The most frequent tasks in the microbiology area is the determination of the weight or mass of desired substances, chemicals. Another important aspect to be considered is the preservation of substances. Thus, the powders, as well as other granular or paste-like substances should Chamber not be placed directly on the platform for weighing of the balance. It is recommended that glazed paper or a small weigh boats is the best choice to weigh. It is recommended to use glazed papers if the material is 15 grams or less needs to be weighed. A the weigh boat pan or small beaker must be utilized if you are weighing a larger amount. Because of the the light weight of glazed papers its weight is subject to a minimal. If the weight is less of 1 gram an electronic balance is recommended. The larger amount (above 1 grams) is not to be considered using electronic balances.
Principle
A chemical’s weight is determined by comparing it with its mass. unidentified chemical is measured by comparing it to an established weight. To make balances, there are various kinds of balances that are commonly used. The single pan balances are commonly employed in laboratories for students. The basic principle behind operation is based on the concept of substitution weighing i.e. replacing the mass of unknown construction with the unknown object, and then removing the necessary amount for balance. The weighted material is put on the pan, and some removed weights are taken off the beam using an internal levers in order to restore balance. Any remaining imbalance shifts the beam slightly when it is projected onto the scale. The amount of weight in the undiscovered is the sum of the weight that is removed, in addition to the reading from the scale. It is also observed that the counter-weight is built to also function as a damper to stop any oscillation in the beam.
Requirements
- Balance
- Weighing materials or chemicals
Procedure
The process of weighing depends on the specific model of balance being used. The most common steps are listed in the following order:
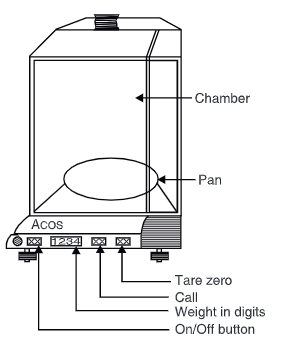
Step 1: Set zero
- Set all controls to zero.
- Move the lever for beam release down until it is in the ‘time weigh position.
- If the scale doesn’t reach zero at the point of zero, you can adjust using the zero control knobs until it does.
Step 2: Weigh the unknown
- Secure the beam and place the object on the pan.
- Move the lever for beam release up to the “course weigh” position, then turn the knob on the largest weight decade, and watch the scale returns to “zero”.
- Reverse off one unit.
- Then, proceed to the next smaller the weights (the following knob on the left) and, after a moment and a pause, switch the lever to release the beam downwards to the ‘fine weigh the position.
- Once the optical scale is close to the other end, adjust your micrometer until the indicator reading appears on the smaller division on the scale.
Results
Check total weight left to right 10 decade knob unit knob-decimal scale indicator – fine adjustment. After the weighing is completed stop the beam and then return all knobs and micrometer drums to zero.
Precautions
- The most important thing to do is reduce the amount of the amount of vibration.
- Be careful not to bump the case of the bench at any point or during weights.
- Avoid touching any area of the balance, or the bench.
- Cleanliness and care are obviously just as crucial for single pan balance as double pan balance is concerned.
- Clean up any spilled chemical and shut the door of the balance case following the application.
- Don’t handle any objects that need to be weighed by naked hands. Make use of the tongs provided, or paper towels or gloves. There aren’t any gloves to be found So you’ll need to employ paper towels when handling objects.
- Never put chemicals near the balance pan. Instead, make use of vessels, weighing papers and filter papers.
- Do not place cold or hot items on your balance. Hot objects may give lower readings due to the buoyancy of air and cold objects have higher readings because of moisture condensation from water vapour.
- Do not spill any chemicals within the enclosure for the analytical balance. If you spill chemical on the top load balance Clean it up immediately. Keep the weighing chamber and the weighing pan in good condition.
- Do not overburden the balance. The typical capacity of the balance’s analytical function is 110 grams.
- Always be mindful of the balance.
- Before you use the balance, be sure you have a clean pan. If it’s dirty, inform your instructor. Clean the pan using a specific brush.
Importance of Weighing in Analysis
- Weighing is one of the essential tasks performed by all QC labs.
- A lot of the time our understanding isn’t at a enough degree
- The importance of this issue or its the complexity of it is often overlooked.
- The quality of the weighing is the determinant of accuracy and quality of the final
- The quality of the weighing determines accuracy and reliability of the results of the final test.
- The USP specifically requires extremely precise results when it comes to weighing analytes quantitative measurements.
