Wheatley Trichrome Staining is the permanent staining method used in parasitology for detecting intestinal protozoans from stool samples, and it is mainly applied when cysts and trophozoites is to be identified in a fixed smear. It is the process in which three dyes are used so that the parasite structures get a clear differential colour from the background. The stain was first developed from Gomori’s tissue stain and it was later modified by Wheatley to include fixative and hydration steps, forming a rapid but simple procedure for intestinal amoeba and flagellates. In a properly stained smear, the nuclear chromatin and karyosome appear in red to purple-red while the cytoplasm is stained in blue-green, and the fecal debris appear green. It is referred to as an important technique because it provides a permanent record and helps in visualising small internal structures which is usually not seen in wet mounts.
Principle of the Wheatley Trichrome Stain
The principle of the Wheatley Trichrome stain is based on differential staining of the protozoan structures so that the internal morphology becomes clearly visible in a permanent smear. It is the process in which Chromotrope 2R and Light Green (or Fast Green) bind to different cell components with the help of a polyacid such as phosphotungstic acid that act as a mordant and differentiator. Chromotrope 2R has a strong affinity for dense cell materials, so the nuclear chromatin, karyosome, chromatoid bodies, bacteria, and ingested RBCs is stained in red to purple-red. The Light Green dye stains the cytoplasm of cysts and trophozoites in blue-green, and the fecal background also takes a green shade. This colour contrast is the major principle because it allows the protozoan structures to stand out from debris, and small features which is usually missed in wet mount examination becomes visible. The stain is applied on stool films that are fresh or fixed in PVA or SAF, and it produces permanent, uniformly stained smears useful for routine parasitological diagnosis.
Preparation of Reagents for Wheatley Trichrome Staining
- Wheatley Trichrome Stain
- Chromotrope 2R (0.6 g) is used as the primary dye.
- Light Green SF (0.3 g) is added for cytoplasmic staining.
- Phosphotungstic acid (0.7 g) acts as the polyacid component.
- Glacial acetic acid (1.0 ml) is mixed with the dry ingredients.
- Distilled water (100 ml) is added after the mixture is allowed to stand for some time.
- The stain prepared is purple in appearance and it is stored in glass or plastic bottles at room temperature.
- It is kept protected from light and it generally remains usable for long periods.
- 70% Ethanol with Iodine (Iodine Alcohol)
- Iodine crystals are dissolved in 70% ethanol until a dark solution is formed.
- The mixture is diluted again with 70% ethanol until a strong tea-coloured solution is produced.
- This reagent is replaced often so that dark iodine deposits does not interfere with the smear.
- Acid–Ethanol Solution (Destaining Agent)
- 90% ethanol (99.5 ml) is mixed with glacial acetic acid (0.5 ml).
- It is used in the differentiation step so that excess stain is removed from the background.
- Other Essential Reagents
- 70% ethanol is used to rinse the smear in various steps.
- 95% ethanol is used during dehydration.
- 100% ethanol is used for final dehydration and it is kept tightly closed so that moisture does not dilute it.
- Xylene or xylene substitute is used for clearing the slides, and it is replaced if it becomes cloudy.
