Wright Giemsa stain is a polychromatic staining method used mainly for blood smears and bone marrow samples. It is the combination of Wright’s stain and Giemsa stain, and it is the process in which eosin Y and methylene blue with its oxidation products (azure dyes) are used together to produce different colour reactions in the cells. It is the Romanowsky effect that gives the characteristic purple colour to the nucleus and neutrophil granules, and this effect is not produced when the individual dyes are used alone. The acidic dye eosin binds with the basic components like hemoglobin to give orange-pink colour, while the basic methylene blue derivatives binds with the acidic components like nucleic acids to give blue-purple colour.
It is the modified form of the original Romanowsky stain. Romanowsky first used eosin and aged methylene blue in 1891 for staining malaria parasites. Later Wright introduced a method of heating methylene blue to make a polychrome dye and mixing it with eosin Y, and this mixture was dissolved in methanol which also acted as the fixative.
Afterwards Giemsa added glycerol to stabilise the stain, and this combination improved the basophilic and nuclear staining. These are the reasons why Wright Giemsa stain gives sharper nuclear details and brighter cytoplasmic granules compared to the individual stains.
It is used for routine hematology because it gives clear nuclear details and helps in identifying different blood cells, abnormal cells, Auer rods in leukemic blasts, and toxic granulation in neutrophils. It is also used for detection of blood parasites like Plasmodium. The staining quality depends on pH of buffer solution, and it is usually 6.4–6.8. When the pH becomes alkaline the smear appears more blue, and when it becomes acidic the smear appears more red. It is important that methanol fixation is proper because the dye complex is unstable in water, and the process requires correct timing to avoid precipitation or distortion of cells.
Wright Giemsa Stain Principle
Wright Giemsa stain is the polychromatic neutral stain in which eosin Y and polychromed methylene blue are used together to produce differential colouring of blood cells. It is the methanol that keeps the dye mixture dissolved and it is also the fixative, and when it is applied on the smear it dehydrates the cells and fixes the proteins to the slide. This is referred to as the process where the fixation occurs before the aqueous staining step, and this helps in preventing the loss of cellular details.
The principle is based on the electrostatic interaction between the dye ions and the cellular components. The acidic cell structures which have negative charge attract the basic cationic dyes like azure and methylene blue, and these structures become blue or purple which is called basophilia. The basic cell structures having positive charge attract the acidic eosin Y and these become red or orange, and this is eosinophilia. It is this acid–base binding that makes the different cells to appear in different colours.
The Romanowsky effect is the important feature in this stain. It is the interaction between eosin Y and azure B that produces the characteristic purple shades which are not given by the dyes alone. This effect helps in showing the nuclear chromatin and the neutrophil granules in violet or reddish-purple colour. The Giemsa component makes this effect stronger, and the nuclear borders become sharper with bright cytoplasmic granules.
The pH of the buffer is the critical factor in this staining method. The usual buffer is at pH 6.4–6.8 because at this pH the ionization of dyes and proteins is proper. When the pH becomes low the smear becomes more red, and the nuclei look pale. When the pH becomes high the smear becomes more blue, and the nuclear details get obscured. It is the process where the dye forms precipitate in water, and so the aqueous step must be controlled carefully to allow proper staining without artefacts.
Requirements
- Giemsa stain powder is required for preparing the staining solution.
- Absolute methanol (acetone-free) and glycerol are used for dissolving the stain powder and making the stock stain.
- Absolute methanol (100%) is used as the fixative for thin blood smears.
- Buffer salts like disodium phosphate (Na₂HPO₄) and sodium phosphate monobasic (NaH₂PO₄·H₂O) are needed for preparing the buffer.
- Distilled or deionized water is used because tap water affects the pH.
- Triton X-100 solution (5%) is sometimes added for proper wetting of the smear.
- Immersion oil is required for microscopic examination after staining.
- Coplin jars or staining jars are required to hold the stain and buffer.
- Brown glass bottles are used for storing the stock stain to protect it from light.
- Glass beads may be added to the bottle to help in mixing the stain.
