What is Lysine Iron Agar (LIA)?
- Lysine Iron Agar (LIA) is a type of differential medium that is commonly used in microbiology laboratories to test organisms for their ability to deaminate or decarboxylate lysine. It was initially developed by Edwards and Fife in 1961 as a presumptive identification tool for Salmonella species, including lactose-fermenting Salmonella arizonae, which has been linked to foodborne gastroenteritis outbreaks.
- The purpose of Lysine Iron Agar is to differentiate between different types of bacteria based on their metabolic activities. It consists of peptone and yeast extract, which provide essential nutrients for bacterial growth, and dextrose, which serves as a fermentable carbohydrate. Ferric ammonium citrate and sodium thiosulphate are included in the medium as indicators of hydrogen sulphide (H2S) formation.
- The LIA medium is prepared by solidifying the agar in test tubes. The medium is then stabbed to the base of the butt and streaked on the slant. The inoculated medium is then incubated under appropriate conditions to allow bacterial growth and metabolic reactions to occur.
- Lysine deamination is an aerobic process that takes place on the slant of the LIA medium. Bacteria that deaminate lysine produce an alpha-ketocarboxylic acid, which reacts with the iron salt present near the surface of the medium in the presence of oxygen. This reaction results in the formation of a reddish-brown compound, indicating lysine deamination.
- On the other hand, lysine decarboxylation is an anaerobic process that occurs in the butt of the LIA medium. Bacteria capable of decarboxylating lysine produce the amine cadaverine, leading to an alkaline reaction and the development of a purple color in the butt of the medium.
- Additionally, LIA can be used to distinguish between coliform organisms such as Escherichia and Shigella. Some strains of lactose-fermenting Salmonella species can rapidly ferment lactose, which may suppress hydrogen sulphide (H2S) production on other media, such as Triple Sugar Iron Agar. By using LIA in conjunction with other selective media, a more accurate discrimination between different bacterial species can be achieved.
- In summary, Lysine Iron Agar is a differential medium that allows microbiologists to detect lysine deamination and decarboxylation reactions in bacteria. It is particularly useful for presumptively identifying lactose-fermenting Salmonella species, including those associated with foodborne outbreaks. The medium’s distinctive color changes and gas production patterns aid in the differentiation of various bacterial species based on their metabolic capabilities.
Principle of Lysine Iron Agar (LIA)
The principle of Lysine Iron Agar (LIA) lies in its ability to differentiate bacteria based on their metabolic activities, specifically lysine deamination and decarboxylation reactions. The composition of LIA includes lysine, peptones, a small amount of glucose, ferric ammonium citrate, and sodium thiosulfate.
LIA has a dual nature, with an aerobic slant and an anaerobic butt. To perform the test, the LIA medium is stabbed to the base of the butt and streaked on the slant. The reactions that take place in the medium provide valuable information about the metabolic capabilities of the bacteria being tested.
When the bacteria in the medium ferment glucose, organic acids are produced, resulting in an acidic environment in the butt of the medium, which is indicated by a yellow color.
If the organism being tested produces lysine decarboxylase, it acts on lysine and forms a compound called cadaverine. Cadaverine has the ability to neutralize the organic acids produced by glucose fermentation. As a result, the butt of the medium reverts to an alkaline state, turning purple.
If the organism does not produce lysine decarboxylase, the butt of the medium remains acidic, maintaining its yellow color.
In addition to the decarboxylation reaction, LIA is also capable of detecting lysine deamination. When oxidative deamination of lysine occurs, a compound is formed. In the presence of ferric ammonium citrate and the coenzyme flavin mononucleotide, this compound reacts and produces a burgundy color on the slant of the medium.
If deamination does not occur, the slant of the LIA medium remains purple. The pH indicator used in LIA, bromocresol purple, is yellow at or below pH 5.2 and purple at or above pH 6.8. This indicator helps to visualize the pH changes in the medium.
Peptone and yeast extract present in the medium provide essential nutrients for bacterial growth. Dextrose serves as a source of fermentable carbohydrates. Ferric ammonium citrate and sodium thiosulfate act as indicators for the formation of hydrogen sulfide (H2S). Bacterial cultures that produce hydrogen sulfide cause blackening of the medium due to the production of ferrous sulfide.
