Auramine–Rhodamine staining is the process used to demonstrate acid-fast bacilli from clinical specimens and it is considered a modified form of the Acid-Fast staining technique. It is the process where fluorochrome dyes bind with the mycolic acid of the bacterial cell wall and it is visualized under a fluorescent microscope. It is the preferred screening method for detecting Mycobacterium tuberculosis and other acid-fast organisms because it is more sensitive and the examination of the smear is faster.
It is the process which uses Auramine O and Rhodamine B dyes that have a high affinity for the mycolic acids (Mycolic acids are long-chain fatty acids present on the Mycobacterium spp cell wall and they are the major lipid components essential for the bacterial survival).
When these dyes bind with the cell wall the bacilli become acid-fast and they resist decolorization by acid-alcohol. This is referred to as the property of acid-fastness. A counterstain like potassium permanganate is used in the final step to reduce background fluorescence and allow clear visualization of the stained bacilli.
It is the technique where the stained bacilli appear as bright yellow-green or orange-red rods against a dark background. It is observed at lower magnification because fluorescent staining gives high contrast and allows scanning of larger smear areas in less time. This is considered a presumptive test because the stain cannot differentiate between viable and dead cells and species identification needs culture or molecular methods.
Among the important changes developed in this technique was the introduction of fluorescent dyes by Hagemann (1937) and further analysis by Truant, Brett and Thomas (1962) showing increased sensitivity in detecting acid-fast bacilli. A combination of Auramine and Rhodamine produced better staining results in comparison to using them separately and it was faster than Ziehl-Neelsen staining. It is also noted that these dyes can stain some Sporozoa parasites under fluorescence.
Principle of Auramine- Rhodamine Staining
The principle of Auramine–Rhodamine staining is based on the strong affinity of the fluorochrome dyes for the mycolic acids present on the cell wall of acid-fast bacilli. It is the process where Auramine O and Rhodamine B penetrate the lipid-rich mycobacterial cell wall and form a stable dye–lipid complex. This complex is resistant to removal by acid-alcohol, and this resistance is referred to as acid-fastness. It is the property that allows the acid-fast bacilli to retain the primary stain even after decolorization, while the non-acid-fast organisms lose the dye during this step.
A counterstain like potassium permanganate is applied to reduce the background fluorescence, helping in darkening debris and non-specific materials. When the smear is viewed under a fluorescent microscope, the stained bacilli absorb ultraviolet light and emit bright yellow-green or reddish-orange fluorescence. This gives a high contrast image which helps in rapid detection of the acid-fast organisms in the specimen.
Reagents required
The reagents used in this staining technique are arranged into the primary stain, the decolorizing agent, the counterstain, and the rinse water. These reagents help in demonstrating the acid-fast bacilli by forming the dye–lipid complex on the cell wall and removing non-specific background materials.
Primary Fluorochrome Stain– It is the staining solution that contains the fluorochrome dyes used to stain and demonstrate the acid-fast bacilli. The active dyes are Auramine O and Rhodamine B. Auramine O gives a yellow-green fluorescence and Rhodamine B improves the staining intensity with a reddish-orange effect. These dyes are dissolved in glycerol and distilled water, and phenol is commonly added as a mordant helping the dye to penetrate the waxy mycolic acid layer. It is noted that phenol is corrosive and poisonous, therefore some preparations use phenol-free formulations.
Decolorizing Agent– It is the mild acid-alcohol solution used to remove the primary stain from the non-acid-fast cells. The solution generally contains 0.5% HCl in 70% ethanol. This acid-alcohol is weaker compared to that used in Ziehl–Neelsen staining and the reagents cannot be interchanged because the staining principle is different.
Counterstain (Quenching Agent) – This is the reagent used to darken the background material so that the fluorescent bacilli can be clearly demonstrated. Potassium permanganate (0.5% aqueous solution) is mainly used for quenching the background. It reduces the non-specific fluorescence from debris. Other dyes like acridine orange or thiazine red can be used but potassium permanganate is the standard reagent.
Rinse Water– Distilled or deionized water is used for preparing the reagents and also for rinsing the slides. It is important because chlorine present in tap water can interfere with fluorescence and also because tap water may contain environmental mycobacteria which may cause false-positive reactions.
