transgenic) recombinations. The V sections of both the light and heavy chains can be replaced during receptor modification.
- In numerous animal taxa, Ig germline diversity is absent or extremely low. Only one or a few functional V, D, and J segments are available for recombination, ensuring that all immature B cells have the same antigen specificity and bind self antigen.
- During cell division, DNA crossing over events with nearby pseudogenes (gene segments containing stop codons) cause modifications to the V region sequences.
- This technique generates several Ig V regions. Once cells cease to connect to themselves, they develop and migrate to the periphery.
Heterogeneity of B Cell
- During foetal development, bone marrow stem cells generate the B-1 B cell, a B cell with characteristics distinct from those of typical B cells. B-1 Cells have membrane CD5.
- They are self-renewing, meaning that they can make additional adult naïve cells similar to themselves by division in the lymphoid tissues of the periphery.
- Conventional B-2 cells can only divide in response to antigen and form memory or plasma cells in the periphery; bone marrow progenitors must be used to generate new naive B-2 cells.
- B-1 BCR is significantly less varied than B-2 BCR. B-1 BCR is generated preferentially from a subset of Ig gene segments, lacks extra N nucleotides at segment junctions, and is mostly selective for common bacterial carbohydrate antigens.
- B-1 cells largely produce IgM and experience minimal somatic hypermutation. B-1 cells and their produced antibodies are termed polyreactive because they respond to antigens found on numerous pathogens and bind various antigens with low affinity.
- B-1 cells produce the majority of the IgM observed in unvaccinated mice. B-1 cells produced after birth contain a greater diversity of Ig than those produced during foetal development, but not as much as B-2 cells.
- Eventually, stem cells in bone marrow cease making B-1 cells. The gamma/delta T cell is an early-developing T cell type that is similar to the gamma/delta T cell.
- B cells alter their location in accordance with their maturation stages, with each site providing a microenvironment optimal for the B cell at that point of its life. Just under the bone, stem cells generate lymphoid progenitors and pro-B cells in the bone marrow.
- As B cells mature, they migrate toward the core of the bone marrow. Mature naive B cells exit the bone marrow and use selectins to bind addressins on the endothelium of blood vessels to enter peripheral lymphoid tissues, passing via T cell areas and entering B cell areas (follicles).
- Peyer’s patches, tonsils, and the appendix consist primarily of enormous follicles. The milieu in the MALT follicles (including the T cell cytokines produced there) instructs the B cells to generate IgA, whereas the microenvironment in the lymph nodes and spleen instructs the B cells to manufacture IgG.
- B cells that contact antigen and receive the proper T cell assistance in the T cell regions develop germinal centres in the follicles, where they rapidly divide, undergo somatic hypermutation, and are selected for B cells with greater affinity receptors.
- Antibody-secreting plasma cells are largely present in the medullary cords of the lymph nodes, the red pulp of the spleen, the bone marrow (particularly IgG-secreting plasma cells), and the mucosal lamina propria (IgA-secreting plasma cells).
- Memory B cells are mostly present in the border zone of the spleen, the sub-capsular sinus of the lymph nodes, and under the intestinal epithelium in the Peyer’s patches and crypt epithelium of the tonsils; a small number are also detected in the blood.
- These spontaneously occurring malignancies have assisted immunologists in comprehending B cell development.
- Each form of tumour has its own unique Ig gene recombination status and homing characteristics. In nearly every instance, these tumours arise from a single B cell transformed into a cancer cell.
- Monoclonality permits clinicians to identify tumour cells and monitor their treatment responses.
- Some B cell malignancies contain DNA translocations that result in the activation of oncogenes. A translocation is the transfer of a section of one chromosome to another.
- Oncogenes are genes often linked with regulated cell division; disruption of their function by translocation can lead to uncontrolled proliferation.
- In Africa, Epstein Barr Virus (EBV), which often causes a benign childhood sickness or a more serious infectious mononucleosis in young people in the United States, is linked to Burkitt’s lymphoma, a type of B cell malignancy.
- Myc is translocated in Burkitt’s lymphoma cells under the direction of a H or L chain promoter. Because these promoters are active in B cells, a B cell with this translocation and additional mutations may experience uncontrolled proliferation.
- There appears to be a connection between Burkitt’s lymphoma and malaria. bcl-2 is another gene that is activated by translocation to Ig locus.