- Filter paper (Whatman No. 1) is used for filtering the stain to avoid precipitates.
- Clean and dry microscope slides are used for preparing smears.
- A timer is needed because staining depends on accurate timing.
- Fresh blood samples are needed to make the smears immediately.
- Thin smears are fixed with methanol, and thick smears are left unfixed to allow RBC lysis.
- A quality-control smear is kept for checking the stain performance.
- Buffer pH is important, usually pH 7.2 for malaria detection and pH 6.8 for general blood cell staining.
- The working stain (like 2.5% solution) is prepared freshly because it is unstable after dilution.
- Staining time depends on concentration, such as 45–60 minutes for 2.5% stain or around 10 minutes for 10% stain.
- The stock stain is shaken daily for 30–60 minutes for about 14 days so that the stain matures properly.
Wright Giemsa Stain Procedure
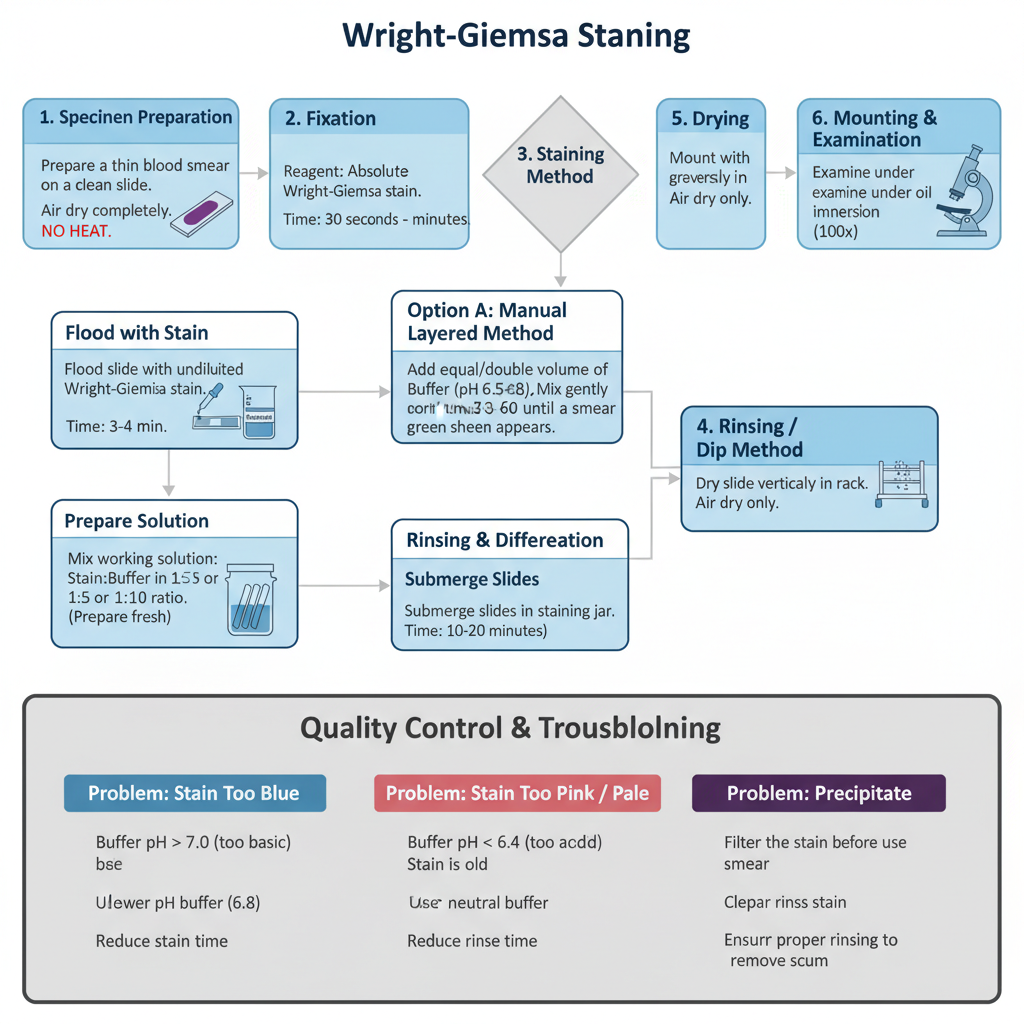
I. Specimen Preparation
- It is required to prepare a thin blood smear on a clean slide. The smear is one cell thick at the viewing area and it moves from a thick region to a feathered edge.
