Acid fast stain is a differential staining method that is used to identify the acid fast bacteria which retain the primary dye even after treatment with acid alcohol. It is the process where the organisms having a high amount of mycolic acids and waxes in their cell wall show resistance to decolourisation. These are hydrophobic substances and it creates a barrier that do not allow the normal stains to enter so these organisms remain unstained in simple staining methods. It is the property called acid fastness and these bacteria appears red because the carbolfuchsin stain is retained while the non-acid fast cells take up the counterstain and appear blue or green.
This stain is mainly applied for the detection of Mycobacterium tuberculosis causing tuberculosis and Mycobacterium leprae causing leprosy. Some species of Nocardia and certain parasites like Cryptosporidium are also detected by this stain. It is the process where three important methods are used which are Ziehl–Neelsen method (hot method), Kinyoun method (cold method) and the fluorescent dye method using Auramine O. In the Ziehl–Neelsen method heat is used to drive the stain through the waxy cell wall while the Kinyoun method uses higher concentration of phenol instead of heat. The fluorochrome method makes the bacteria appear bright under UV light which helps in fast screening of samples.
Even though it is widely used this staining has some limitations because it needs a high number of bacteria to give a positive result and it cannot differentiate between living and dead acid fast cells. It is therefore mostly used as an initial diagnostic tool and the final confirmation is generally done by culture or molecular techniques.
Principle of Acid Fast Stain
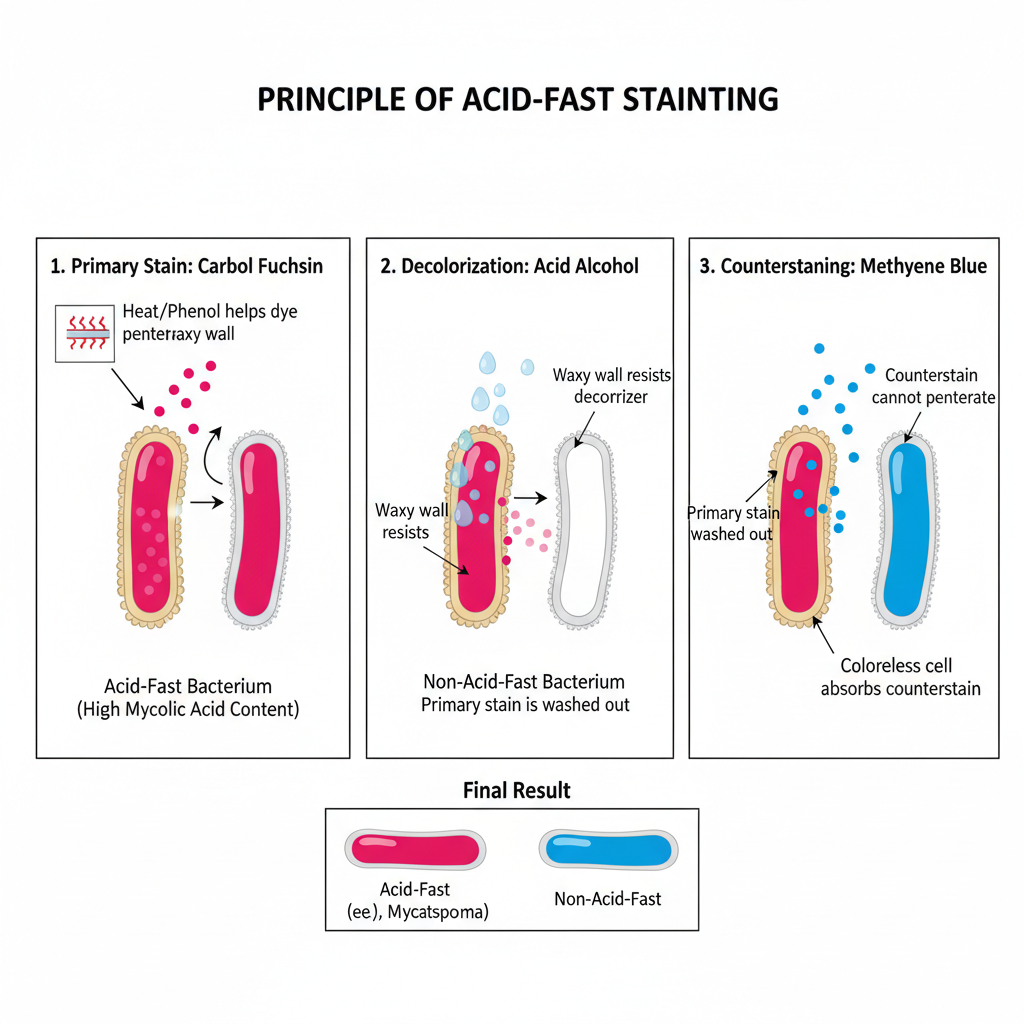
The principle of acid fast stain is based on the special nature of the cell wall of acid fast bacteria where a high amount of lipid and mycolic acids are present. It is the process where the waxy hydrophobic layer do not allow the normal stains to enter so a phenolic dye is used to penetrate the cell wall. It is explained through penetration of the dye, retention of the dye inside the waxy layer and resistance to decolourisation by acid alcohol. In the first step the primary stain carbol fuchsin enters the cell because heat is applied in the Ziehl–Neelsen method which softens the waxy layer or in the Kinyoun method the phenol acts as a solvent. It helps in dissolving the lipid content so the dye moves inside the cell wall.
