What is Yersinia Pestis?
- Yersinia pestis, a gram-negative coccobacillus bacterium, is the causative agent of the disease commonly referred to as the plague. This bacterium belongs to the genus Yersinia, which is part of the enterobacteria family, encompassing three distinct human pathogens. Among these, Yersinia pestis stands out due to its historical significance and its unique biological characteristics.
- Morphologically, Y. pestis is a facultative anaerobic organism, implying its ability to thrive in both oxygen-rich and oxygen-deprived environments. Its shape is a fusion of the spherical attributes of cocci and the elongated features of bacilli, appearing as short ovals when observed microscopically. Unlike many bacteria, Y. pestis is non-motile, meaning it lacks the capability to move autonomously within its surroundings. To propagate, it necessitates a host organism, classifying it as an obligate parasite.
- Historically, Y. pestis has been a significant pathogen, responsible for devastating pandemics such as the Plague of Justinian and the Black Death, the latter being the most lethal pandemic recorded in human history. The disease manifests in three primary forms: pneumonic, septicemic, and bubonic. Intriguingly, Y. pestis is not only a parasite to humans but also to the Oriental rat flea (Xenopsylla cheopis), which acts as its primary vector. This dual parasitic relationship categorizes Y. pestis as a hyperparasite.
- The discovery of this bacterium can be credited to Alexandre Yersin of the Pasteur Institute in Paris, during a plague outbreak in Hong Kong in 1894. While initially named Pasteurella pestis, the bacterium was later renamed Yersinia pestis in 1944 in honor of its discoverer.
- In contemporary times, the World Health Organization reports between one thousand to two thousand cases of the plague annually. However, with the advent of antibiotics, the prognosis for affected individuals has significantly improved. Notably, during the Vietnam War era, Asia witnessed a surge in cases, likely due to ecological disturbances and increased human-animal interactions. Presently, over 95% of reported cases originate from sub-Saharan Africa and Madagascar. Beyond its impact on humans, Y. pestis also affects various non-human mammals, including the black-tailed prairie dog and the endangered black-footed ferret in the United States.
- In summary, Yersinia pestis is a scientifically intriguing bacterium with a rich historical background. Its unique characteristics and its role in past pandemics make it a focal point in the study of infectious diseases.
Yersinia Pestis Definition
Yersinia pestis is a gram-negative coccobacillus bacterium responsible for causing the disease known as the plague, historically linked to significant pandemics such as the Black Death. This facultative anaerobic organism is an obligate parasite, primarily transmitted to humans through the bite of infected fleas.
General characteristics of Yersinia Pestis
Yersinia pestis, a bacterium responsible for the plague, exhibits several distinct morphological and biochemical characteristics:
- Morphology and Staining: Y. pestis is a Gram-negative coccobacillus with dimensions approximately 1.5 X 0.7 mm. The bacteria often arrange themselves singly, in short chains, or in small clusters. A notable feature is its bipolar staining when subjected to Giemsa or methylene blue stains. This results in a unique appearance where the two ends of the bacterium are densely stained, leaving the central region clear.
- Polymorphism: In aged cultures, Y. pestis displays polymorphism, meaning it can adopt various shapes. These can range from coccoid and club-shaped to filamentous and even giant forms. The presence of 3% NaCl in the culture medium can further enhance this involution.
- Mobility and Sporulation: The bacterium is inherently non-motile, does not form spores, and is not acid-fast.
- Capsular Formation: Y. pestis possesses capsules, though they are more prominently observed in cultures maintained at 37°C compared to the optimal growth temperature of 27°C.
- Biochemical Properties: Y. pestis is a facultative anaerobic bacterium, meaning it can grow in both oxygen-rich and oxygen-deprived environments. It exhibits bipolar staining, which imparts a “safety pin” appearance due to its antiphagocytic slime layer. Biochemically, it tests negative for urease production, lactose fermentation, and indole synthesis. This differentiates it from other bacteria, even within the Yersinia genus.
- Phylogenetics: Y. pestis shares a close genetic relationship with Yersinia pseudotuberculosis. Another relative, though more distantly related, is Yersinia enterocolitica.
In summary, Yersinia pestis is a bacterium with a unique set of characteristics that distinguish it from other pathogens. Its morphology, staining properties, and biochemical attributes make it a subject of interest in the realm of microbiology.
Taxonomy of Yersinia Pestis
The genus Yersinia is a member of the family Enterobacteriaceae and comprises a diverse group of species. Currently, there are at least 18 recognized or proposed species within this genus. Among these, Yersinia pestis stands out as the type species, with its close relatives being the enteropathogenic species Y. pseudotuberculosis and Y. enterocolitica. The genus also includes species that are primary pathogens of fish, such as Y. ruckeri, and others that function as secondary invaders or saprophytes.
- Genomic Characteristics:
- The DNA guanine-cytosine (GC) content of Y. enterocolitica is slightly higher (47-48.5 mol%) compared to Y. pestis and Y. pseudotuberculosis (46-47 mol%).
- DNA hybridization studies have revealed that Y. pestis shares approximately 9% homology with E. coli, 23% with Y. enterocolitica, and a significant 83% with Y. pseudotuberculosis. This high degree of homology with Y. pseudotuberculosis suggests a recent divergence of Y. pestis from this species, estimated to be around 10,000 years ago.
- Plasmid-Encoded Virulence:
- The human pathogenic species (Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica) possess a shared 70-kb virulence plasmid. In Y. pestis, this plasmid is termed pCD, while in the enteropathogenic yersiniae, it is referred to as pYV.
- Taxonomic Relationships:
- Analysis of 16S rRNA genes has shown that the Yersinia genus forms a coherent cluster. Within this, Y. pestis, Y. pseudotuberculosis, and certain isolates of Y. kristensenii form one subline, while Y. enterocolitica constitutes another.
- Two primary systems of nomenclature have emerged for Y. pestis. One system divides the species into subspecies based on host range and biochemical reactions. The other system, based on populations, incorporates a rooted phylogenetic tree constructed from single nucleotide polymorphisms (SNPs) evaluated in global strains.
- Subspecies Classification:
- The most widely recognized subspecies is Y. pestis subspecies pestis, which can cause lethal disease in a broad range of mammals. This subspecies is globally distributed and poses a potential risk to inhabitants of most continents.
- Another subspecies, Y. pestis subspecies microtus, is restricted to central Asia and primarily affects rodents of the Superfamily Muroidea. This subspecies is often termed ‘enzootic’.