- The smear is allowed to air dry. Heat should not be used because it changes the cell morphology.
- Fixation is done immediately after complete drying. Delay in fixation causes cell degradation and the stain intensity becomes poor.
II. Fixation
- The reagent used is absolute methanol (methyl alcohol).
- The slide is immersed in methanol or the slide is flooded with methanol.
- The fixation time is generally between 30 seconds to 3 minutes.
- It is the process that dehydrates the cells and keeps the cellular proteins stable during the aqueous staining step.
III. Staining Method Options
Two staining approaches is used in laboratories. These are the Manual layered method and the Immersion method.
Option A: Manual Layered Method
- The slide is placed horizontally and the smear is flooded with undiluted Wright–Giemsa stain for 3–4 minutes for dye penetration.
- An equal or double volume of phosphate buffer (pH 6.5–6.8) or distilled water is added directly on the stain.
- The stain and buffer is mixed gently until a metallic green sheen appears on the top.
- The mixture is allowed to stand for 6–8 minutes which is almost double the initial stain time.
Option B: Immersion / Dip Method
- A working solution is prepared by mixing stain with buffer in 1:5 or 1:10 ratio.
- Slides are submerged in the staining jar.
– The staining time is 10–20 minutes depending on dilution and expected stain depth.
– Rapid stains use dipping in undiluted stain for 60 seconds and then buffer for 60 seconds. - Working stain solution is unstable. It should be prepared fresh or replaced every 6 hours.
IV. Rinsing and Differentiation
- The slide is rinsed with distilled water or phosphate buffer (pH 6.5–6.8).
- Rinsing is continued until the thin smear edges become faint pinkish–red which usually needs 30–60 seconds.
- Excessive rinsing decolorizes the smear. In the layered method, the slide is flooded with water first so that the scum does not stick to the smear.
V. Drying and Mounting
- The slide is dried vertically in a rack. Air drying is recommended and heat is avoided.
- For permanent slide preparation, the slide may be dipped in xylene and mounted using a mounting medium and cover glass.
- Microscopic examination is done under oil immersion (100x) with immersion oil.
VI. Quality Control and Troubleshooting
- Buffer pH controls color quality.
- pH 6.4–6.8 gives pink/tan RBCs and purple nuclei.
- pH 7.2 is used during parasite study such as Plasmodium because it improves dot visibility.
- Precipitate removal when black or purple granules appear:
- Filter the stain.
- Clean jars and staining equipment.
- Ensure proper rinsing.
- Stain may be diluted by 5% with methanol if separation occurs.
Color correction
- Too Blue: buffer is too basic (pH > 7.0) or smear thickness is high. A lower pH buffer (6.8) is used or staining time is reduced.
- Too Pink / Pale: buffer is acidic (pH < 6.4) or stain is old. A neutral buffer is used or a fresh stain is prepared.