When the heat is removed the waxy layer solidifies and the dye becomes trapped inside forming a stable complex with the mycolic acids. This is referred to as retention and it is the main reason why these bacteria hold the stain so tightly. In the next step acid alcohol is used for decolourisation. Non acid fast bacteria do not have such lipid layers so the dye is removed easily and these cells become colourless. Acid fast bacteria resist the entry of acid alcohol because the lipid layer do not allow the solvent to enter, so the dye is retained.
The counterstaining step colours the non acid fast organisms either blue or green as methylene blue or malachite green is used. These organisms therefore appear in contrast to the bright red acid fast cells. It is the process that helps in detecting Mycobacterium tuberculosis, Mycobacterium leprae and some species of Nocardia. This principle is used in routine diagnosis even though it is not very sensitive and it is mostly used as an initial rapid test before further confirmation.
Acid Fast Stain Reagents
- Primary Stains
- Carbol fuchsin is the main dye used for penetrating the waxy mycolic acid layer.
- Ziehl–Neelsen stain uses lower concentration of basic fuchsin and phenol and depends on heat to soften the waxy layer.
- Kinyoun stain uses higher concentration of basic fuchsin and phenol which helps in penetrating the cell wall without heating.
- Fluorochrome stains (Auramine O or Rhodamine B) are used in fluorescent microscopy where the stained bacteria appear yellow or orange under UV light.
- Fite stain primary reagent is used specially for M. leprae or Nocardia where a mixture of xylene and peanut oil protects the waxy wall before staining with carbol fuchsin.
- Decolorizers
- Acid alcohol (3% HCl in 95% ethanol) is the common decolorizer. It removes the stain from non acid fast organisms but not from acid fast ones.
- Modified decolorizers are used for partially acid fast organisms.
- Sulfuric acid (1%) is used for Nocardia or Cryptosporidium and higher concentration is sometimes used for M. leprae.
- Weak acid alcohol such as 0.5% HCl in 70% ethanol is used in fluorochrome staining methods.
- Counterstains
- Methylene blue is widely used and it stains the non acid fast cells blue.
- Malachite green or brilliant green are also used and these give a green background that contrasts with the red acid fast cells.
- Potassium permanganate (KMnO₄) is used in fluorochrome staining as a quenching agent which decreases background fluorescence.