In conclusion, Yersinia pestis is a bacterium with a rich taxonomic history, closely related to other species within the Yersinia genus. Its classification and understanding have been refined over the years through advanced genomic and phylogenetic studies, providing insights into its evolutionary trajectory and relationships with other species.
Characteristic Features of Yersinia Pestis
Yersinia pestis, a member of the gamma-proteobacteria class, possesses distinct genomic and phenotypic features that contribute to its pathogenicity and classification:
- Genomic Evolution:
- The genome size of Y. pestis ranges between 4.60–4.65 Mb. It is believed to have emerged approximately 20,000 years ago through reductive evolution from its close relative, Yersinia pseudotuberculosis, which has a genome size of 4.72 Mb.
- Genome analysis reveals the presence of numerous insertion sequences, evidence of lateral gene transfer, intragenomic recombination, and remnants indicative of a past enteric lifecycle.
- Plasmid Composition:
- A common 70–75-kb plasmid found in all pathogenic Yersinia species encodes a type III secretion system, known as Yops (“Yersinia Outer Proteins”), and the V antigen.
- A larger 100–110-kb plasmid is responsible for encoding the capsular F1 glycoprotein antigen and the Yersinia murine toxin (Ymt).
- A smaller 9.5-kb plasmid encodes the plasminogen activator Pla, which is believed to facilitate the systemic spread of Y. pestis from peripheral sites, and pesticin, a bacteriocine that aids in iron capture within mammalian hosts.
- Phenotypic Characteristics:
- Yersinia pestis is an aerobic, non-sporulated, Gram-negative bacillus. It is oxidase-negative, urease-negative, and catalase-positive.
- It exhibits slow growth at 28°C and pH 7.4.
- The bacterium is known for its lipopolysaccharide endotoxin and iron uptake mechanisms, which contribute to the pigmentation of colonies when grown on Congo red medium.
- Biochemical Profiling and Subspecies:
- Based on biochemical profiling, nine biovars of Y. pestis have been proposed, including the well-known Y. pestis subsp. pestis. Other proposed subspecies, such as angola, altaica, and caucasica, have been isolated from various rodent species in different regions.
- Distinct biotypes of Y. pestis subsp. pestis have been identified based on their glycerol and nitrate reduction capabilities. For instance, the Antiqua biotype is both glycerol and nitrate reduction-positive, while the Orientalis biotype is glycerol-negative but nitrate reduction-positive.
- Unique to the Orientalis isolates of Y. pestis subsp. pestis is their ability to stabilize a specific bacteriophage in their chromosome, which contributes to their pathogenicity.
In summary, Yersinia pestis is a bacterium with a rich set of genomic and phenotypic features that have evolved over time, enabling it to be a potent pathogen. Its distinct plasmids, biochemical properties, and subspecies classifications further underscore its complexity and adaptability.
Habitat of Yersinia Pestis
Yersinia pestis, the causative agent of the plague, predominantly resides in a variety of wild animals. Notably, animals such as dogs, squirrels, rabbits, and mice serve as natural reservoirs for this bacterium. These animals play a crucial role in the maintenance and dissemination of Y. pestis in the environment, facilitating its transmission and perpetuation in various ecosystems.
Epidemiology of Yersinia Pestis
Yersinia pestis, the bacterium responsible for the plague, has a unique epidemiological profile that distinguishes it from other pathogens. Several key aspects of its epidemiology are as follows:
- Current Distribution: Despite historical pandemics, the plague remains dormant in many regions. However, due to the third pandemic, the bacterium is now more widespread in global sylvatic reservoirs than ever before. This extensive presence in wild animals suggests that complete eradication of the disease in the foreseeable future is highly improbable.
- Host-Parasite Dynamics: A fundamental principle in epidemiology posits that a parasite should not severely harm its host, as doing so would jeopardize the environment essential for its survival. While this principle holds true for many pathogens, including Y. enterocolitica and Y. pseudotuberculosis, Y. pestis is an exception. For transmission to occur, Y. pestis must fatally infect its host, prompting flea vectors to seek new hosts for sustenance.
- Impact on Vectors: Notably, Y. pestis not only affects its mammalian hosts but also its flea vectors. The bacterium can lead to the death of blocked fleas, making it unique in its ability to cause mortality in both its primary host and its vector.
- Transmission Mechanism: The transmission dynamics of Y. pestis are distinct. The bacterium has evolved to create a severe septicaemia in its mammalian hosts, leading to rapid death. This acute progression often outpaces the host’s immune response, resulting in fatality before any significant immune intervention can occur.
- Environmental Adaptation: Y. pestis has carved a specialized ecological niche for itself. This niche is so well-defined and protected that the bacterium remains largely unaffected by environmental changes, with temperature being a notable exception. Its evolution has been directed more towards colonizing fleas and inducing severe septicaemia in hosts rather than adapting to external environmental pressures.
In essence, the epidemiology of Yersinia pestis is characterized by its unique transmission dynamics, its impact on both hosts and vectors, and its specialized ecological niche. This bacterium’s ability to cause rapid and severe infections in its hosts, combined with its widespread presence in wild reservoirs, underscores the challenges in managing and eradicating the disease.
Structure of Yersinia Pestis
- Yersinia pestis, the causative agent of the plague, exhibits a distinct cellular structure that is crucial for its pathogenicity and survival. When cultured under optimal conditions or within a host, the cells of Y. pestis are characterized as small, robust, and resemble the typical morphology of Gram-negative rods.
- A distinguishing feature of Y. pestis is its lack of certain genes found in enteropathogenic yersiniae. These genes, which are responsible for the regulation or production of flagella and the lipopolysaccharide (LPS) O-group structure, are either entirely absent or remain unexpressed in Y. pestis.
- As a result, the plague bacilli are inherently non-motile. Additionally, at room temperature, these cells exhibit a “rough” morphological appearance. However, when exposed to host temperatures, the colonies may present a smoother appearance due to the temperature-dependent synthesis of Caf1, a specific protein.
- Another intriguing aspect of Y. pestis is its coloration. Cell-free extracts of both Y. pestis and Y. pseudotuberculosis, when cultivated at 37°C, display a pronounced red-brown hue. This distinct coloration is attributed to the presence of KatY, a significant protein endowed with catalase-peroxidase activity. Interestingly, this protein appears to be redundant in its function.
- In contrast, colonies of Y. pestis remain colorless post-growth at 37°C. However, when incubated at 26°C, these colonies can absorb specific pigmented molecules, such as haemin and Congo red. This unique property, termed Pgm1, is encoded by the haemin storage locus (Hms) found within a specific chromosomal sequence.