Procedure for Wheatley Trichrome Staining
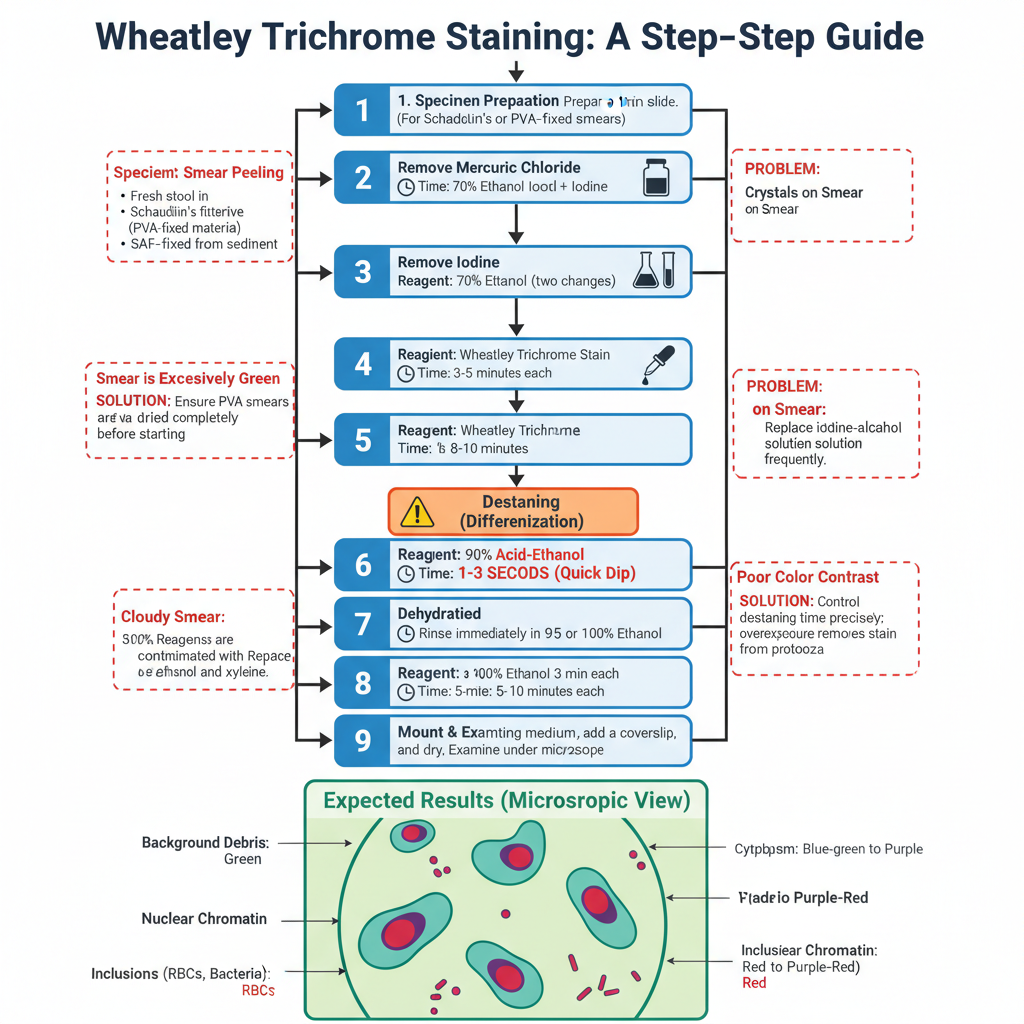
- Preparation of the Specimen
- A thin and well-fixed smear is required for proper staining.
- Fresh stool is smeared and immediately placed in Schaudinn’s fixative for at least 30 minutes.
- PVA-fixed material is mixed well and excess PVA is absorbed on paper before preparing the smear, then dried completely.
- SAF-fixed specimens are smeared from the sediment and dried, and these usually does not require iodine–alcohol treatment.
- Removal of Mercuric Chloride (for PVA or Schaudinn’s fixed smears)
- Slides are placed in 70% ethanol with iodine for 5–10 minutes.
- This step removes mercury crystals which otherwise form artifacts.
- Removal of Iodine
- Slides are transferred through two changes of 70% ethanol for 3–5 minutes.
- Incomplete removal gives a green smear later in the procedure.
- Staining of the Smear
- Slides are placed in undiluted Wheatley Trichrome stain for about 8–10 minutes.
- Destaining (Differentiation)
- Slides are dipped for 1–3 seconds in 90% acid–ethanol.
- Overexposure removes stain from protozoans, giving a poor smear.
- Stopping the Destaining
- Slides are rinsed immediately in 95% ethanol (or 100% ethanol).
- This stops the action of the acid.
- Dehydration of the Smear
- Slides are placed in two changes of 95% ethanol for 3 minutes each.
- Slides are then placed in two changes of 100% ethanol for 3 minutes each.
- Clearing of the Smear
- Slides are placed in two changes of xylene (or substitute) for 5–10 minutes.
- Cloudiness at this step indicates incomplete dehydration.
- Mounting of the Preparation
- A mounting medium is applied and a coverslip is placed gently.
- Slides are dried either overnight or for 1 hour at 37°C before microscopic examination.
- Expected Results
- Background debris stains green.
- Cytoplasm of cysts and trophozoites appears blue-green with slight purple tint.
- Nuclear chromatin and karyosomes stain red to purple-red.