In summary, the principle of Lysine Iron Agar involves observing color changes in the medium, indicating the metabolic activities of bacteria. These changes are associated with lysine deamination and decarboxylation reactions, glucose fermentation, and hydrogen sulfide production. The medium’s composition and indicators help to differentiate bacterial species based on their metabolic capabilities.
Composition of Lysine Iron Agar (LIA)
| Ingredients | Gms/liter |
| Peptone | 5.000 |
| Yeast extract | 3.000 |
| Dextrose (Glucose) | 1.000 |
| L-Lysine | 10.00 |
| Ferric ammonium citrate | 0.500 |
| Sodium thiosulphate | 0.040 |
| Bromocresol purple | 0.020 |
| Agar | 15.000 |
Final pH (at 25°C): 6.7±0.2
Preparation of Lysine Iron Agar (LIA)
To prepare Lysine Iron Agar (LIA), the following steps are typically followed:
- Measure out 34.56 grams of LIA powder and add it to 1000 ml of distilled water.
- Heat the mixture while stirring to ensure the medium completely dissolves.
- Continue heating until the solution reaches boiling point, ensuring thorough mixing.
- Once the medium is completely dissolved and homogeneous, it is ready for dispensing.
- Transfer the LIA medium into tubes, ensuring proper labeling for identification purposes.
- The tubes containing the LIA medium are then sterilized using an autoclave. The autoclaving process involves subjecting the tubes to high-pressure steam at 121°C (15 lbs pressure) for approximately 15 minutes. This sterilization step is crucial for eliminating any potential contaminants.
- After autoclaving, the tubes should be allowed to cool in a slanted position. This positioning will enable the medium to solidify into slants with deep butts.
- Once the LIA tubes have cooled and solidified, they are ready for use in bacterial culture and testing.
Following these steps ensures the proper preparation and sterilization of Lysine Iron Agar, providing a suitable medium for the growth and metabolic reactions of bacteria during laboratory experiments and tests.
Method of Use of Lysine Iron Agar (LIA)
The following is the method of using Lysine Iron Agar (LIA) for bacterial culture and interpretation of results:
- Prior to inoculation, allow the LIA medium to adjust to room temperature. This is important to ensure optimal growth conditions for the bacteria.
- From a pure culture plate, select a well-isolated colony using a sterile straight inoculating needle.
- Inoculate the LIA Slant by inserting the needle into the center of the tube twice, reaching the bottom of the tube. This is done to introduce the bacteria into the anaerobic butt of the medium. Then, streak the slant using a fishtail motion to distribute the bacteria along the slanted surface of the medium.
- After inoculation, tightly cap the LIA tube and place it in an incubator set at 35°C. Incubate the tubes for the specified duration, typically 18 to 24 hours. This allows sufficient time for bacterial growth and metabolic reactions to occur.
- Once the incubation period is complete, remove the tubes from the incubator and examine them. Observe and interpret the results based on the observed color changes and other indicators.
- Interpretation of results typically involves observing the color changes in the butt and slant regions of the LIA medium. Changes in color, such as yellow, purple, or burgundy, can provide information about the metabolic activities of the bacteria.
- Compare the observed results with known patterns or reference guides to identify the bacterial species or make presumptive identifications based on the characteristic reactions seen in LIA.
By following these steps, the LIA medium can be effectively used for the growth, metabolic testing, and interpretation of bacterial cultures. The incubation period allows the bacteria to exhibit specific reactions, aiding in the identification or differentiation of bacterial species.
Result Interpretation on Lysine Iron Agar (LIA)
Interpretation of results on Lysine Iron Agar (LIA) involves observing the color changes and reactions in the medium. Here is a summary of the result interpretations on LIA:
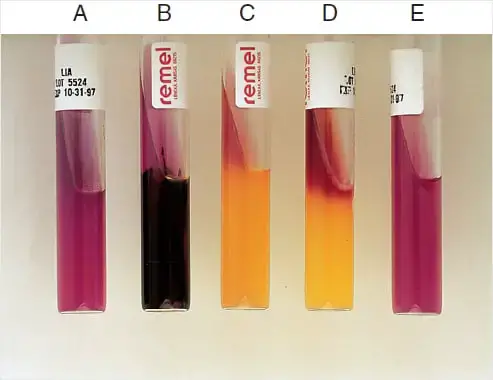
- Purple slant/purple butt (K/K): This indicates an alkaline slant and alkaline butt. It signifies that the organism is negative for lysine deaminase but positive for lysine decarboxylase.