Wright Giemsa Stain result
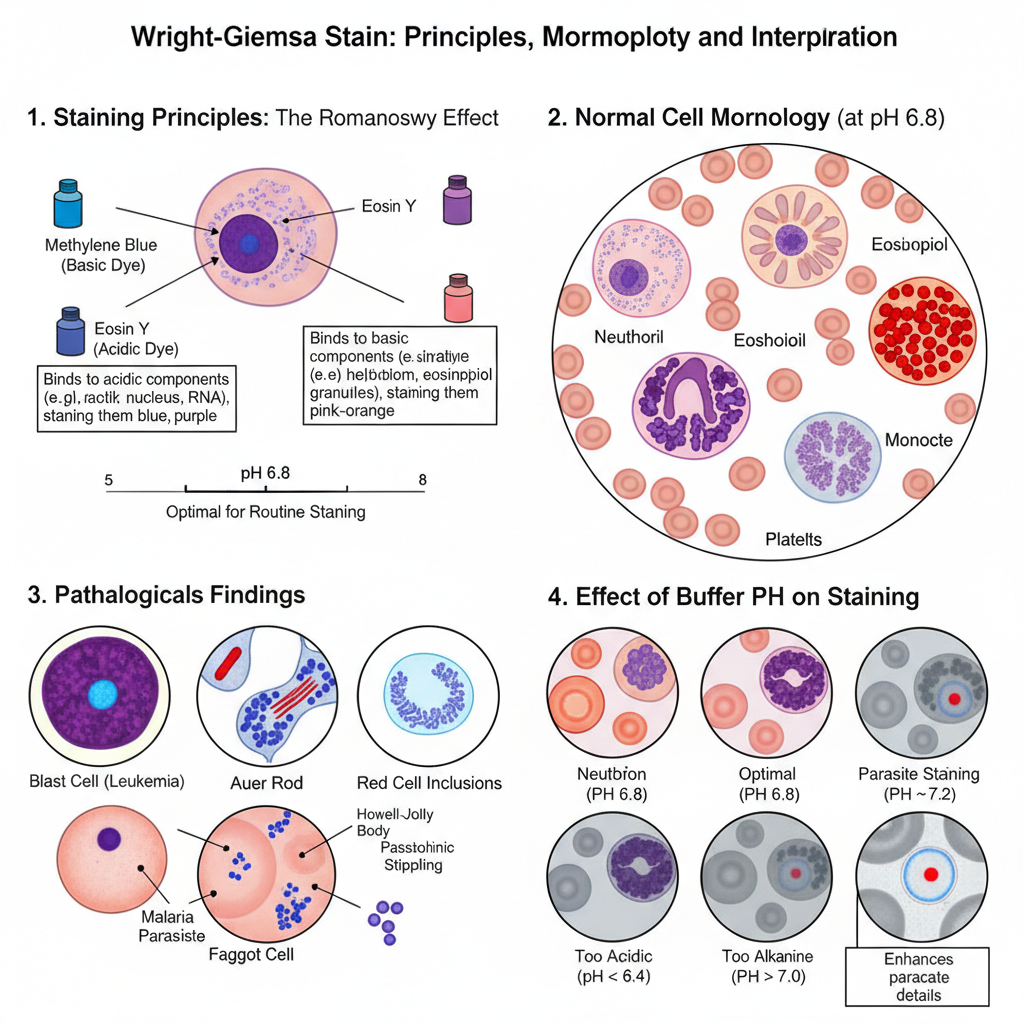
Normal Staining Reaction
- The stain shows a polychromatic effect known as the Romanowsky effect. It is the process in which different cell parts take different shades of purple depending on their pH affinity.
- Methylene blue and its oxidation products bind to acidic components and eosin Y binds to basic components.
- At proper staining conditions (around pH 6.8) the cells show characteristic colours which are used for routine hematological evaluation.
Some of the main appearances are–
- Erythrocytes: These appear pink–tan or orange–red. A central pallor is present covering one-third of the cell.
- Neutrophils: Nucleus is dark purple or blue-purple and segmented. Cytoplasm is faint pink or pale tan with fine lavender or lilac granules.
- Eosinophils: Nucleus is purple and mostly bilobed. Granules are large, uniform, and red-orange or red-brown.
- Basophils: Granules are large and coarse. These stain deep purple or violet-black and may hide the nucleus.
- Lymphocytes: Nucleus is round or indented and stains dense dark purple. Cytoplasm is sky-blue or dark blue and may show few azurophilic granules.
- Monocytes: Nucleus is purple and kidney-shaped or horseshoe-shaped with lacy chromatin. Cytoplasm is blue-grey with vacuoles and fine azurophilic dust.
- Platelets: Small irregular bodies having red-purple granules in light blue cytoplasm.