- Inclusions like bacteria and RBCs also stain red to purple-red.
- Common Problems and Correction
- Smear peeling occurs when PVA smears are not fully dried, so longer drying is needed.
- Cloudy smear is due to water contamination in ethanol or xylene, so reagents must be changed.
- Crystals appear when mercury is not removed; iodine–alcohol must be replaced frequently.
- Excess green staining is due to incomplete iodine removal; longer 70% ethanol rinsing is needed.
- Poor colour contrast occurs when destaining is not controlled and needs careful timing.
Results and Interpretation of Wheatley Trichrome Stain
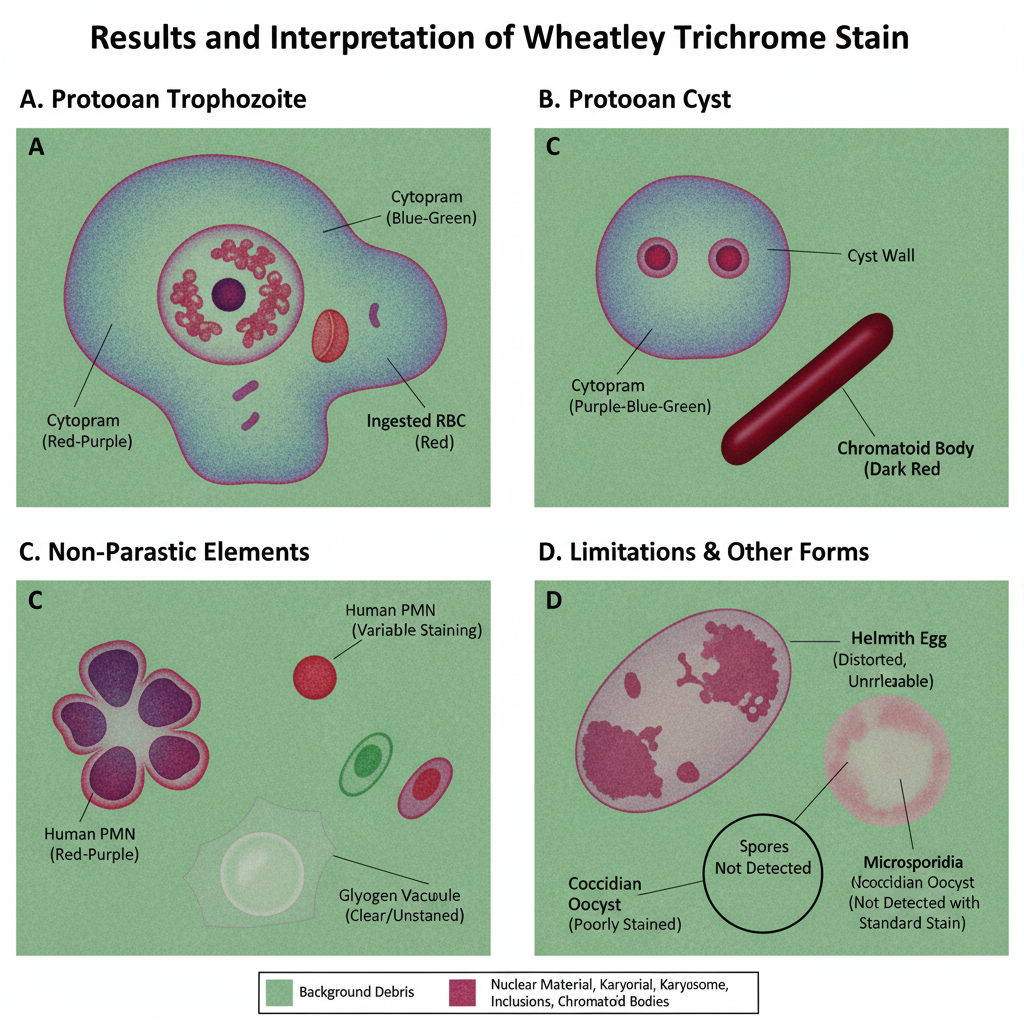
- General Appearance of the Smear
- The background debris and fecal material stain in a uniform green colour.
- This contrast is important because it helps the protozoan structures to stand out clearly from artifacts.
- Protozoan Trophozoites
- The cytoplasm stains blue-green and it may show a slight purple shade.