Modified/Rapid Kit Reagents– Some rapid kits contain a combined decolorizer and quencher, reducing the staining steps. In sputum digestion-decontamination steps, reagents like NALC–NaOH or hypochlorite-based solutions may be used before staining depending on the sample condition.
Procedure of Auramine- Rhodamine Staining

The procedure is carried out in several steps beginning with smear preparation, staining, decolorization, counterstaining, and examination under a fluorescent microscope. It is the process used to demonstrate acid-fast bacilli by forming a dye–lipid complex and then removing non-specific materials which helps in clear visualization.
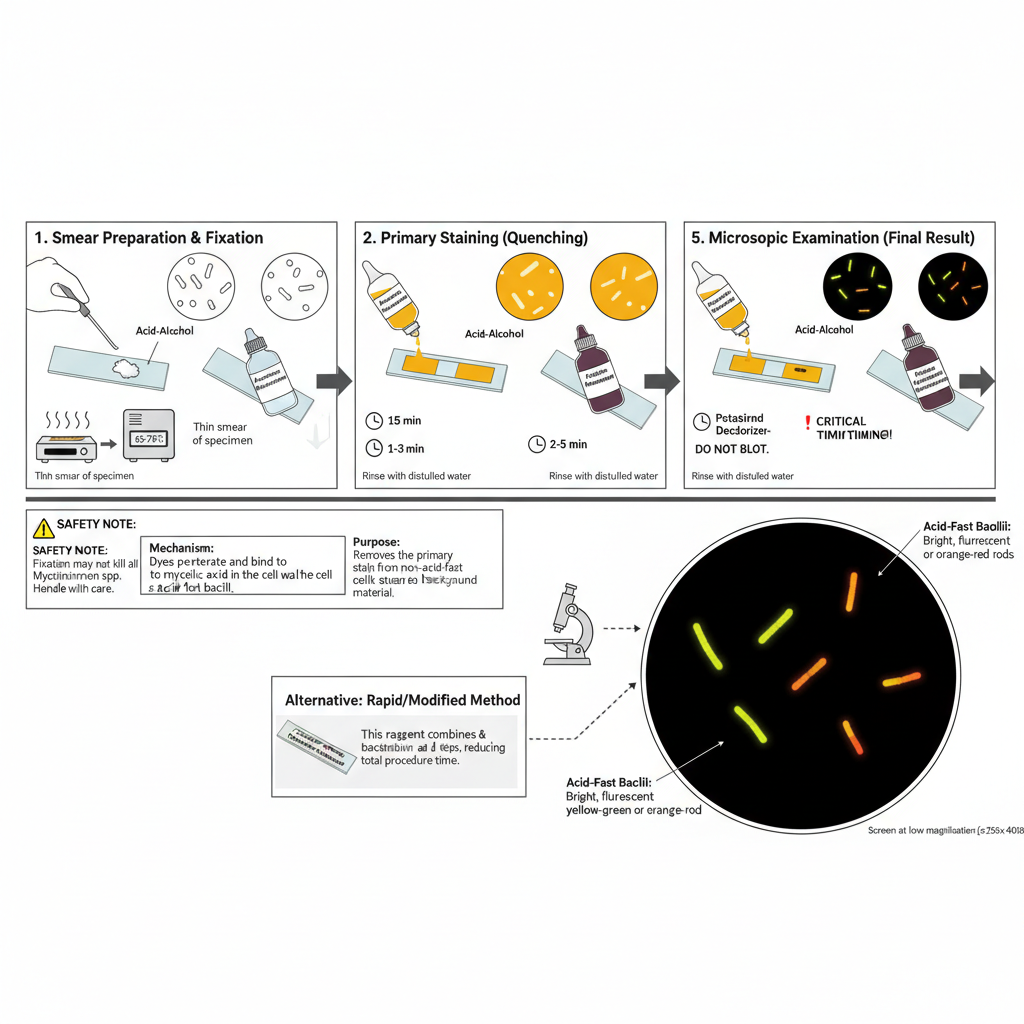
1. Specimen Preparation and Fixation
It is the first step where the specimen such as sputum is spread on a clean slide. A thin smear is made because thick smears may flake or may not decolorize properly. The smear is then fixed by heat so that it can adhere well on the slide surface. Slides are warmed at about 65–75°C for some time or passed through flame lightly. It is noted that fixation does not kill all Mycobacterium spp and therefore handling must be careful.