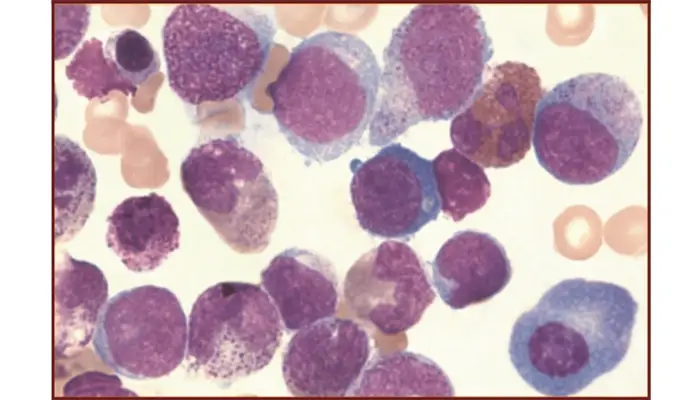
Pathological Findings
It is the stain used to detect abnormal cells and inclusions during disease diagnosis.
- Blast cells: Large cells with large nucleus, visible nucleoli and blue cytoplasm are seen in different leukemias.
- Auer rods: These appear as red rod-shaped structures in myeloblasts and indicate Acute Myeloid Leukemia.
- Faggot cells: When many Auer rods remain together inside the cytoplasm. It is characteristic of Acute Promyelocytic Leukemia.
Red cell inclusions are–
- Howell–Jolly bodies which are purple and round remnants of DNA.
- Basophilic stippling which appear as fine or coarse blue granules all over the RBC.
- Pappenheimer bodies which are iron-containing granules and stain blue-purple.
Parasites– Plasmodium parasites are seen as structures with a red chromatin dot and blue cytoplasm. Wright–Giemsa detects them, but detailed features like Schüffner’s dots are better seen with Giemsa stain.
Effect of Buffer pH
pH is the factor that controls the final colour balance of the smear.
Some of the important pH observations are–
- pH 6.8: This is the standard pH. RBCs show pink/tan colour and nuclear details remain clear.
- pH 7.2: Used for parasite examination. RBCs may appear grey or slightly washed, but parasite nuclei stain red-purple and Schüffner’s dots become clearer.
- pH < 6.4: The smear becomes too acidic. RBCs are bright red or orange. Nuclear stain becomes pale and eosinophil granules become very bright red.
- pH > 7.0: The smear becomes too blue. RBCs look grey or blue and nuclear details become darker and obscured.
Precaution of Wright Giemsa Stain
- The stain should be filtered before use because the dye complex is unstable and forms precipitates.
- Working stain must be freshly prepared since diluted stain becomes unstable after few hours.
- Stock stain bottles should be tightly closed and stored in brown bottles to prevent moisture and light exposure.
- Buffer pH must be maintained between 6.4–6.8 and distilled water should be used in preparing buffers.
- The smear must be fixed immediately after air drying with absolute methanol.
- Heat should not be used for drying or fixation because it distorts cells.
- Slides should be clean and free from oil, dust or lint.
- Smears should be thin to ensure proper stain penetration.
- Rinsing should be gentle and controlled and avoid over rinsing.
- Staining should be done in covered jars to prevent evaporation of methanol.
- Timing must be followed strictly to avoid excessive basophilia or weak eosinophilia.
- Methanol should be handled in well ventilated areas because it is toxic.
- Automated stainers should be cleaned regularly to avoid dye deposits.
- Wright Giemsa stain is not optimal for identifying features like Schüffner’s dots, so a confirmatory Giemsa stain is required in suspected malaria.
Uses of Wright Giemsa Stain
- It is used for examining peripheral blood smears to differentiate the various white blood cells.
- It helps to study nuclear and cytoplasmic features like chromatin pattern and granules.
- It is used to detect abnormal red blood cell morphology in different types of anemia.
- The Giemsa component enhances reddish-purple granules which improves cell differentiation.
- It is used for identifying blast cells in blood and bone marrow for leukemia diagnosis.