Procedure of Acid Fast Stain
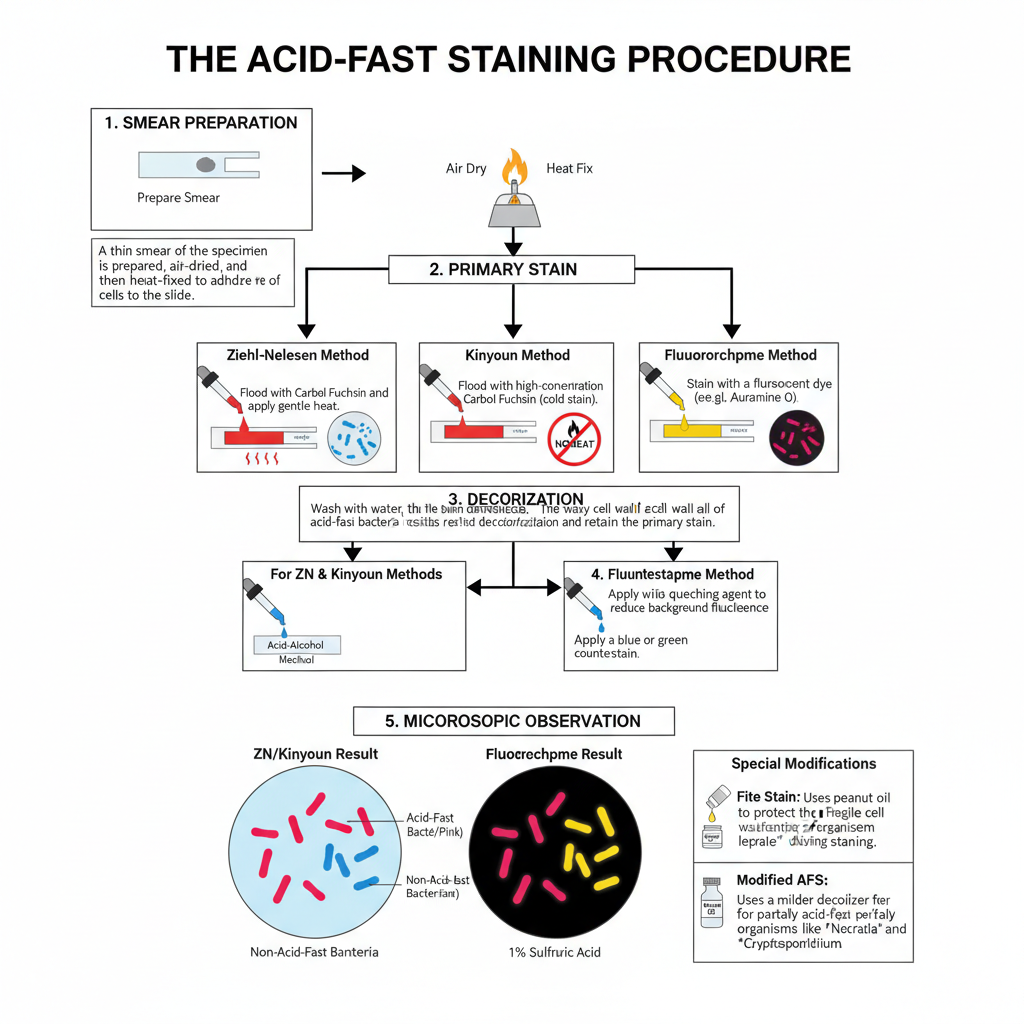
- Preparation of smear
- A small portion of the specimen is taken on a clean slide and it is spread to make a thin smear.
- It is allowed to air dry.
- The smear is then fixed by passing over gentle flame or by using a warmer. Heat helps in sticking the cells to slide and maintaining the structure.
- Application of primary stain
- The smear is flooded with Carbol fuchsin.
- In Ziehl–Neelsen method the slide is heated until vapour is seen but boiling is avoided.
- In Kinyoun method no heating is done because phenol content is high.
- In Fluorochrome stain the slide is kept with Auramine O or Auramine–Rhodamine dye for required time.
- Decolorization step
- The slide is gently washed with water.
- Acid-alcohol is added over the smear.
- Non-acid fast cells lose the primary stain while acid-fast cells retain the stain due to waxy cell wall.
- Decolorization is continued till no more colour comes out.
- Counterstaining
- The slide is washed with water to stop the acid action.
- Methylene blue or malachite green is added in Ziehl-Neelsen and Kinyoun method.
- In Fluorochrome stain potassium permanganate is used to reduce background fluorescence.
- Drying and observation
- The slide is washed, dried and observed under microscope.
- Acid-fast cells appear red (or yellow–orange in fluorescence) and non-acid fast cells appear blue or green.
- Special modifications
- In Fite stain peanut oil or mineral oil is used to protect delicate organisms.
- Modified acid-fast staining uses mild decolorizer like 1% sulphuric acid for partially acid-fast organisms.