2. Primary Staining
The smear on the slide is flooded with the Auramine–Rhodamine staining solution. It is kept for around 15 minutes so that the dyes penetrate the mycolic acid layer. Heating is not required in this staining step because the dyes have strong affinity for the cell wall. After staining, the slide is rinsed with distilled water to remove the excess stain because chlorine in tap water may interfere with the fluorescence.
3. Decolorization
In this step the smear is flooded with acid–alcohol which removes the stain from non-acid-fast cells. It is generally kept for about 2–3 minutes. The acid-fast bacilli retain the dye because of their acid-fastness. The slide may be gently swirled to help remove loosely bound stain. It is then rinsed again with water to stop the decolorizing effect.
4. Counterstaining (Quenching)
The slide is flooded with potassium permanganate solution which helps in darkening the background. This is kept for about 2–5 minutes. Strict timing is important because more time may reduce the fluorescence of the bacilli. After counterstaining the slide is rinsed with water and allowed to air dry. The smear should not be blotted because it may get damaged.
5. Microscopic Examination
The dried slide is observed under a fluorescent microscope that has suitable filters. The smear can be screened at low magnification (250x–400x) because fluorescence gives a strong contrast. The acid-fast bacilli appear as bright yellow-green or orange-red rods against a dark background.
6. Rapid/Modified Methods
Some rapid staining kits combine the decolorizing and quenching steps into one reagent. In these methods the smear is stained briefly, rinsed, and then treated with the combined reagent, helping in reducing the total time needed for staining.

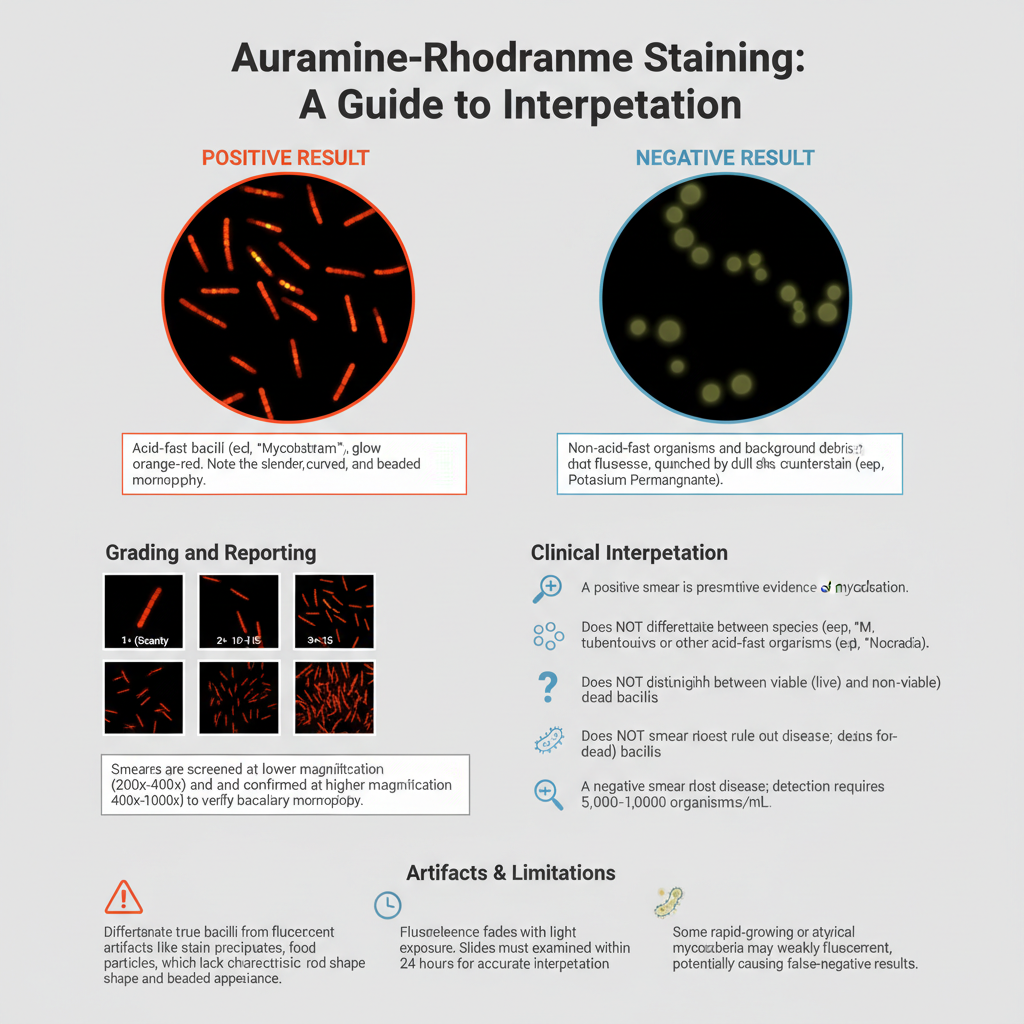
Result and Interpretation of Auramine- Rhodamine Staining
- Positive Test — Acid-fast microorganisms glow orange-red against a black backdrop.