- This sequence is prone to spontaneous deletions, leading to the loss of the entire Pgm locus. As a result, strains that lack this locus are termed Pgm2, while those that only lose the ability to absorb haemin or Congo red are designated as Hms−.
- In summary, the structural attributes of Yersinia pestis are intricately linked to its genetic makeup, which in turn influences its pathogenic capabilities and interactions with the host environment. The bacterium’s ability to adapt its structure based on temperature and other external factors underscores its evolutionary prowess and resilience.
Transmission of Yersinia Pestis
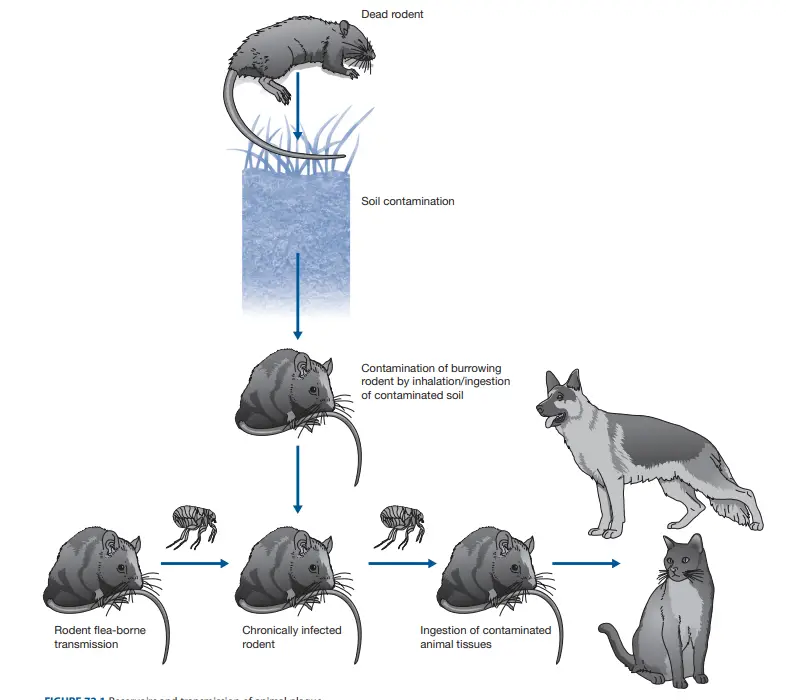
Yersinia pestis, the causative agent of plague, has multiple modes of transmission. Here’s a detailed breakdown:
- Flea Bites:
- The most common mode of transmission is through the bite of infected fleas. When a flea feeds on an infected rodent, it can ingest the bacteria. When the flea later bites a human, it can transmit the bacteria, leading to bubonic plague.
- The primary vector is the rat flea, Xenopsylla cheopis, but other fleas can also transmit the disease.
- Direct Contact:
- Humans can become infected through direct contact with contaminated tissues or fluids from an infected animal, especially when handling the carcasses of infected animals.
- This can lead to septicemic plague if the bacteria enter the bloodstream.
- Inhalation:
- Pneumonic plague, a severe form of the disease, can be transmitted between humans through the inhalation of respiratory droplets from a person who has pneumonic plague. This form of transmission can lead to rapid outbreaks in populations.
- Consumption:
- Though rare, humans can get infected by consuming undercooked meat of infected animals.
- Other Vectors:
- Besides fleas, other biting insects have occasionally been implicated in the transmission of the plague.
- Domestic Animals:
- Cats and dogs can bring infected fleas into homes, increasing the risk of human infection. Additionally, cats can develop pneumonic plague, which can then be transmitted to humans.
Prevention primarily involves controlling rodent and flea populations, avoiding contact with wild animals, and taking precautions in areas where plague is endemic.
Virulence factors of Yersinia Pestis
Yersinia pestis, the causative agent of the plague, possesses a multifaceted array of virulence factors that facilitate its pathogenicity. These factors, encoded both chromosomally and on plasmids, play pivotal roles in the bacterium’s ability to infect and cause disease. Here are the key virulence factors:
- Plague Toxin:
- Endotoxin: A di-polysaccharide present in the cell wall, the endotoxin is instrumental in many of the systemic manifestations of diseases caused by Y. pestis.
- Murine Toxins: These proteinaceous toxins exhibit characteristics of both exotoxins and endotoxins. Released upon cell lysis, they are thermolabile and can be toxoided. While they affect rats and mice, they are inactive in guinea pigs, rabbits, and primates. Their effects encompass local edema, neurosis, and systemic impacts on the vascular system and liver.
- F1 Antigens:
- A heat-labile protein, the F1 antigen is produced exclusively by virulent strains at 37°C. Governed by a plasmid, it impedes phagocytosis and is crucial for bestowing protective immunity in both humans and mice.
- V and W Antigens:
- V Antigen: A 90 Kilo-Dalton protein.
- W Antigen: A 145 K-Da acidic lipoprotein.
- Both antigens are synthesized by virulent Y. pestis strains at 37°C in calcium-deficient conditions. They deter phagocytosis and the intracellular destruction of the bacterium within macrophages.
- Type III Secretion System (TTSS):
- Comprising numerous proteins, the TTSS aids in the secretion of Y. pestis virulence factors into host cells. It empowers the bacterium to evade phagocytic killing and curtails cytokine production, thereby diminishing the host’s inflammatory immune response.
- Enzymatic Virulence Factors:
- Y. pestis produces enzymes like coagulase, fibrinolysin, plasmalogen activator protease, which bolster its virulence. The enzyme PIA degrades specific complement components, thwarting opsonization and phagocytic migration. Additionally, these enzymes dissolve fibrin clots, enabling rapid bacterial spread.
- Iron Absorption:
- The bacterium’s virulence is augmented by its capacity to assimilate organic iron through a siderophore-independent mechanism.
- Surface Component:
- An unidentified surface component of Y. pestis binds hemin and basic aromatic dyes, resulting in pigmented colonies in culture.
- Persuasive Synthesis:
- The bacterium’s ability for persuasive synthesis has also been linked to its virulence.
In summary, the virulence of Yersinia pestis is a result of a combination of toxins, antigens, secretion systems, enzymes, and other factors. These elements collectively enable the bacterium to invade, evade host defenses, and proliferate, leading to the severe manifestations of the plague.
Pathogenesis of Yersinia Pestis
Yersinia pestis, the causative agent of the plague, has a complex pathogenesis that allows it to effectively invade and proliferate within the human host. Here’s an in-depth look into its pathogenic process:
- Initial Infection:
- The infection in humans typically begins with the bite of an ectoparasite. This bite induces a localized inflammatory response at the site of entry.