- Purple slant/yellow butt (K/A): This result shows an alkaline slant and an acid butt. It suggests that the organism is negative for both lysine deaminase and lysine decarboxylase, but it ferments glucose.
- Red slant/yellow butt (R/A): This represents a red slant and an acid butt. It indicates that the organism is positive for lysine deaminase but negative for lysine decarboxylase. It also ferments glucose.
- Black precipitate: This indicates hydrogen sulfide (H2S) production, resulting in the formation of black precipitates in the medium. It suggests sulfur reduction by the organism.
Additionally, specific bacterial species may exhibit characteristic growth patterns and reactions on LIA:
- Citrobacter freundii: Luxuriant growth; acidic butt (yellow); alkaline slant (purple or no color change); positive H2S production (blackening of medium).
- Escherichia coli: Luxuriant growth; alkaline butt (purple or no color change); alkaline slant (purple or no color change); negative H2S production.
- Proteus mirabilis: Luxuriant growth; acidic butt (yellow); deep red slant indicating lysine deamination; positive H2S production (blackening of medium).
- Salmonella Arizonae, Enteritidis, Typhimurium: Luxuriant growth; alkaline butt (purple or no color change); alkaline slant (purple or no color change); positive H2S production (blackening of medium).
- Shigella flexneri: Luxuriant growth; acidic butt (yellow); alkaline slant (purple or no color change); negative H2S production.
Interpretation of gas production is often irregular or suppressed on LIA, and gas bubbles or cracks may be observed in the medium.
By analyzing the color changes, reactions, and specific growth patterns, microbiologists can make preliminary identifications or differentiations of bacterial species based on their metabolic capabilities using LIA.
a revised version of the table:
| Color | Result | Interpretation |
|---|---|---|
| Purple slant/purple butt | Alkaline slant/alkaline butt (K/K) | Lysine deaminase negative; Lysine decarboxylase positive |
| Purple slant/yellow butt | Alkaline slant/acid butt (K/A) | Lysine deaminase negative; Lysine decarboxylase negative; Glucose fermentation |
| Red slant/yellow butt | Red slant/acid butt (R/A) | Lysine deaminase positive; Lysine decarboxylase negative; Glucose fermentation |
| Black precipitate | H2S production | Sulfur reduction Proteus spp. are capable |
| Organisms | Growth |
| Citrobacter freundii | Luxuriant growth; butt: acidic reaction, yellowing of the medium; slant: alkaline reaction, purple or no color change; positive reaction for H2S, blackening of medium |
| Escherichia coli | Luxuriant growth; butt: alkaline reaction, purple or no color change; slant: alkaline reaction, purple or no color change; negative reaction for H2S |
| Proteus mirabilis | Luxuriant growth; butt: acidic reaction, yellowing of the medium; slant: deep red, lysine deamination; positive reaction for H2S, blackening of medium |
| Salmonella Arizonae | Luxuriant growth; butt: alkaline reaction, purple or no color change; slant: alkaline reaction, purple or no color change; positive reaction for H2S, blackening of medium |
| Salmonella Enteritidis | Luxuriant growth; butt: alkaline reaction, purple or no color change; slant: alkaline reaction, purple or no color change; positive reaction for H2S, blackening of medium |
| Salmonella Typhimurium | Luxuriant growth; butt: alkaline reaction, purple or no color change; slant: alkaline reaction, purple or no color change; positive reaction for H2S, blackening of medium |
| Shigella flexneri | Luxuriant growth; butt: acidic reaction, yellowing of the medium; slant: alkaline reaction, purple or no color change; negative reaction for H2S |
Quality Control of Lysine Iron Agar (LIA)
Quality control of Lysine Iron Agar (LIA) involves assessing various parameters to ensure the consistency and reliability of the medium. Here is a summary of the quality control measures for LIA:
- Appearance: The LIA powder should have a light yellow to greyish yellow color and be a free-flowing homogeneous powder.