- Negative Test — Organisms that are not acid-fast will not fluoresce or may appear pale yellow, in stark contrast to the bright organisms that are acid-fast.
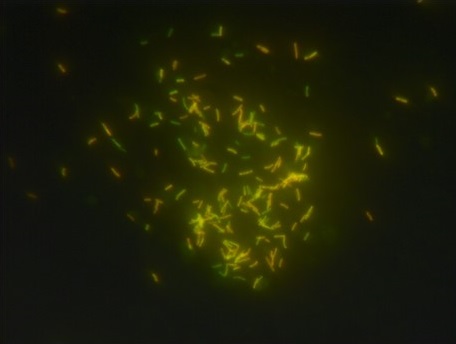
Microscopic Appearance
- It is observed that the acid-fast bacilli appear as bright fluorescent rods seen against the dark background.
- The organisms stained with Auramine generally show yellow-green colour while Auramine–Rhodamine combination gives orange or reddish-orange appearance.
- These bacilli is usually slender and curved showing beaded appearance where staining intensity changes along the rod.
- The non-acid-fast organisms is quenched by the counterstain (potassium permanganate) and appear as dull shadow without fluorescence.
Clinical Interpretation
- A positive fluorescent smear indicates the presence of acid-fast bacilli and it is considered as presumptive evidence of mycobacterial infection.
- It is the process that detects all acid-fast species including NTM and sometimes Nocardia, so it cannot be used to differentiate species.
- This stain cannot distinguish viable and dead bacilli and therefore treated patients may still show positive smear.
- A negative smear means AFB is not seen but it does not rule out tuberculosis because detection requires around 5000–10000 organisms/ml.
Grading and Reporting
- The smear is reported in semi-quantitative pattern as scanty, 1+, 2+, 3+ or 4+ based on the bacilli seen per microscopic field.
- This technique is screened at lower magnification (200x–400x) allowing larger field view and faster scanning.
- The reaction is confirmed under higher magnification (400x–1000x) to verify true bacillary morphology before reporting as positive.
Artifacts and Limitations
- It is important to differentiate true AFB from artifacts like stain precipitates, fibres or food particles that may fluoresce but do not show rod-shape.
- Some rapid-growing mycobacteria is weakly fluorescent and may produce false-negative results.
- The fluorescence fades with time or with light exposure and therefore the slides is examined within 24 hours for correct interpretation.
Applications of Auramine- Rhodamine Staining
- It is mainly used for rapid screening of Acid-Fast Bacilli (AFB) from clinical specimens, especially for detecting Mycobacterium tuberculosis in respiratory samples.
- The stain is applied in laboratories that process high specimen load because the fluorescent method allows scanning of smears at lower magnification and covers larger field areas in short time.
- It is used for examining sputum, urine, cerebrospinal fluid, other body fluids, and tissue samples in order to demonstrate acid-fast organisms.
- In histological sections (formalin-fixed paraffin tissues), it is used to show acid-fast bacilli in conditions like tuberculous lymphadenitis where sensitivity is higher than routine staining.
- It is used for non-tuberculous mycobacteria detection and also identifies Mycobacterium leprae due to the acid-fast nature of these organisms.