- Lymphatic Spread:
- Following the initial infection, Y. pestis migrates via the lymphatic system towards the regional lymph nodes.
- Bubo Formation:
- The bacterium experiences exponential growth within the lymph node, leading to the formation of a characteristic swollen and painful lymph node termed a “bubo.”
- Systemic Dissemination:
- Y. pestis doesn’t remain localized. It spreads through the lymphatic and circulatory systems, reaching vital organs such as the spleen and liver. This widespread dissemination can lead to a severe and rapidly progressing septicemia.
- If the bacterium reaches the lungs, it can cause secondary pneumonic plague, a highly contagious and often fatal form of the disease.
- Further dissemination to the meninges results in meningitis, characterized by inflammation of the protective membranes covering the brain and spinal cord.
- Coagulation and Shock:
- The hematogenous spread of Y. pestis to various organs and tissues can trigger intravascular coagulation, leading to the formation of small blood clots throughout the body. This, coupled with the effects of bacterial endotoxins, can precipitate endotoxic shock, a life-threatening condition.
- Immune Evasion:
- One of the reasons Y. pestis is so virulent is its ability to shield itself from the human immune system. It achieves this through serum resistance and by evading the innate immune responses of the host.
- Intracellular Proliferation:
- Y. pestis is not just an extracellular pathogen. It is a facultative intracellular bacterium, meaning it can invade and multiply within host cells, specifically macrophages. This intracellular lifestyle further aids in its evasion from the immune system and contributes to its pathogenicity.
In essence, the pathogenesis of Yersinia pestis is a multifaceted process involving initial infection, systemic spread, immune evasion, and intracellular proliferation. Its ability to rapidly multiply within human tissues and evade the host’s immune defenses underscores its potency as a pathogen and the severity of the diseases it causes.
Animal Infection of Yersinia Pestis
Yersinia pestis, the causative agent of plague, exhibits a multifaceted interaction with various animal species and their associated ectoparasites. Here’s a comprehensive overview of its impact on the animal kingdom:
- Persistence in Soil:
- Yersinia pestis has the capability to survive in soil for extended periods, spanning several months. This characteristic potentially aids in the prolonged existence of plague foci and might serve as a primary infection source for burrowing rodents.
- Rodent Reservoirs:
- Over 200 rodent species can become infected with Y. pestis. Among these, plague-resistant species are perceived as reservoirs, harboring the bacterium without manifesting disease symptoms.
- Ectoparasite Vectors:
- While various ectoparasites, including ticks, can be naturally infected with Y. pestis, fleas play a pivotal role in its transmission.
- A staggering 131 flea species have been identified as primary vectors transmitting the bacterium among rodents.
- The transmission efficacy of fleas is enhanced due to the bacterium’s ability to block the flea’s proventriculus. This blockage, caused by the expression of the plasminogen activator Pla by the ingested bacteria, leads to increased hunger in fleas, prompting them to feed more frequently. This, in turn, results in the contamination of the bite site with regurgitated, bacteria-laden blood.
- Mechanisms of Suppression:
- Yersinia pestis has evolved sophisticated mechanisms to inhibit inflammatory responses in hosts that are resistant to plague. This suppression allows the bacterium to achieve high concentrations in the blood, reaching up to 10^8 organisms/ml. Such high concentrations facilitate efficient transmission, even with the minuscule volume of blood (approximately 10^-4 ml) ingested by a flea.
- Carnivore Infection:
- Carnivorous animals can contract the infection upon consuming infected rodents. However, certain species, such as dogs and other canids, exhibit resistance to the disease.
- Life Cycle Complexity:
- The intricate life cycle of Yersinia pestis, encompassing soil, ectoparasites, and mammals, leads to fluctuating prevalence rates of animal plague, known as enzoonosis. This can occasionally culminate in sudden, large-scale mortality events in animals susceptible to plague, termed epizoonoses.
In summary, Yersinia pestis maintains a complex relationship with the animal kingdom, involving various species and their ectoparasites. Understanding these interactions is crucial for effective plague management and control.
Human Plague of Yersinia Pestis
Yersinia pestis, the bacterium responsible for the plague, has a multifaceted transmission pattern in humans. Here’s a detailed examination of its transmission routes and implications:
- Bubonic Plague via Flea Bites:
- The primary mode of transmission for bubonic plague is through the bite of an infected flea. When their primary hosts, typically rodents, die off in large numbers, these fleas seek alternative blood sources, including humans. Given the flea’s necessity for daily blood meals for survival, the risk of human infection escalates significantly post a massive die-off of plague-infected rodents.
- Inoculation through Skin Lacerations:
- Direct contact with, and especially the skinning of, dead animals can lead to the transmission of Y. pestis if there are skin lacerations present. Such exposure can result in either bubonic or septicemic forms of the plague.
- Primary Pneumonic Plague:
- Inhalation of Y. pestis from large droplets can lead to primary pneumonic plague. Such transmission typically occurs during close contact (less than 1.5 meters) with infected cats or humans who are coughing up significant amounts of bloody sputum. On average, every primary inhalational case results in approximately 1.3 secondary cases.
- Ingestion-based Transmission:
- Consuming raw or undercooked food contaminated with Y. pestis can lead to infection. Notably, camel meat and liver have been frequently identified as sources of food-borne transmission.
- Human Ectoparasitic Transmission:
- The role of human ectoparasites in the transmission of Y. pestis is gaining attention, especially in the context of historical plague epidemics. Transmission through the anthropophilic flea, Pulex irritans, and the human body louse, Pediculus humanus, has been proposed, especially in cases where multiple family members are infected. Experimental evidence further supports the potential role of the human body louse in plague transmission.
In essence, the human plague caused by Yersinia pestis can manifest through various transmission routes, each with its unique set of challenges and implications. Understanding these pathways is crucial for effective prevention and control measures in affected regions.
Geographic Distribution Of Human Plague
The global distribution of the human plague, caused by the bacterium Yersinia pestis, is influenced by various ecological and environmental factors. Here’s a comprehensive overview of its geographic spread:
- Plague Foci and Soil Reservoirs:
- The existence of soil reservoirs and the relative resilience of different rodent species contribute to the consistent re-emergence of plague in established foci across all continents, with the exceptions of Antarctica and Oceania.