- Nuclear chromatin and the karyosome stain in red to purple-red.
- Ingested bacteria or RBCs inside the trophozoites also stain red to purple-red.
- Protozoan Cysts
- Cysts show a blue-green cytoplasm similar to trophozoites but often appear slightly more purple.
- Chromatoid bodies, chromatin and karyosomes stain red to purple-red, which is useful for species identification.
- Non-Parasitic Cellular Elements
- Human cells such as macrophages, PMNs and RBCs stain red to purple.
- Yeasts may stain green, blue or red-purple depending on the specimen.
- Glycogen masses appear as clear vacuoles because clearing agents dissolve the glycogen.
- Helminth Eggs and Larvae
- These can stain red to purple but are usually distorted in shape.
- They are not reliably identified in this stain and are better seen in wet mount preparations.
- Limitations of Interpretation
- Coccidian parasites like Cryptosporidium and Cyclospora stain poorly or inconsistently.
- Microsporidia spores are not detected with the standard Wheatley stain and require modified trichrome with higher chromotrope concentration.
- Diagnostic Value
- A properly stained smear shows clear contrast between background and protozoan structures.
- The red to purple-red staining of nuclear features is the main indicator that helps differentiate similar species like Entamoeba histolytica and Entamoeba coli.
Limitations of Wheatley Trichrome Stain
- It is not suitable for detecting coccidian parasites like Cryptosporidium parvum and Cyclospora cayetanensis. These organisms do not stain well and often are not visible on trichrome-stained smear.
- Microsporidia spores is not visible in the standard Wheatley method. A modified trichrome stain is required for proper visualization.
- Helminth eggs and larvae may retain excessive stain. These appear too dark or distorted, so this stain is not recommended for their diagnosis and wet preparations is preferred.
- The quality of staining is highly dependent on the fixative used. Improper fixation results in organisms that fail to stain or appear mostly red with poor differentiation.
- Mercuric chloride–based fixatives (like Schaudinn’s or PVA) need an iodine–alcohol step. If the mercuric crystals is not removed completely, crystalline deposits may obscure the smear.
- Some non-mercury fixatives produce smears with faint or fuzzy morphology. These can create dense backgrounds and distort protozoa, making identification difficult.
- Entamoeba coli cysts are difficult to fix properly. Even with acceptable quality control, these cysts may show poor morphology.
- The technique is time-consuming as the procedure can take around 60 minutes. It requires trained laboratory personnel to perform and interpret correctly.
- The differentiation step using acid–ethanol is critical. If slides remain for more than 3 seconds, over-destaining occurs and nuclear detail is lost.
- Inadequate removal of iodine may cause the smear to appear predominantly green. This reduces contrast and affects interpretation.
- The identification of protozoa needs high-magnification oil immersion. The examination is labor-intensive, and smaller organisms may be missed if the smear is not scanned thoroughly.
Applications of Wheatley Trichrome Stain
- It is used for the definitive identification of intestinal protozoa. The stain highlights cytoplasmic and nuclear structures that is not visible clearly in wet mounts.
- Trophozoites and cysts are differentiated easily because the cytoplasm stains blue-green to purple and nuclear bodies stain red to purple-red.
- Among the important organisms identified, the stain helps in differentiating Entamoeba histolytica from non-pathogenic species based on nuclear morphology. Giardia intestinalis trophozoites and cysts is also clearly visible.
- Small protozoa like Endolimax nana, Iodamoeba buetschlii and Chilomastix mesnili are detected because the stain shows their characteristic nuclear structures.
- It is the process used to identify human cells such as macrophages, PMNs, and RBCs. Ingested erythrocytes inside amoebae stain red, supporting diagnosis of E. histolytica.
- Yeast cells stain green to red, which helps in differentiating them from parasitic cysts. Budding forms and pseudohyphae can also be recognized.
- The stain gives a contrasting background that appears green, so protozoa stand out clearly with their blue-green or red staining.
- It is applied for studying free-living amoebae in specific contexts. Acanthamoeba trophozoites show nucleus and acanthopodia clearly, and cyst walls can be visualized.
- For Naegleria, trophozoite morphology is recognized, though cyst nuclei may not stain well. Permanent staining helps maintain reference slides.