- The stain also detects Nocardia species because their cell wall contains mycolic acids and shows partial acid-fastness.
- Modified Auramine-phenol fluorescence staining is used in parasitology for demonstrating Cryptosporidium and Isospora oocysts in fecal smears which appear fluorescent against dark background.
- It is used in clinical decision making because positive smear gives early indication of tuberculosis and infectiousness allowing isolation and treatment initiation.
- The staining is also utilized to estimate bacterial load based on smear positivity which indicates the potential for transmission.
- It is used to monitor patient response during therapy as smear conversion from positive to negative indicates decrease in bacillary count.
Limitations of Auramine- Rhodamine Staining
- It provides only presumptive detection because all acid-fast organisms show fluorescence and the stain cannot differentiate M. tuberculosis from NTM or Nocardia.
- The stain cannot distinguish viable bacilli from dead bacilli, so smear may remain positive in patients who already became culture-negative during treatment.
- Some rapidly growing mycobacteria is weakly fluorescent and may produce false-negative results.
- Dormant or persistent bacilli may have altered mycolic acid content leading to reduced stain uptake.
- A relatively high bacterial load (5000–10000 AFB/ml) is required for detection so the method may miss cases having low bacillary count.
- Sensitivity is lower for some specimen types like gastric aspirates which show more false-positive and false-negative patterns.
- The technique requires fluorescence microscope and proper filter sets which increases the cost and infrastructure requirement.
- The fluorescence fades with time and slides must be observed within 24 hours for accurate interpretation.
- Overexposure to counterstain (potassium permanganate) can quench fluorescence making bacilli less visible.
- Water used in staining must be chlorine-free because chlorine interferes with fluorescence and may lead to false-negative results.
- The chemicals used in staining like Auramine O, Rhodamine B, phenol, acid-alcohol and potassium permanganate are irritants and some are possible carcinogens, creating safety concerns during handling.
Advantages of Auramine- Rhodamine Staining
- It is more sensitive compared to the Ziehl–Neelsen method and shows higher detection rate of acid-fast bacilli.
- The stain detects low bacterial load because the fluorochrome dyes bind strongly with mycolic acids making few organisms visible easily.
- It allows rapid screening since slides is examined at lower magnification (250x–400x) without the need of oil immersion in the initial step.
- Larger field area is seen in single view, so examination time decreases and many slides can be processed in short duration.
- Some rapid protocols of this stain reduce the staining time because decolorizing and quenching steps is combined.
- The contrast produced is very clear as fluorescent rods appear bright on dark background helping in easy observation.
- It reduces eye strain of the microscopist because the glowing bacilli stand out clearly and interpretation becomes simpler.
- The microscope stage remains cleaner since oil is not used during screening and movement between slides becomes convenient.
- It is applicable for various specimen types like sputum, tissue sections and concentrated fluids making the technique versatile.
FAQ
Q1. What is Auramine–Rhodamine staining used for?
A. It is used for rapid detection and screening of acid-fast bacilli, mainly Mycobacterium tuberculosis, from different clinical specimens.
Q2. What is the principle of Auramine–Rhodamine staining?
A. It is the process in which fluorochrome dyes bind with mycolic acids of acid-fast organisms and these organisms fluoresce brightly under fluorescence microscope against dark background.
Q3. How does acid-fast staining work?
A. Acid-fast staining works by allowing dyes to penetrate the waxy, lipid-rich cell wall of acid-fast organisms, and after decolorization with acid-alcohol these organisms retain the dye because of high mycolic acid content.
Q4. What organisms are detected by Auramine–Rhodamine staining?
A. These are used to detect Mycobacterium tuberculosis, non-tuberculous mycobacteria, Mycobacterium leprae, and sometimes Nocardia species.
Q5. What dyes are used in the Auramine–Rhodamine staining method?
A. The method uses Auramine O and Rhodamine B fluorochrome dyes.
Q6. What is the procedure for Auramine–Rhodamine staining?
A. In this method the smear is stained with Auramine–Rhodamine dye, then decolorized with acid-alcohol, and counterstained with potassium permanganate before observing under fluorescent microscope.
Q7. How do you interpret the results of Auramine–Rhodamine staining?