- Re-emergence in Historical Hotspots:
- Over the past 15 years, several regions have witnessed outbreaks of human plague after dormant periods spanning 30 to 50 years. These regions include:
- India (1994)
- Zambia (1996)
- Indonesia (1997)
- Algeria (2003, 2009)
- Congo (2005)
- Uganda (2006)
- Libya and China (2009)
- Over the past 15 years, several regions have witnessed outbreaks of human plague after dormant periods spanning 30 to 50 years. These regions include:
- Recent Data on Plague Cases:
- According to the World Health Organization’s 2009 update:
- Congo reported 966 cases in 2007.
- Zambia had 700 cases.
- Madagascar reported 591 cases.
- Uganda had 257 cases.
- Tanzania reported 59 cases.
- The USA had 7 cases.
- Mongolia reported a single case.
- According to the World Health Organization’s 2009 update:
- Plague as a Re-emerging Disease:
- Contrary to the large historic urban outbreaks observed in previous centuries, the plague is now predominantly contracted during outdoor professional and recreational activities. In the USA, activities involving close, prolonged contact with pet dogs, such as sharing a bed, have been significantly linked to the disease.
- European Context:
- While Europe hasn’t reported recent human cases, animal plague remains active, especially along the western banks of the Caspian Sea.
- Seasonal Patterns:
- Human plague exhibits a seasonal pattern, which varies across different geographic areas. Even within a single country, the seasonal trends can differ.
In summary, the human plague, while having historical roots, continues to pose a threat in various parts of the world. Its geographic distribution is influenced by a combination of ecological, environmental, and human behavioral factors. Understanding these patterns is vital for effective public health interventions and disease control.
Genome of Yersinia Pestis
The genome of Yersinia pestis, the causative agent of the plague, provides a comprehensive understanding of its pathogenicity and evolutionary adaptations. This genome has been meticulously annotated, revealing insights into its virulence mechanisms, chromosomal configurations, and plasmid content.
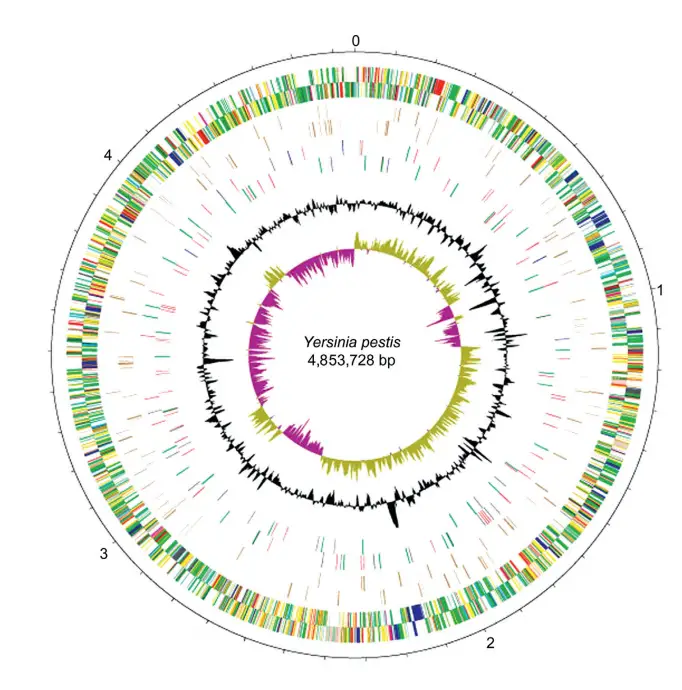
Chromosomal Features:
- The chromosome of Y. pestis strain CO92 is a model for genomic studies. It showcases genes categorized by function, polarity, pseudogenes, IS elements, and variations in G+C content.
- The chromosome has undergone multiple genomic rearrangements, including translocations and inversions. These changes, however, do not seem to impact the bacterium’s virulence.
- Lateral gene transfers have introduced genes into the genome, including those encoding insecticidal toxins. Some of these toxins remain inactive in Y. pestis, potentially to preserve its flea vector.
- Many surface structures, such as lipopolysaccharide (LPS), flagella, and fimbriae, were present in the ancestral Y. pestis but have since been inactivated. This inactivation likely enhances the bacterium’s ability to invade host tissues, a crucial step for causing acute disease.
- The chromosome houses a significant number of IS elements, far more than its close relative, Y. pseudotuberculosis. These IS elements play a role in genomic plasticity and adaptation.
Plasmid Overview:
- Y. pestis possesses three notable plasmids that contribute to its virulence:
- pPCP: This plasmid encodes for the plasminogen activator (Pla), which is crucial for the bacterium’s invasiveness. Pla facilitates the bacterium’s spread within the host. Most strains of Y. pestis carry this plasmid, making it a hallmark of its pathogenicity.
- pCD: Also known as pYV in other Yersinia species, this plasmid is present in all Yersinia strains pathogenic to humans. It encodes for virulence effectors called Yops and the V antigen. The presence of this plasmid imparts specific growth requirements to the bacterium, such as the need for calcium at 37°C.
- pMT: Exclusive to Y. pestis, this plasmid encodes the capsular antigen (Caf1) and a phospholipase toxin lethal to rodents. While Caf1 enhances virulence in certain hosts, it’s not essential for human infection. The phospholipase toxin, on the other hand, is vital for colonizing fleas, the primary vector for plague transmission.
In summary, the genome of Yersinia pestis is a testament to its evolutionary journey as a potent pathogen. Its chromosomal features and plasmids offer a blueprint of its virulence strategies, making it one of the most studied bacteria in the realm of infectious diseases.
Physiology of Yersinia Pestis
Yersinia pestis, the causative agent of the plague, exhibits unique physiological characteristics that differentiate it from other bacterial species. Understanding its growth patterns and nutritional requirements provides insights into its pathogenicity and survival mechanisms.
Growth Dynamics:
- Contrary to earlier beliefs, Y. pestis does not grow more rapidly at ambient temperatures (around 26°C) compared to the host body temperature (37°C). The misconceptions might have arisen due to a lack of understanding of its specific growth requirements.
- When cultivated under optimal conditions, including a 10% CO2 concentration, Y. pestis forms visible colonies within 24 hours, similar to the growth rate of enteropathogenic yersiniae. For consistent growth in liquid cultures, it is advisable to subculture the bacteria multiple times in the same medium before conducting experiments. Under these conditions, both Y. pestis and enteropathogenic yersiniae have a doubling time of approximately 70 minutes at both 26°C and 37°C.