- Bcl-2 protein shields B-lineage cells from programmed cell death; therefore, cells containing translocated bcl-2 live beyond their natural lifespan and may develop cancer.
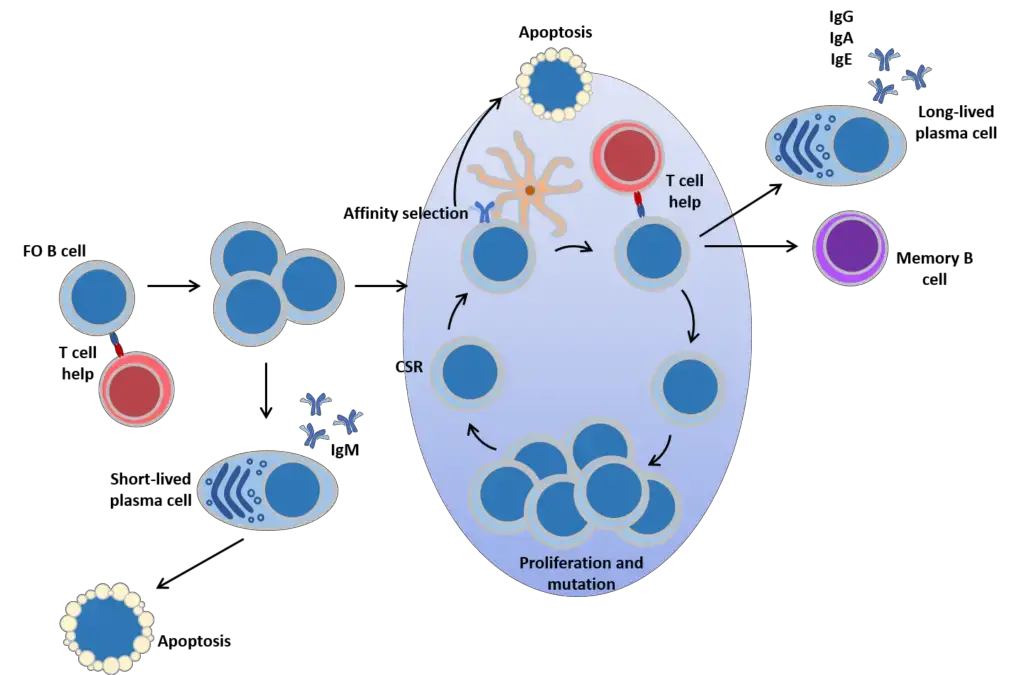
B cell responses to antigen
- Mature FO In quest of antigen, B cells travel between secondary lymphoid organs. Upon encountering a cognate antigen, B cells aided by T cells may undergo a variety of developmental pathways.
- First, the cells are capable of plasmacytic differentiation, the formation of extrafollicular plasmablasts, and the formation of IgM-secreting plasma cells.
- These cells lack somatic mutations in their Ig genes and have a short lifespan, yet they offer a quick first response to antigen.
- The second developmental possibility is the formation of a germinal centre, a specialised structure in which B cells undergo cycles of proliferation accompanied by affinity maturation: an iterative process of Ig gene mutation and selection that results in a B cell pool with the highest affinity for antigen binding.
- In addition, the cells undergo class-switch recombination. Immunoglobulin class switching to IgG, IgA, and IgE is a significant mechanism for diversifying B cell responses and adapting antibody function to immunological stress.
- Exiting the GC are memory B cells and plasma cells bearing somatically altered and generally high affinity BCRs of switched isotypes.
B cell (B lymphocyte) Applications
- Antigen Presentation:
- Function as professional antigen-presenting cells (APCs).
- Express antigen-MHC complexes and T cell receptors involved in T-cell activation.
- Assist in coordinating the immune response.
- Associated with inactivating T cells in the non-specific or innate immune system.
- Cytokine Secretion:
- Produce cytokines for cell-cell communication during immune responses.
- Cytokines facilitate the recruitment of white blood cells, such as phagocytes.
- Enhance the immune response by promoting antigen elimination.
- Antibody Production:
- Primary function of B cells.
- Produce antibodies as part of the antibody-mediated humoral immune response.
- Antibodies fight against multiple antigens of different origins.
- Provide protection to the body against potential harm.