- It helps to observe features like Auer rods which are important in classifying AML.
- It is used for studying the maturation stages of cells for leukemia classification systems.
- It is used for rapid detection of blood parasites like Plasmodium and Trypanosomes.
- It is helpful for screening, but a dedicated Giemsa stain is preferred for species identification.
- It is used for staining bone marrow aspirates to study cellularity and myeloid-erythroid ratio.
- It helps in identifying defects in hematopoietic development.
- It can be used in cytology for staining urine samples to detect eosinophils.
- It is used in cytogenetics for staining chromosomes to identify defects.
- It is used for staining fine-needle aspirate samples to detect tumorous cells.
Advantages of Wright Giemsa Stain
- It produces strong polychromatic staining because of the Romanowsky effect.
- The Giemsa component increases basophilic and nuclear staining intensity.
- It shows sharper nuclear borders and bright reddish-purple granules in the cytoplasm.
- It helps in clear identification of RBCs, WBCs, and platelets by showing their characteristic features.
- It is important in diagnosing anemia, infections, and leukemia.
- It helps in detecting special features like Auer rods which are diagnostic for AML.
- It is used for rapid screening of blood parasites when quick reporting is needed.
- It is low cost and easy to prepare in most laboratories.
- It takes less time than stains like MGG while giving strong colour reactions.
- It can be used for many types of smears including blood, bone marrow, urine samples, and cytogenetic preparations.
- Compared with Wright stain it gives stronger nuclear staining.
- Compared with Giemsa it is faster for routine hematology work.
- Compared with MGG it is quicker and still gives good staining intensity.
Limitations of Wright Giemsa Stain
- It is not optimal for parasite identification because some features like Schüffner’s dots are not clearly seen, and confirmation with a dedicated Giemsa stain is often required.
- It may fail to differentiate species of Plasmodium accurately, especially in doubtful or negative smears.
- The dye complex is chemically unstable and forms precipitates easily in aqueous phase.
- Dark granular precipitates may appear on the smear and can hide important cell structures.
- The working stain becomes unstable after dilution and must be freshly prepared within short time intervals.
- Old or oxidized stain gives weak colouring and poor nuclear clarity.
- It is highly sensitive to buffer pH and staining time.
- Alkaline pH makes the smear too blue and obscures nuclear details.
- Acidic pH makes the smear too pink and weakens the nuclear staining.
- Delay in fixation causes cell distortion and poor staining reaction.
- The procedure needs liquid reagents, several washing steps, and careful timing which increases the effort and time.
- It produces chemical waste which requires proper disposal.
- Manual handling and reagent dependence make scalability difficult in remote or low-resource settings.
- BioGnost. (2018, February 9). Wright-Giemsa solution: Instructions for use [Product Insert]. https://www.biognost.com/wp-content/uploads/2020/02/Wright-Giemsa-IFU-V4-EN4.pdf
- Centers for Disease Control and Prevention. (n.d.). Plasmodium ovale. DPDx – Laboratory Identification of Parasites of Public Health Concern. https://www.cdc.gov/dpdx/resources/pdf/benchAids/malaria/Povale_benchaidV2.pdf
- Centers for Disease Control and Prevention. (n.d.). Staining for malaria parasites. DPDx – Laboratory Identification of Parasites of Public Health Concern. https://www.cdc.gov/dpdx/resources/pdf/benchaids/malaria/malaria_staining_benchaid.pdf