Results of acid-fast staining
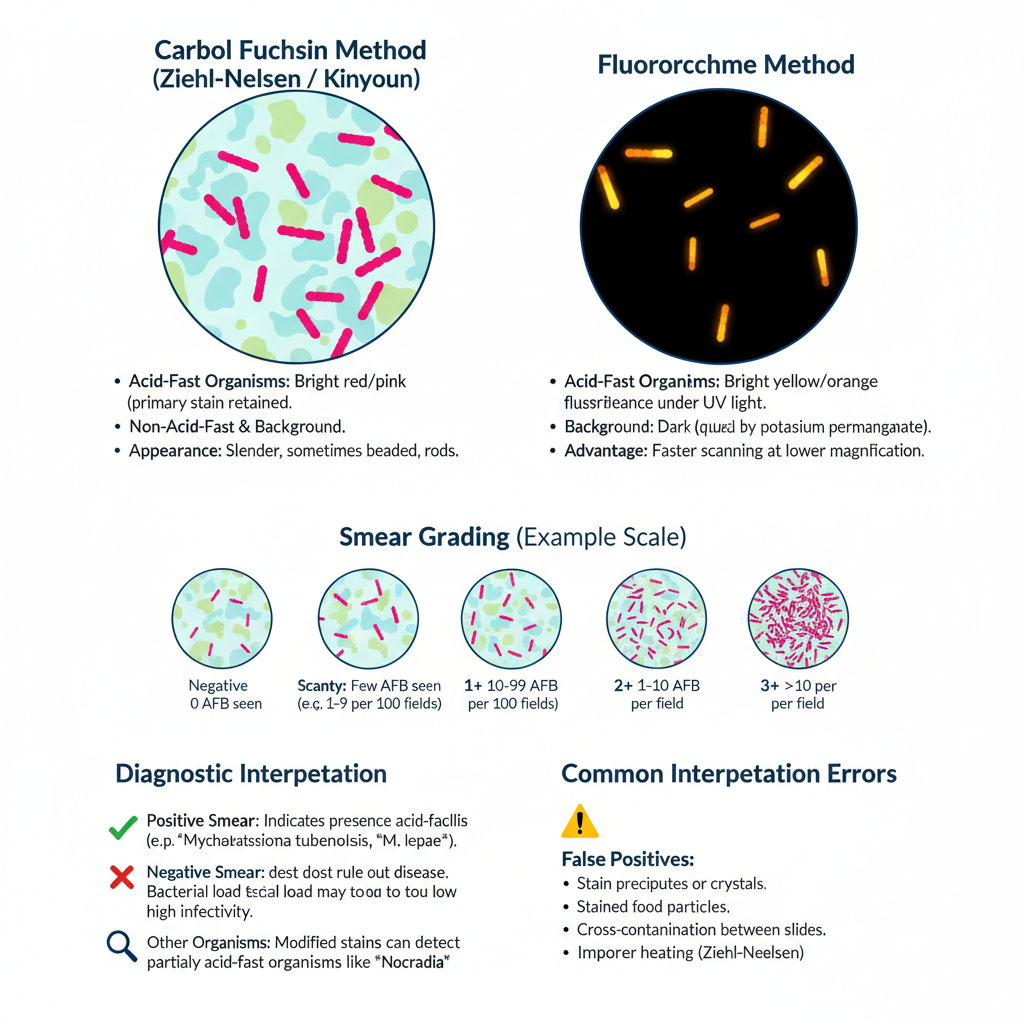
- Appearance in Carbol fuchsin methods (Ziehl-Neelsen and Kinyoun)
- Acid-fast organisms appear bright red or pink because the primary stain remains trapped in the waxy cell wall.
- These are usually slender rod-shaped cells and sometimes show beaded forms.
- Non-acid-fast organisms take the counterstain and appear blue or green.
- Background material like mucus or epithelial cells also appears blue or green giving contrast to the red bacilli.
- Appearance in Fluorochrome method
- Acid-fast organisms show bright yellow or orange fluorescence under UV light.
- The background remains dark because potassium permanganate is used for quenching.
- These glowing rods can be seen at lower magnification and scanning of slide becomes faster.
- Grading of the smear
- Negative result is when no acid-fast bacilli is seen in the required number of microscopic fields.
- Scanty result is given when only a few organisms is present and the exact number is written.