Nutritional Requirements:
- Y. pestis has a temperature-dependent nutritional requirement for calcium (Ca^2+). Additionally, it can utilize potassium D-gluconate as an energy source, which helps maintain a neutral pH during growth. This ability to utilize D-gluconate is beneficial as it can tolerate concentrations up to 0.04 M.
Genomic Degeneration:
- Since its divergence from Y. pseudotuberculosis, Y. pestis has undergone significant chromosomal degeneration. This evolutionary process has led to the loss of several genes that influence its overall physiology. These genetic changes have implications for its metabolic capabilities and pathogenic potential.
Phenotypic Traits and Virulence:
The virulence of Y. pestis is influenced by its phenotypic traits. Specific combinations of its plasmids and genetic markers determine its lethality in different hosts. For instance, strains of Y. pestis carrying certain plasmids exhibit varying levels of lethality when introduced into mice through different routes of infection. The presence or absence of these plasmids and genetic markers can significantly alter the bacterium’s ability to cause disease.
In conclusion, the physiology of Yersinia pestis is a complex interplay of its growth dynamics, nutritional requirements, genomic changes, and phenotypic traits. These factors collectively contribute to its ability to cause one of the most devastating diseases in human history.
Catabolism and Anabolism of Yersinia Pestis
Catabolism:
- Yersinia pestis, a bacterium responsible for the plague, employs glycolytic and terminal oxidation pathways similar to those observed in E. coli. However, a distinguishing feature of Y. pestis is the absence of Zwf and AspA in most of its biovars, except the most primitive enzootic ones.
- The absence of Zwf hinders the synthesis of pentose and the generation of NADPH through the hexose monophosphate pathway. AspA, on the other hand, is crucial for the conversion of L-aspartate to fumarate, an intermediate in the tricarboxylic acid cycle.
- These deficiencies have implications for the bacterium’s response to low calcium. For instance, using D-gluconate as an energy source instead of D-glucose can prevent cell lysis under specific conditions.
- Additionally, the absence of added sodium in calcium-deficient media restricts the secretion of L-aspartate but allows for vegetative growth. This sodium-dependent removal of L-aspartate, which is catabolically inert, likely explains the bacterium’s nutritional need for CO2.
Anabolism:
- In terms of anabolic processes, Y. pestis exhibits slow growth at 5°C on enriched solid media. On chemically defined solid minimal media at 26°C, it requires a set of specific nutrients, including inorganic salts, sulfur, a fermentable carbohydrate, L-methionine, and either glycine or L-threonine.
- The need for glycine or L-threonine arises due to the loss of formyltetrahydrofolate hydrolase (PurU), a phenomenon also observed in E. coli. Depending on the biovar or population, additional nutritional requirements may be present. For instance, biovar orientalis typically needs L-phenylalanine due to a genetic insertion within the pheA gene.
- However, other biovars might not have this insertion and can synthesize L-phenylalanine independently. The role of L-isoleucine and L-valine in the growth of biovar orientalis remains an area of interest.
- Notably, Y. pestis is not inhibited by L-valine in defined media, unlike E. coli, possibly due to a deletion in the acetolactate synthase regulatory subunit (IlvN). Different biovars of Y. pestis subspecies microtus have varying nutritional requirements.
- While some only need inorganic salts and a fermentable carbohydrate, others have requirements similar to epidemic strains but with distinct metabolic lesions. The genetic basis for these anabolic blocks in both enzootic and epidemic strains is intricate and requires further exploration.
In summary, the catabolic and anabolic pathways of Yersinia pestis are complex and influenced by specific genetic and environmental factors. Understanding these metabolic processes provides insights into the bacterium’s survival, growth, and pathogenicity.
Yersinia Pestis Symptoms
The clinical manifestations of Yersinia pestis infection vary based on the form of the disease it induces. The three primary forms are bubonic, septicemic, and pneumonic plague. The incubation period, which is the time between exposure to the bacterium and the onset of symptoms, typically ranges from one to six days for all forms.
- Bubonic Plague:
- Origin: This is the most prevalent form of the plague, resulting from the bite of an infected flea. The bacteria travel through the bloodstream and localize in the lymph nodes, where they proliferate.
- Symptoms: Initial symptoms include a sudden onset of fever, headache, and chills. The hallmark sign is the development of one or more swollen, painful lymph nodes, termed buboes. As the bacteria multiply, the lymph nodes may become pus-filled and tender to touch.
- Septicemic Plague:
- Origin: This form arises when Y. pestis directly invades the bloodstream, leading to a rapid and widespread infection.
- Symptoms: Unlike the bubonic form, septicemic plague might not always present with buboes. However, it can lead to both bubonic and pneumonic forms due to its dissemination to lymph nodes and lungs. A notable symptom is the appearance of signs of necrosis, particularly in extremities like the fingers, toes, and nose.
- Pneumonic Plague:
- Origin: This is the most severe form and occurs when the bacteria infect the lungs, either directly through inhalation of infected droplets or secondary to a bubonic or septicemic infection.
- Symptoms: Initial symptoms mirror those of the bubonic form, including fever, headache, and chills. However, these are swiftly followed by respiratory symptoms such as coughing, chest pain, and difficulty breathing. Notably, pneumonic plague is the only form that can spread from person to person through respiratory droplets.
In summary, Yersinia pestis can manifest in various forms, each with its distinct set of symptoms. Recognizing these symptoms is crucial for timely diagnosis and treatment, given the bacterium’s potential for rapid progression and high fatality rate.
Diagnosis of Yersinia Pestis
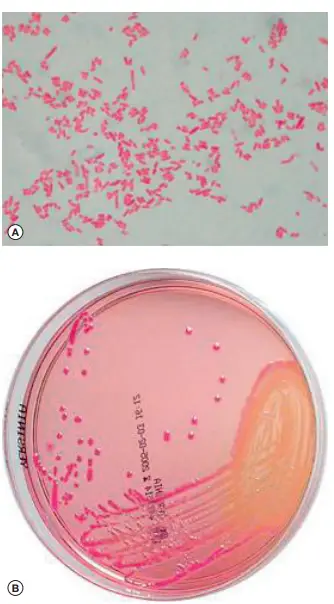
Yersinia pestis, the causative agent of plague, necessitates a meticulous diagnostic approach, especially in patients presenting with febrile adenitis from regions known for active plague epizoonoses. Here’s a comprehensive overview of the diagnostic methods:
- Specimen Collection:
- Initial steps involve collecting whole blood in successive blood culture bottles, sputum, urine, and lymph node fluid. The latter can be obtained using saline for aspiration.