A. Acid-fast bacilli appear as bright yellow-green or reddish-orange fluorescent rods, and non-acid-fast material is quenched and appears dark. Presence of fluorescent rods is interpreted as positive.
Q8. What is acid-fastness in microbiology?
A. Acid-fastness is the property in which organisms resist acid-alcohol decolorization because of high mycolic acid in the cell wall.
Q9. What is the difference between Auramine–Rhodamine stain and Ziehl–Neelsen stain?
A. Auramine–Rhodamine uses fluorescent dyes and allows screening at low magnification while Ziehl–Neelsen uses carbol fuchsin and requires oil immersion, and fluorescence method is more sensitive.
Q10. What are the advantages of Auramine–Rhodamine staining?
A. It is more sensitive, faster to screen, has larger field view, produces clear contrast, and does not need oil immersion during initial screening.
Q11. What are the limitations of Auramine–Rhodamine staining?
A. It cannot differentiate species, cannot distinguish viable from dead bacilli, requires fluorescence microscope, fluorescence fades with time, and chemicals used may be hazardous.
Q12. What is the role of potassium permanganate in Auramine–Rhodamine staining?
A. It is the counterstain that quenches background fluorescence making the field dark so that fluorescent bacilli become clearly visible.
Q13. What equipment is needed for Auramine–Rhodamine staining?
A. It requires fluorescence microscope with proper filters, staining reagents, chlorine-free water, slides, and heating or staining rack depending on the procedure.
Q14. Is Auramine–Rhodamine stain carcinogenic?
A. Auramine O and Rhodamine B are considered possible carcinogens and must be handled carefully.
Q15. What is the “Truant method of staining”?
A. It is the fluorescent staining method using Auramine–Rhodamine dyes for detecting acid-fast bacilli and is widely used as the standard fluorochrome technique.
- Alpha-Tec Systems. (n.d.). Rapid auramine O fluorescent stain set [Instructions for use]. Calibre Scientific.
- Annam, V., Kulkarni, M. H., & Puranik, R. B. (2009). Comparison of the modified fluorescent method and conventional Ziehl–Neelsen method in the detection of acidfast bacilli in lymphnode aspirates. CytoJournal, 6(13). https://doi.org/10.4103/1742-6413.53887
- Apan, T. Z., İşeri, L., & Köksal, F. (2011). The comparison between the modified auramine rhodamine flourochrome staining and Ziehl-Neelsen staining method from sputum specimens in the presence with radiometric Bactec Tb system. Kırıkkale Üniversitesi Tıp Fakültesi Dergisi, 13(1), 24–29.
- Association of Public Health Laboratories. (n.d.). AFB smear microscopy [Training module].
- BioGnost. (2019). TB auramine-rhodamine reagent [Instructions for use].
- Edmund Optics. (n.d.). Fluorescence filter set for TRITC rhodamine dye.
- Key Diagnostics. (n.d.). Mycobacterium: Modified auramine O stain.
- Merck KGaA. (2023). Microscopy AFB-fluor phenol-free 1.01597.0007 [Instructions for use]. Sigma-Aldrich.
- Mokobi, F. (2022, September 5). Auramine-Rhodamine staining. Microbe Notes. https://microbenotes.com/auramine-rhodamine-staining/
- Nelson, M., Hebbeln, K., Jepson, R., Sellers, G., Cochran, C., & Aldous, W. (2022). Verification of the Scientific Devices modified auramine O stain set in a low incidence TB setting [Poster presentation]. Association of Public Health Laboratories.
- Newport. (n.d.). HPF1330 fluorescence optical filter set.
- Strumpf, I. J., Tsang, A. Y., Schork, M. A., & Weg, J. G. (1976). The reliability of gastric smears by auramine-rhodamine staining technique for the diagnosis of tuberculosis. American Review of Respiratory Disease, 114(5), 971–976. https://doi.org/10.1164/arrd.1976.114.5.971
- [Title from source text]. (n.d.). Auramine-rhodamine staining: Principles, procedures, and role in modern mycobacterial diagnostics.
- UK Health Security Agency. (2025). Staining procedures (UK Standards for Microbiology Investigations TP 39 Issue 3.1). Royal College of Pathologists.
- WebPath. (n.d.). Auramine-rhodamine fluorescence – Acid fast bacteria. University of Utah.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.