- B Cell-Based Immunotherapy:
- B cells contribute to immune regulation through cytokine production and antigen presentation.
- B cells can be consistently generated from peripheral blood.
- Relatively insensitive to tumor-derived immunosuppressive mechanisms.
- Do not induce tolerance by themselves.
- Well tolerated with minimal toxic side effects.
FAQ
What are B cells?
B cells, also known as B lymphocytes, are a type of white blood cell that plays a vital role in the immune system.
What is the function of B cells?
The primary function of B cells is to produce antibodies and mediate the humoral immune response. They also serve as antigen-presenting cells and produce cytokines.
How do B cells produce antibodies?
B cells have specialized receptors on their surface called B cell receptors (BCRs). When these receptors encounter an antigen, the B cell is activated, leading to the production and secretion of specific antibodies.
What is the role of B cells in the immune response?
B cells are responsible for the production of antibodies that can neutralize pathogens, mark them for destruction by other immune cells, and provide immunity against future infections.
Can B cells present antigens to other immune cells?
Yes, B cells can act as antigen-presenting cells (APCs) and present antigens to T cells, initiating an immune response and coordinating the activities of other immune cells.
How are B cells activated?
B cells can be activated by direct recognition of antigens through their B cell receptors or through interaction with helper T cells.
What are memory B cells?
Memory B cells are a subset of B cells that are formed after an initial encounter with an antigen. They “remember” the antigen and mount a more rapid and robust response upon re-exposure, providing long-term immunity.
What is the relationship between B cells and autoimmune diseases?
In autoimmune diseases, B cells may produce antibodies that mistakenly target the body’s own tissues or cells, leading to immune system dysfunction and tissue damage.
Can B cells be used in immunotherapy?
Yes, B cells have been investigated as a potential target for immunotherapy, particularly in B cell malignancies such as certain types of lymphoma and leukemia.
How are B cells different from T cells?
B cells and T cells are both types of lymphocytes but have different functions. B cells primarily produce antibodies, while T cells are involved in cell-mediated immune responses and can directly kill infected cells.
References
- Textbook of Microbiology & Immunology by Subhash Chandra Parija
- Peter J. Delves, Seamus J. Martin, Dennis R. Burton, and Ivan M. Roitt(2017). Roitt’s Essential Immunology, Thirteenth Edition. John Wiley & Sons, Ltd.
- Judith A. Owen, Jenni Punt, Sharon A. Stranford (2013). Kuby Immunology. Seventh Edition. W. H. Freeman and Company
- Althuwaiqeb SA, Bordoni B. Histology, B Cell Lymphocyte. [Updated 2022 May 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560905/
- Cano RLE, Lopera HDE. Introduction to T and B lymphocytes. In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A, et al., editors. Autoimmunity: From Bench to Bedside [Internet]. Bogota (Colombia): El Rosario University Press; 2013 Jul 18. Chapter 5. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459471/
- https://en.wikipedia.org/wiki/B_cell
- https://www.immunology.org/public-information/bitesized-immunology/cells/b-cells
- https://www.akadeum.com/b-cell/
- https://ashpublications.org/blood/article/112/5/1570/25424/B-lymphocytes-how-they-develop-and-function
- https://www.britannica.com/science/B-cell
- https://www.thermofisher.com/in/en/home/technical-resources/cell-lines/b/cell-lines-detail-34.html?ef_id=Cj0KCQjwk5ibBhDqARIsACzmgLRWKn5R2qVEXn9c-DG1_U9f-LTh1TpHEXejPUvxUdOGr8e8Ce6sICUaAvtEEALw_wcB:G:s&s_kwcid=AL!3652!3!605589671972!!!g!!!382790548!126051992563&cid=bid_clb_cce_r01_co_cp0000_pjt0000_bid00000_0se_gaw_dy_pur_con&gclid=Cj0KCQjwk5ibBhDqARIsACzmgLRWKn5R2qVEXn9c-DG1_U9f-LTh1TpHEXejPUvxUdOGr8e8Ce6sICUaAvtEEALw_wcB
- https://microbenotes.com/b-cells-b-lymphocytes/
- https://microbenotes.com/b-cell-b-lymphocyte/
- https://www2.nau.edu/~fpm/immunology/Exams/Bcelldevelopment-401.html
- https://courses.lumenlearning.com/wm-biology2/chapter/t-and-b-lymphocytes/