- Gelling: After preparation, the medium should form a firm gel that is comparable to a 1.5% Agar gel.
- Color and Clarity of prepared medium: The LIA medium should have a purple color and appear clear to slightly opalescent when prepared in tubes as slants.
- pH: The pH of a 3.45% w/v aqueous solution of LIA should be 6.7 ± 0.2.
- pH Range: The pH range of the prepared LIA medium should be between 6.50 and 6.90.
- Cultural Response: The cultural characteristics of specific reference strains should be observed after incubation at 35-37°C for 18-24 hours. This includes growth patterns, reactions in the butt and slant regions, and H2S production.
- Organism Inoculum: The recommended inoculum size for the reference strains should be 50-100 CFU (colony-forming units).
- Growth: The reference strains should exhibit luxuriant growth on the LIA medium.
- Butt and Slant Reactions: The reference strains should display the expected acid or alkaline reactions in the butt and slant regions, as specified in the results.
- H2S Production: The reference strains that are expected to produce H2S should show a positive reaction, resulting in the blackening of the medium.
By regularly performing these quality control measures, laboratories can ensure that their LIA medium is reliable, consistent, and suitable for bacterial identification and differentiation based on lysine metabolism and H2S production.
Uses of Lysine Iron Agar (LIA)
Lysine Iron Agar (LIA) has several uses in the field of microbiology. These uses include:
- Differentiation of enteric organisms: LIA is recommended for the differentiation of enteric organisms based on their ability to decarboxylate or deaminate lysine and form hydrogen sulfide (H2S). By observing the color changes in the medium, microbiologists can identify specific metabolic capabilities of the tested bacteria.
- Detection of lactose fermenting and non-fermenting Salmonella: LIA is a sensitive medium for the detection of both lactose fermenting and lactose non-fermenting Salmonella species. This makes it a valuable tool for identifying Salmonella strains involved in foodborne outbreaks or other infections.
- Differentiation of Gram-negative bacilli, especially Enterobacteriaceae: LIA is particularly useful in distinguishing different types of Gram-negative bacilli, especially among the Enterobacteriaceae family. The medium’s ability to detect lysine deamination, decarboxylation, and H2S production helps in the differentiation and identification of various bacterial species within this group.
- Presumptive identification of Proteus and Providencia species: LIA can be used for the presumptive identification of Proteus and Providencia species. These bacteria typically exhibit a characteristic reaction on LIA, producing a red slant and an alkaline butt. This specific color pattern aids in the preliminary identification of these species.
Overall, Lysine Iron Agar is a versatile medium that finds application in the differentiation and identification of enteric organisms, Salmonella strains, Gram-negative bacilli (particularly Enterobacteriaceae), and the presumptive identification of Proteus and Providencia species. Its ability to detect lysine metabolism and H2S production contributes to its usefulness in microbiological laboratories for various diagnostic and research purposes.
Limitations of Lysine Iron Agar (LIA)
Lysine Iron Agar (LIA) has some limitations that should be taken into consideration when using this medium. These limitations include:
- Additional testing for complete identification: While LIA can provide preliminary identification based on lysine metabolism and H2S production, it is recommended to perform further biochemical, immunological, molecular, or mass spectrometry testing on colonies from pure culture for complete and accurate identification of bacterial species.
- Importance of proper stabbing: Stabbing the butt of the LIA medium is crucial for valid test results. Failure to stab the butt properly can invalidate the test and compromise the accuracy of the interpretation.
- Sensitivity for H2S detection: LIA may not be as sensitive as other iron-containing media, such as Sulfide Indole Motility (SIM) Medium and Triple Sugar Iron Agar (TSIA), in detecting hydrogen sulfide (H2S) production. Therefore, if H2S production is a critical aspect of the identification, other media should be used as complementary or alternative tests.