- 1+ grade is when 10 to 99 bacilli is observed in 100 fields.
- 2+ grade is when bacilli is seen in most fields and around 1 to 10 bacilli per field.
- 3+ grade is when more than 10 bacilli per field is seen indicating heavy load.
- Diagnostic meaning
- Positive smear indicates presence of acid-fast bacilli but it cannot differentiate the species.
- Mycobacterium tuberculosis and Mycobacterium leprae are the major organisms detected.
- Some other partially acid-fast organisms like Nocardia and some parasite oocysts can also show positive reaction in modified stains.
- High smear grades are associated with high infectivity.
- Negative smear does not exclude disease because low bacterial load may remain undetected.
- Common errors during interpretation
- False negative results occur when smear is too thick or when decolorization is excessive, and in improper heating during Ziehl-Neelsen method.
- False positive results occur when stain precipitates or when food particles are stained and appear as red bodies.
- Cross-contamination between slides also produces incorrect positive readings.
Uses of acid-fast staining
- It is used for the diagnosis of Mycobacterial infections including Mycobacterium tuberculosis from sputum, bronchial wash, gastric aspirates, urine and CSF.
- It is used to detect Mycobacterium leprae from skin biopsy or slit-skin smear as the major agent of leprosy.
- It helps in identifying different Non-tuberculous mycobacteria (NTM) like M. avium complex, M. kansasii and M. xenopi which produce pulmonary diseases in immunocompromised patients.
- It is used for monitoring treatment as the smear conversion from positive to negative suggest the therapy is working whereas persistent positivity indicate failure or resistance.
- It helps in grading the infectivity of patients as the smear grading (Scanty, 1+, 2+, 3+) is used to estimate the bacterial load.
- It is used to detect other acid-fast bacteria such as Nocardia species which are partially acid-fast and are associated with infections of lung, skin and brain.
- It is used in modified form to detect intestinal parasites like Cryptosporidium, Isospora and Cyclospora oocysts from stool samples.
- It is used in histopathological examinations to visualize acid-fast bacteria in tissue sections and special stains like Fite stain is used for detecting leprosy bacilli in paraffin-embedded tissue.
Limitations of acid-fast staining
- It has low sensitivity because a high number of bacilli is required in the specimen for detection and smear-negative cases can still have active infection.
- It cannot differentiate the species as all acid-fast organisms appear similar, so Mycobacterium tuberculosis cannot be separated from NTM or other partially acid-fast organisms.
- It cannot determine the viability of the organism because both living and dead bacilli are stained, which affects its use in treatment monitoring.
- It cannot detect drug resistance as the method is only morphological and resistant strains appear the same as susceptible ones.
- It is affected by technical issues since poor specimen quality or thick-thin smears can produce false negative or false positive results.
- It is time-consuming as the Ziehl–Neelsen method need careful heating and detailed microscopic examination of many fields.
- It has safety concerns because heating of phenol containing stains releases fumes which irritate mucous membranes and must be handled carefully.
Advantages of acid-fast staining
- It is a rapid method as the smear can give early indication of mycobacterial infection within hours to 1–2 days while culture needs long time for growth.
- It is economical and can be performed in basic laboratories since only simple reagents and a microscope is required, so it is widely used in high-burden areas.
- It helps in assessing infectivity because the smear grading (Scanty, 1+, 2+, 3+) indicates the bacterial load which is used for isolation decisions.
- It is useful in treatment monitoring because reduction in smear positivity indicate the fall in bacillary load during therapy.
- It detects a broad range of acid-fast organisms including Mycobacterium tuberculosis, M. leprae, NTM and partially acid-fast organisms like Nocardia and intestinal parasite oocysts by modified techniques.
- It is useful in diagnostic algorithms when smear is positive but TB-specific tests are negative, indicating possible NTM disease.
- It has method-specific advantages as fluorescent staining (Auramine-O) increases sensitivity and reduces scanning time, and the Kinyoun method avoid heating, reducing phenol fumes and improving laboratory safety.
Examples of Acid-Fast Stain
- Ziehl–Neelsen stain (Hot method)
- It is the classical method where heat is used to help the Carbol fuchsin dye enter the waxy cell wall.
- The smear is steamed while primary stain is present.
- Acid-fast cells appear bright red and the background becomes blue or green after counterstaining.