- In certain cases, cerebrospinal fluid (CSF) might be collected based on clinical indications.
- Environmental specimens could encompass ectoparasites from the patient’s body and clothes, as well as samples from animals suspected to be infection sources.
- Laboratory Precautions:
- While clinical and environmental specimens can be processed in a Biosafety Level (BSL)-2 laboratory, the culture and handling of Y. pestis mandate a BSL-3 laboratory setting due to its high pathogenicity.
- Point-of-Care Diagnosis:
- A rapid diagnostic approach involves the use of a dipstick immunochromatographic assay that detects the F1 capsular antigen. This assay can be applied to various samples, including urine, whole blood, bubo pus, and sputum.
- Direct microscopic examination post Gram staining can identify Gram-negative bacilli with a unique “hairpin appearance” due to bipolar staining.
- Molecular Diagnostics:
- PCR-based assays offer a precise method for detecting Y. pestis.
- Isolation and culture require incubation at 32°C in a 5% CO2 atmosphere for several days. Specialized selective media, such as cefsulodin-Irgasan-novobiocin (CIN) and beef heart-Irgasan-novobiocin (BIN) agar, aid in the growth of the bacterium.
- Rapid identification can be achieved using immunochromatographic detection of the F1 antigen on bacterial colonies. Peptidic profiling through mass spectrometry is another effective identification method.
- Antibiotic Susceptibility Testing:
- Essential due to the emergence of antibiotic-resistant strains, especially in regions like Madagascar. Some strains have shown resistance to multiple antibiotics, including those typically used for treatment and prophylaxis.
- Typing of Y. pestis:
- Traditional methods involve testing for nitrate reduction, glycerol assimilation, and rhamnose assimilation to determine the biotype.
- Advanced molecular typing techniques include single nucleotide polymorphism analysis, regions of deletion analysis, and multiple loci variable number of tandem repeats (VNTR) analysis, among others.
- Serology:
- Modern serological techniques are valuable for gauging seroprevalence in sentinel animals. They can also be employed for rapid diagnosis during outbreaks when serum is the primary available specimen.
In conclusion, the diagnosis of Yersinia pestis is multifaceted, combining traditional culture methods with advanced molecular techniques. Given the severity of the disease, timely and accurate diagnosis is paramount to ensure effective treatment and control of outbreaks.
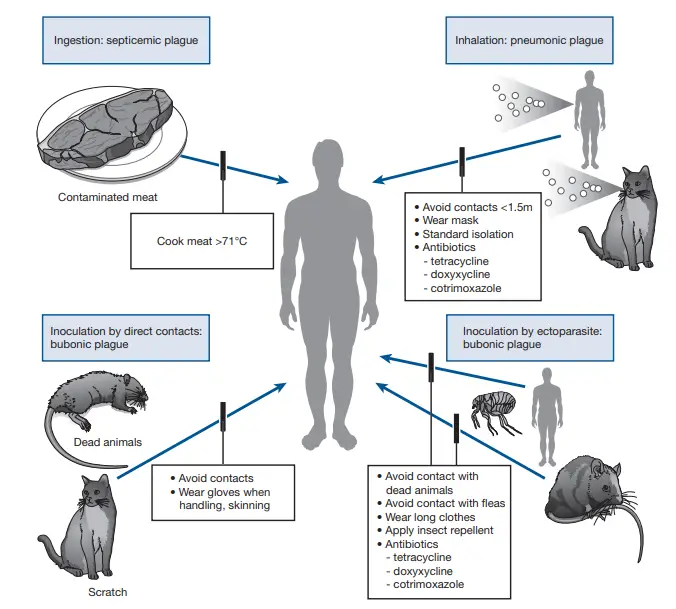
Treatment of Yersinia Pestis
Yersinia pestis, the causative agent of plague, demands prompt and effective treatment to mitigate its severe consequences. Here’s a detailed overview of the therapeutic approaches:
- Immediate Antibiotic Administration:
- It is imperative to initiate an effective antibiotic regimen as soon as plague is suspected in a patient.
- Historically, streptomycin has been the reference antibiotic, especially in regions like Madagascar. However, its availability has dwindled in many countries.
- Alternative Antibiotics:
- Gentamicin, either as a standalone treatment or combined with a tetracycline, serves as a suitable alternative to streptomycin.
- Studies have indicated that gentamicin and doxycycline are comparably effective in treating patients, barring those in the terminal stage of the disease.
- Other Potential Treatments:
- Fluoroquinolones and cephalosporins have demonstrated efficacy in animal models. However, their use in human patients is not widely recommended due to limited clinical data supporting their effectiveness. Notably, there’s a singular case where plague was successfully treated using ciprofloxacin.
- Duration of Treatment:
- The antibiotic regimen should persist for a duration of 10 days to ensure complete eradication of the bacterium.
- Typically, patients exhibit clinical improvement within 2-3 days post the initiation of antibiotic treatment. However, it’s not uncommon for fever to linger for a few additional days.
- Supportive Therapy:
- In cases where the patient develops septic shock or progresses to septicemic plague, supportive therapy becomes crucial. This involves measures to stabilize the patient and manage the complications arising from the systemic spread of the infection.
In summation, the treatment of Yersinia pestis infections necessitates a swift and targeted approach. While antibiotics remain the mainstay of therapy, the choice of drug and the need for supportive care depend on the severity and progression of the disease. Proper treatment is vital to reduce morbidity and mortality associated with this formidable pathogen.
Prevention And Control of Yersinia Pestis
Yersinia pestis, the bacterium responsible for plague, poses significant public health challenges. Effective prevention and control measures are paramount to mitigate its spread and impact. Here’s an overview of the strategies:
- Primary Prevention Before Exposure:
- Refrain from visiting regions with active epizootic plague.
- Steer clear of visibly ill or deceased animals and promptly report such sightings to health authorities.
- Minimize exposure to rats, fleas, and other rodents. This includes:
- Wearing protective clothing and using repellents to deter ectoparasites when outdoors.
- Applying DEET-based insect repellents to lower extremities.
- Treating clothes and bedding with repellents and insecticides.
- Employing gloves when handling deceased animals and ensuring thorough cooking of meat.
- Vaccination:
- Several vaccines, including the live attenuated Y. pestis EV76 strain and the F1 fraction, have been developed.
- However, the F1 fraction vaccine has shown limited efficacy and has been associated with side effects in a significant proportion of recipients.
- Current evidence is insufficient to conclusively determine the effectiveness and long-term impacts of these vaccines in plague control.