- It is used for detecting Blastocystis hominis. The central body form is visible, and the stain helps in noting the organism quantity on the smear.
- It is preferred for producing permanent fecal smears in diagnostic parasitology. This allows long-term storage and detailed microscopic study when required.
Advantages of Wheatley Trichrome Stain
- The stain gives superior morphological detail. It is the process where nuclear chromatin, karyosomes and chromatoid bodies stain red to purple-red, helping in clear identification of cysts and trophozoites.
- Small protozoa which is missed in wet mounts can be visualized easily. Organisms like Endolimax nana or Entamoeba hartmanni appear clearly in the permanent stained smear.
- It provides distinct color contrast because of the three-dye system. Protozoa stain blue-green to purple with red nuclear structures while the background debris usually stains green.
- These color differences help in separating parasites from fecal debris, yeast cells and artifacts.
- The procedure is simpler and faster compared to iron hematoxylin stain. A complete staining can be finished in about 55 minutes which is useful for high workload laboratories.
- It forms a permanent record that does not fade quickly. The stained slides can be stored for reference, teaching or later examination.
- It works well with different fixatives. Mercuric chloride-based fixatives and non-mercury alternatives like SAF can give consistent results when the method is followed properly.
FAQ
How to prepare the Stool For Wheatley Trichrome Stain?
Prepare the sample of fresh stool. Using applicator sticks, place small amounts of faeces on clean, sterile microscope slides. producing extremely tiny streaks of the sample. Do not dry the slides, and immediately place them in Schaudinn’s fixative for 30 minutes. If the stool sample is watery, place three to four drops of PVA on the smeared slide (slide with a stool sample) and mix well. Allow the slide to dry for several hours at 35° – 37°C or overnight at room temperature.
What is Wheatley Trichrome Stain used for?
Wheatley Trichrome Stain is used for the detection, identification, and distinction of intestinal protozoa in faecal specimens.
How does the Wheatley Trichrome Stain work?
The Wheatley Trichrome Stain works by staining permanent stained smears of faecal specimens with trichrome dye, which provides high contrast and makes cellular features visible for the identification of protozoa.
What type of faecal specimens can be used with Wheatley Trichrome Stain?
PVA-fixed or Schaudinn’s solution-preserved faecal specimens can be used with Wheatley Trichrome Stain.
Who developed Wheatley Trichrome Stain?
Wheatley Trichrome Stain was initially developed by Gomori for staining tissue slices and cytological smears. Wheatley refined the technique in 1951 by adding fixation and dehydration processes.
What are the two dyes used in Wheatley Trichrome Stain?
The two dyes used in Wheatley Trichrome Stain are Chromotrope 2R, which stains nuclear chromatin, chromatoid bodies, karyosomes, parasite eggs and larvae, bacteria, and swallowed erythrocytes a reddish-purple, and light green or fast green dyes, which produce a blue-green colour in the cytoplasm of fixed cysts, trophozoites, and other cellular components.
What are the potential health hazards associated with this product?
The potential health hazards associated with this product include irritation to the eyes, skin, and respiratory tract.
Has the toxicology of this substance been thoroughly researched?
No, the toxicology of this substance has not been thoroughly researched.
Who should administer this product?
This product should only be administered by trained personnel in the field of in vitro diagnostics.
How should specimens and materials be sterilized after usage?
After usage, specimens, containers, and test materials should be appropriately sterilized to protect against the threats of microbiological hazards.
What should be done if the dark-blue liquid has become purple or if the expiration date has passed?
If the dark-blue liquid has become purple or if the expiration date has passed, this product should not be used and should be disposed of properly.
What is the status of Wheatley Trichrome Stain lot numbers?
All Wheatley Trichrome Stain lot numbers have been checked and determined to be acceptable.
What should be done when testing positive and negative controls?
The testing of positive and negative controls should adhere to the laboratory’s defined quality control procedures.
Can patient outcomes be published if abnormal quality control results are observed?
No, patient outcomes should not be published if abnormal quality control results are observed.
When should positive control slides be examined?
Positive control slides should be examined before the use of new permanent stain lot numbers and at least once per week thereafter.
How should the results of the Wheatley Trichrome Stain be verified?
The results of the Wheatley Trichrome Stain can be verified by examining stained smears of faeces containing leukocytes or epithelial cells in the absence of positive specimens.