- Centers for Disease Control and Prevention. (2016, May 3). Staining blood smears. DPDx – Laboratory Identification of Parasites of Public Health Concern. https://www.cdc.gov/dpdx/diagnosticprocedures/blood/staining.html
- Doddagowda, S. M., Shashidhar, H. A., & Prasad, C. S. B. R. (2017). Leishman-Giemsa cocktail – Is it an effective stain for air dried cytology smears. Journal of Clinical and Diagnostic Research, 11(3), EC16–EC18. https://doi.org/10.7860/JCDR/2017/25553.9490
- Ethos Biosciences. (n.d.). 5 ways to correct precipitate in hematology stains. https://www.ethosbiosciences.com/correct-precipitate-in-hematology-stains
- Ethos Biosciences. (n.d.). How to set-up and conduct a Wright’s stain. https://www.ethosbiosciences.com/how-to-set-up-and-conduct-a-wrights-stain
- Ethos Biosciences. (n.d.). Improve the definition & intensity of a Wright’s or Wright-Giemsa stain. https://www.ethosbiosciences.com/improve-wrights-or-wright-giemsa-stain
- Ethos Biosciences. (n.d.). Lab technician’s guide to troubleshooting: 20 common issues with biological stains [eBook]. Astral Diagnostics. https://irp.cdn-website.com/240a28f1/files/uploaded/AstralDX_Troubleshooting%20stains-web.pdf
- Ethos Biosciences. (n.d.). Why is my Wright’s or Wright-Giemsa stain too blue? https://www.ethosbiosciences.com/why-is-wright-or-wright-giemsa-stain-too-blue
- Hardy Diagnostics. (2024, June 3). How to do a Wright-Giemsa stain. https://hardydiagnostics.com/blog/how-to-do-a-wright-giemsa-stain
- Li, Y., Yang, W., Wang, W., Lin, D., Wei, H., Wang, Y., Liu, B., Wang, H., Xiao, J., Ru, Y., Dong, S., Wang, J., & Mi, Y. (2021). Auer rods in mixed phenotype acute leukemia, T/myeloid: A report of three cases. Leukemia Research Reports, 15, 100236. https://doi.org/10.1016/j.lrr.2021.100236
- Lynch, E. C. (1990). Peripheral blood smear. In H. K. Walker, W. D. Hall, & J. W. Hurst (Eds.), Clinical methods: The history, physical, and laboratory examinations (3rd ed.). Butterworths. https://www.ncbi.nlm.nih.gov/books/NBK263/
- Merck & Co. (n.d.). Wright-Giemsa stain of peripheral blood smear. MSD Manual Professional Edition. https://www.msdmanuals.com/professional/multimedia/image/wright-giemsa-stain-of-peripheral-blood-smear
- Mokobi, F. (2022, September 5). Romanowsky stains- Principle, types, applications. Microbe Notes. https://microbenotes.com/romanowsky-stains/
- Noul. (2025, April 18). Wright-Giemsa stain to NGSI – The future of blood staining methods. https://noul.com/en/board_news_blog/wright-giemsa-stain-blood-staining/
- Oktiyani, N., Muhlisin, A., Roebiakto, E., Norsiah, W., & Mahpolah. (2023). Utilization of alternative buffer solutions for staining thin blood smears by the Giemsa, Wright stain and Romanowsky method. Tropical Health and Medical Research, 5(1), 34-45. https://tropicalhealthandmedicalresearch.com/index.php/JAK/article/download/76/65/545
- ResearchGate. (2020, December 14). My Wright-Giemsa stain on macrophages isn’t showing any cytoplasm, just nucleus. Any tips to improve my staining? [Online forum post]. https://www.researchgate.net/post/My_Wright-Giemsa_Stain_on_macrophages_isnt_showing_any_cytoplasm_just_nucleus_Any_tips_to_improve_my_staining
- Rowley Biochemical Inc. (n.d.). B-149 Wright-Giemsa stain method. https://rowleybio.com/wp-content/uploads/B-149-Wright-Giemsa-Stain.pdf
- Schiffer, C. A., & Stone, R. M. (2003). Morphologic classification and clinical and laboratory correlates. In D. W. Kufe, R. E. Pollock, & R. R. Weichselbaum (Eds.), Holland-Frei cancer medicine (6th ed.). BC Decker. https://www.ncbi.nlm.nih.gov/books/NBK13452/
- The Wright-Giemsa stain: Physicochemical principles, standardized protocol, and diagnostic interpretation. (n.d.). [Review article].
- Wikipedia. (n.d.). Romanowsky stain. Retrieved April 26, 2025, from https://en.wikipedia.org/wiki/Romanowsky_stain