- It is commonly used in many laboratories because the reagents are simple and the method is reliable.
- Kinyoun stain (Cold method)
- This method does not use heating, instead the phenol concentration in the dye is increased.
- The high phenol content helps the dye to penetrate the cell wall at room temperature.
- Acid-fast cells appear red or purple and non-acid-fast cells appear blue or green.
- It is used because it avoids heating and reduces exposure to phenol vapour.
- Auramine–Rhodamine stain (Fluorochrome method)
- Fluorescent dyes like Auramine and Rhodamine bind with the mycolic acids of the cell wall.
- No heating is used in this method.
- Acid-fast bacilli glow yellow or orange when seen under fluorescence microscope.
- It is helpful where many samples is tested because scanning at low magnification becomes easy.
- Fite stain
- It is used for delicate acid-fast organisms like Mycobacterium leprae.
- A mixture of oil and xylene is used to protect the cell wall during tissue processing.
- The organisms appear red in stained tissue sections.
- This method is mostly applied in histology work.
- Modified acid-fast stain
- A weaker acid like 1% sulphuric acid is used for decolorization.
- It is applied for partially acid-fast organisms such as Nocardia, Cryptosporidium, and Cyclospora.
- The oocysts appear pink or red while background structures remain blue or green.
- It helps in detecting parasites that lose stain easily in the standard strong-acid method.
FAQ
What is acid-fast stain used for?
It is used to detect acid-fast organisms which retain the primary dye even after acid-alcohol treatment. It is mainly applied for identifying Mycobacterium species in clinical samples.
How does acid-fast staining work?
It works by using a strong lipid–soluble dye that enters the waxy cell wall. The dye remains fixed because the cell wall resists decolorization with acid-alcohol.
What bacteria are identified by acid-fast stain?
The main bacteria identified are Mycobacterium tuberculosis, Mycobacterium leprae, and partially acid-fast organisms like Nocardia in modified methods.
Why are some bacteria considered acid-fast?
These bacteria have high mycolic acid content in cell wall which binds the dye tightly. Because of this, the stain is not removed by acid-alcohol.
What are the steps in the acid-fast staining procedure?
The steps are smear preparation, primary staining, decolorization, and counterstaining. Heating may be used in Ziehl–Neelsen method while Kinyoun method does not use heating.
What diseases are diagnosed using acid-fast stain?
Diseases caused by Mycobacterium tuberculosis and Mycobacterium leprae can be diagnosed. It also helps in detecting infections by some acid-fast parasites in modified staining.
What are the different types of acid-fast stains?
Ziehl–Neelsen stain, Kinyoun stain, Auramine–Rhodamine fluorescent stain, Fite stain, and modified acid-fast stain are the common types.
What reagents are used in acid-fast staining?
Carbol fuchsin is the primary dye, acid-alcohol is the decolorizer, and methylene blue or malachite green is the counterstain. In fluorescent method, Auramine and Rhodamine dyes are used.
What do acid-fast stain results mean?
Acid-fast cells appear red or fluorescent depending on the method. Non-acid-fast cells take the counterstain. The presence of red bacilli indicates acid-fast organisms in the specimen.
What is the role of mycolic acid in acid-fastness?
Mycolic acid is a waxy lipid that forms a barrier in the cell wall. It allows the dye to enter but prevents its removal during decolorization.
Is acid-fast stain a differential stain?
Yes, it is a differential stain because it separates acid-fast organisms from non-acid-fast organisms based on their staining reaction.
What is the principle of acid-fast staining?
The principle is that acid-fast organisms retain the primary dye even when treated with strong acid-alcohol, due to the presence of waxy lipid in the cell wall.
What is the difference between Ziehl–Neelsen and Kinyoun staining?
Ziehl–Neelsen uses heat to help dye penetration while Kinyoun increases phenol concentration so no heating is required. Both give red acid-fast cells and blue or green background.
How do you prepare for an acid-fast stain test?
A clean slide is taken, a thin smear is made, it is air-dried and heat-fixed. The slide is then ready for staining steps.
What is the history of the acid-fast stain?
The method was developed in the late 19th century for staining Mycobacterium. Ziehl and Neelsen modified the technique using Carbol fuchsin and heat which became the standard procedure.