- Proteus sp. and H2S production: Proteus species that produce H2S may not result in blackening of the LIA medium. This is because the acid produced by these organisms during glucose fermentation can suppress H2S formation. Therefore, additional testing, such as Triple Sugar Iron (TSI) Agar, should be used as a follow-up identification method to confirm H2S production in these cases.
- Variability in carbohydrate fermentation: Some species or strains of bacteria may exhibit delayed reactions or completely fail to ferment the carbohydrate in the expected manner. This can result in atypical or inconclusive results on LIA.
- Irregular or suppressed gas production: Gas production, a by-product of certain metabolic cycles, may be irregular or suppressed on LIA. This means that gas bubbles or cracks may not be consistently observed in the medium, making it a less reliable indicator of gas production.
- LIA is not a substitute for TSI: LIA should not be used as a substitute for Triple Sugar Iron (TSI) Agar. While both media provide information about carbohydrate fermentation and H2S production, TSI offers more comprehensive results and is generally preferred for accurate identification.
It is important to be aware of these limitations when using Lysine Iron Agar to ensure proper interpretation of results and to complement the test with other appropriate methods for complete bacterial identification.
FAQ
What is Lysine Iron Agar (LIA)?
Lysine Iron Agar (LIA) is a differential medium used in microbiology to test organisms for their ability to deaminate or decarboxylate lysine, as well as their capacity to produce hydrogen sulfide (H2S).
How is LIA prepared?
To prepare LIA, the medium is dissolved in distilled water, boiled to ensure complete dissolution, dispensed into tubes, and then sterilized by autoclaving. The tubes are cooled in a slanted position to form slants with deep butts.
How is LIA used?
LIA is used by inoculating the medium with a well-isolated colony from a pure culture plate. The medium is inoculated by stabbing the butt of the tube and streaking the slant. The tubes are then incubated at an appropriate temperature.
What do the different color changes in LIA indicate?
The color changes in LIA can provide valuable information about the metabolic capabilities of the bacteria being tested. Purple indicates alkaline conditions, yellow represents acidic conditions, and red suggests lysine deamination.
What is the purpose of LIA?
The purpose of LIA is to differentiate bacterial species based on their metabolic activities, particularly their ability to deaminate lysine, decarboxylate lysine, and produce H2S.
What can LIA detect in terms of Salmonella?
LIA is particularly useful for the detection of lactose fermenting and non-fermenting Salmonella species, making it a valuable tool in identifying Salmonella strains involved in foodborne outbreaks.
What are the limitations of LIA?
Some limitations of LIA include the need for additional testing for complete identification, the importance of proper stabbing for valid results, and its relatively lower sensitivity in detecting H2S compared to other media. It may also have limitations in detecting H2S production in certain bacteria, such as Proteus species.
Can LIA be used as a substitute for Triple Sugar Iron (TSI) Agar?
No, LIA is not a substitute for TSI Agar. While both media provide information about carbohydrate fermentation and H2S production, TSI Agar is generally preferred for accurate identification and comprehensive results.
How long should LIA be incubated before interpreting the results?
The tubes of LIA should be incubated for 18 to 24 hours before interpreting the results. This allows sufficient time for bacterial growth and metabolic reactions to occur.
Can LIA be used for presumptive identification of certain bacterial species?
Yes, LIA can be used for the presumptive identification of specific species such as Proteus and Providencia. These bacteria may exhibit characteristic reactions on LIA, aiding in their preliminary identification. However, confirmatory testing is necessary for accurate identification.
References
- https://exodocientifica.com.br/_technical-data/M377.pdf
- https://www.neogen.com/globalassets/pim/assets/original/10007/ncm0140_ts_en-us.pdf
- http://www.dalynn.com/dyn/ck_assets/files/tech/TL97.pdf
- https://assets.fishersci.com/TFS-Assets/MBD/Instructions/IFU453772.pdf
- https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/223/800/62915dat.pdf
- https://hardydiagnostics.com/media/assets/product/documents/LysineIronAgar-LIA.pdf
- http://faculty.collin.edu/dcain/CCCCD%20Micro/lysine_iron_agar.htm
- https://www.bd.com/resource.aspx?IDX=22842
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.