- Secondary Prevention Post Exposure:
- Administer tetracycline or trimethoprim-sulfamethoxazole to individuals:
- Exposed to fleas during an outbreak.
- In contact with fluids or tissues from an infected animal.
- Residing in a household with a bubonic plague patient.
- In close proximity to a suspected pneumonic plague case or infected pet.
- Recent studies suggest considering doxycycline as a primary therapeutic agent against bioterrorism agents, including Y. pestis.
- In mass casualty scenarios, oral administration of doxycycline, tetracycline, or ciprofloxacin is recommended.
- Administer tetracycline or trimethoprim-sulfamethoxazole to individuals:
- Preventing Human-to-Human Transmission:
- Isolate pneumonic plague patients and adhere to standard isolation protocols for a minimum of four days post the commencement of antibiotic therapy.
- Control Measures:
- Implement active surveillance of sentinel animals.
- In regions with epizootic outbreaks:
- Eliminate rodent food and shelter sources near homes, workplaces, and recreational areas.
- Regularly treat pets with flea control measures.
- During human plague outbreaks:
- Rapidly diagnose and treat confirmed and suspected cases.
- Eradicate human ectoparasites and animal fleas using licensed insecticides.
In essence, the prevention and control of Yersinia pestis necessitate a multifaceted approach, encompassing personal protective measures, vaccination, post-exposure prophylaxis, and community-wide interventions. Proper implementation of these strategies is crucial to curtail the spread and impact of this formidable pathogen.
Quiz
What is the primary disease caused by Yersinia pestis?
A. Tuberculosis
B. Malaria
C. Plague
D. Cholera
Which of the following is NOT a characteristic of Yersinia pestis cells?
A. Small size
B. Gram-negative
C. Motile
D. Robust structure
At what temperature does Yersinia pestis exhibit a smoother colony appearance due to the production of Caf1?
A. 26°C
B. 37°C
C. 42°C
D. 20°C
Which protein in Yersinia pestis is responsible for its distinct red-brown coloration when grown at 37°C?
A. Hms
B. Pgm1
C. Caf1
D. KatY
Which locus in Yersinia pestis is associated with the absorption of haemin and Congo red?
A. Caf1 locus
B. KatY locus
C. Hms locus
D. Pgm2 locus
Which of the following is NOT a transmission route for the plague caused by Yersinia pestis?
A. Flea bites
B. Contaminated water
C. Inhalation
D. Direct contact with infected tissues
Which gene in Yersinia pestis is responsible for the regulation of flagella?
A. Hms gene
B. Caf1 gene
C. KatY gene
D. None of the above
What is the primary reservoir for Yersinia pestis?
A. Birds
B. Rodents
C. Cattle
D. Fish
Which form of the plague is transmitted through respiratory droplets?
A. Bubonic plague
B. Septicemic plague
C. Pneumonic plague
D. Enteric plague
Which of the following is NOT a symptom of the bubonic plague caused by Yersinia pestis?
A. Swollen lymph nodes
B. High fever
C. Cough with bloody sputum
D. Chills
FAQ
What is Yersinia pestis?
Yersinia pestis is a bacterium that causes the disease known as the plague, which includes forms such as bubonic, septicemic, and pneumonic plague.
How is the plague transmitted to humans?
The primary mode of transmission is through the bite of infected fleas. It can also be transmitted through direct contact with infected tissues or fluids, or by inhaling respiratory droplets from a person with pneumonic plague.
What are the symptoms of the plague caused by Yersinia pestis?
Symptoms vary depending on the form of the plague but can include swollen and painful lymph nodes, fever, chills, weakness, and fatigue. Pneumonic plague can also cause respiratory symptoms like coughing and difficulty breathing.
Is there a treatment available for the plague?
Yes, the plague is treatable with antibiotics. Early detection and treatment are crucial for a positive outcome.
How can the spread of the plague be prevented?
Preventive measures include reducing rodent habitats, using insect repellent, treating pets for fleas, and avoiding contact with wild animals.
Is Yersinia pestis a new bacterium?
No, Yersinia pestis has been responsible for several historical pandemics, including the Black Death in the 14th century.
Can Yersinia pestis be used as a bioterrorism agent?
Due to its high virulence and potential for aerosolized transmission, Yersinia pestis is considered a potential bioterrorism agent.
How is a diagnosis of the plague confirmed?
Diagnosis is typically confirmed through laboratory tests, including blood, sputum, or lymph node aspirate cultures.
Are there any vaccines available against the plague?
Currently, there is no widely available vaccine against the plague. Research is ongoing to develop an effective vaccine.
What is the mortality rate of the plague if left untreated?
If left untreated, the bubonic form of the plague has a mortality rate of 50-60%, while the pneumonic form can be fatal within 24 hours of symptom onset.
References
- Brubaker, R. R. (2015). Yersinia pestis. Molecular Medical Microbiology, 1845–1865. doi:10.1016/b978-0-12-397169-2.00103-7
- Haiko, J., Korhonen, T. K., & Laakkonen, L. (2013). Plasminogen Activator of Yersinia pestis. Handbook of Proteolytic Enzymes, 289–293. doi:10.1016/b978-0-12-382219-2.00069-7
- Drancourt, M. (2013). Plague. Hunter’s Tropical Medicine and Emerging Infectious Disease, 584–590. doi:10.1016/b978-1-4160-4390-4.00072-2
- Easterday, W., Kausrud, K., Star, B. et al. An additional step in the transmission of Yersinia pestis?. ISME J 6, 231–236 (2012). https://doi.org/10.1038/ismej.2011.105
- Barbieri, R., Signoli, M., Chevé, D., Costedoat, C., Tzortzis, S., Aboudharam, G., … Drancourt, M. (2020). Yersinia pestis: the Natural History of Plague. Clinical Microbiology Reviews, 34(1). doi:10.1128/cmr.00044-19
- Analysis of 3800-year-old Yersinia pestis genomes suggests Bronze Age origin for bubonic plague by M. Spyrou et al.
- Yersinia pestis in Pulex irritans Fleas during Plague Outbreak, Madagascar by J. Ratovonjato et al. Link to Paper
- Human ectoparasite transmission of the plague during the Second Pandemic is only weakly supported by proposed mathematical models by Sang Woo Park et al. Link to Paper
- Identification of Risk Factors Associated with Transmission of Plague Disease in Eastern Zambia by S. Nyirenda et al. Link to Paper
- https://www.medindia.net/patientinfo/bubonic-plague.htm#symptoms-and-signs
- https://www.lecturio.com/concepts/yersinia-pestis-plague/