- AAT Bioquest. (2023, March 22). What are the fundamental differences between Ziehl-Neelsen technique and the Kinyoun technique? https://www.aatbio.com/resources/faq-frequently-asked-questions/what-are-the-fundamental-differences-between-ziehl-neelsen-technique-and-the-kinyoun-technique
- Bayot, M. L., Mirza, T. M., & Sharma, S. (2023, August 7). Acid fast bacteria. StatPearls [Internet]. NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK537121/
- Bomkamp, R., & Newell, D. (n.d.). Acid fast bacteria and acid fast staining. Leica Biosystems. https://www.leicabiosystems.com/us/knowledge-pathway/acid-fast-bacteria-and-acid-fast-staining/
- Ciulla-Bohling, R. (2025). Acid-fastness. Research Starters. EBSCO.
- Hussey, M. A., & Zayaitz, A. (2008, September 8). Acid-fast stain protocols. American Society for Microbiology.
- Kong, L., Xie, B., Liu, Q., & Hua, L. (2021). Application of Acid-fast staining combined with MTB/RIF GeneXpert in the diagnosis of Nontuberculous Mycobacteria Pulmonary Disease. International Journal of Infectious Diseases, 104(3). https://doi.org/10.1016/j.ijid.2020.12.091
- Mugusi, S., Lema, J., Ponsian, P., Kamori, D., Kimambo, H., Masanja, P., Anthony, A., Ngemera, M., & Kahwa, A. (2025). Diagnostic accuracy of sputum smear microscopy compared with culture in patients with tuberculosis confirmed by Xpert Mycobacterium tuberculosis/rifampicin assay in Tanzania: A cross-sectional study. Journal of International Medical Research, 53(10). https://doi.org/10.1177/03000605251390703
- National Library of Medicine. (n.d.). Acid-fast bacillus (AFB) tests. MedlinePlus. https://medlineplus.gov/lab-tests/acid-fast-bacillus-afb-tests/
- Remel Inc. (2010, March 22). TB Kinyoun carbolfuchsin [Instructions for use]. Thermo Fisher Scientific.
- Sawadogo, T. L., Savadogo, L. G. B., Diande, S., Ouedraogo, F., Mourfou, A., Gueye, A., Sawadogo, I., Nebié, B., Sangare, L., & Ouattara, A. S. (2012). [Comparison of Kinyoun, auramine O, and Ziehl-Neelsen staining for diagnosing tuberculosis at the National Tuberculosis Center in Burkina Faso]. Médecine et Santé Tropicales, 22(3), 302–306. https://doi.org/10.1684/mst.2012.0082
- Sigma-Aldrich. (2024, July 22). Microscopy AFB-Color carbol fuchsin solution: For the microscopic investigation of acid-fast bacteria (AFB) (cold staining) [Instructions for use].
- Sultana, M. M., Naher, A., Paul, S., Tangim, S. F., Hossain, M. E., Khan, T., & Sultana, S. (2022). Sensitivity and specificity of Auramin-Rhodamin stain for diagnosis of pleural tuberculosis. Journal of Enam Medical College, 12(1), 24–28. https://doi.org/10.3329/jemc.v12i1.71689
- UCSF Health. (2023, December 31). Acid-fast stain. University of California San Francisco. https://www.ucsfhealth.org/medical-tests/acid-fast-stain
- UF Health. (2023, December 31). Acid-fast stain. University of Florida Health.
- Van Rie, A., Fitzgerald, D., Kabuya, G., Van Deun, A., Tabala, M., Jarret, N., Behets, F., & Bahati, E. (2008). Sputum smear microscopy: Evaluation of impact of training, microscope distribution, and use of external quality assessment guidelines for resource-poor settings. Journal of Clinical Microbiology, 46(3), 897–901. https://doi.org/10.1128/JCM.01553-07
- Watson, R. (n.d.). Ziehl Neelsen acid-fast stain. The Virtual Edge. University of Wyoming. http://www.uwyo.edu/virtual_edge/units/acidfast_stain.html
- Wikipedia. (2025, January 10). Mycolic acid. https://en.wikipedia.org/wiki/Mycolic_acid
- Wikipedia. (2025, October 22). Ziehl–Neelsen stain. https://en.wikipedia.org/wiki/Ziehl%E2%80%93Neelsen_stain