- Right choice of balances (Analytical/semi-micro/micro ) with desired resolution, accuracy & repeatability is essential to reduce the error and meet the compliance
Type of balances
| Balance name | Resolution | Quantity of decimal digits (gm) |
| Ultra-microbalances | 0.1 µg | 0.0000001 |
| Microbalances | 1 µg | 0.000001 |
| Semi-microbalances | 0.01mg | 0.00001 |
| Analytical balances | 0.1mg | 0.0001 |
| Precision balances | 1g ÷ 1mg | 1g ÷ 1mg |
Which Balance To Use?
It’s All Depends On The Accuracy You’ll Need In The Experiment;
- A majority of tests will inform you how much precision you’ll require. Don’t use the analytical balance in the event that toploading is sufficient.
- If you have to weigh the closest milligram ( + 0.001 grams) or the 10th of one milligram ( + 0.0001 grams) you can utilize the analytical balance; or, if you prefer, use the top loader.
- If the instructions state “weigh precisely about 2 grams of the sample” make use of the balance that is analytical. The clue lies in the word “accurately”.
Some Examples of Experiment
A) Weigh A Metal Block On The Top – Loading Balance
While you weigh the object, you must shield the item to be weighed by removing your hands with a towel.
- Get a metallic object numbered by asking your teacher. Note its numbers on the sheet of report.
- The balance for top loading is on the counter , next to the window inside your laboratory.
- The first step is to check for chemical or dust, then brush the areas you find.
- The balance should be completely level. Find the bubble at the rear of the balance which shows the level. The bubble should lie within the circle. If it’s not, inform your instructor. The instructor will turn the green round feet to ensure that the balloon is inside the circle. (Students are not to play with these leveling knobs.)
- Then turn the balance ON. Wait until it shows “0.00 g” and then put it on the metallic object. Note the reading on the report sheet using INK. If you have copied the wrong data on the report sheet, simply erase it on a single line and add the correct information below or beside it. The reading’s uncertainty of the balance that toploads is + 0.05 G.
- Switch off the balance. Make sure to shut off the top-loading balance after you have finished.
- Repeat the steps 2-6 mentioned above for two different Balances for top-loading, with the same metal block.
B) Weigh The Same Metal Block On The Analytical Balance
Throughout the weighing process , you must shield the object to be weighted from your hands by using an absorbent paper towel.
- Locate the small weighing room close to the laboratory. Find the balances used in the analysis.
- The degree of uncertainty in a reading that is taken on the analytical balance can be as high as + 0.0001 G. The balance’s glass top is a good way to look down glass top and down to where the bottom of the balance. The bubble that is level is visible through a gap on the floor. The bubble has to be placed precisely in the area. If not, you should report the issue with your teacher. The instructor will adjust the leveling and move the bubble into its center. The students should not alter the leveling on their own. The instructor will slowly and cautiously modify the thumb screws that are on the balance’s legs till the bubble sits at the center in the area. The glass case was specifically designed to protect the balance from temperature changes and air currents that can cause drifting. The readings on the balance do not stop it continues to fluctuate. Be aware that your body is more warm than the ambient air and within the balance. Therefore, do not heat the interior of the balance by using your body warmth. Be sure to keep your hands away from the balance whenever you can and keep the windows of the balance closed when working.
- Click on the button. The balance will be calibrated. After approximately 5 seconds, the balance will show “0.0000 G”. Now the balance can be used for use. If the balance doesn’t read zero, you can press button T (TARE) button. The balance should be reading 0.0000 grams.
- Unlock the door and cautiously place the object of metallic on the pan’s middle to prevent corner load errors. Maximum capacity for the balance can be 110g. Don’t over load the balance by putting in things that exceed the maximum capacity. In fact, overloading the balance can cause damage.
- The balance windows should be closed, then within 5 seconds, record your readings using INK in the sheet of report.
- Remove the object carefully and shut the doors on the glass case.
- Press”OFF” or press the “OFF” button.
- Repeat steps 2-7 on two additional analytical balances , using the same block of metal.
- Give your information to your instructor and ask for his or her signature on the sheet of report.
C) Weigh By Difference Three Samples Of Nacl Using The Analytical Balance.
Clean three 125ml Erlenmeyer flasks. Make sure that the surfaces of Erlenmeyer flasks have dried completely. Then, number them 1, 2 and 3 with a markers on the white spot of the flask. Remember: During the process of weighing, keep the Erlenmeyer flasks and your hands by using a paper towel.
- Make use of the plastic spoon to transfer around two spoonfuls of NaCl(s) into the clean, dry 50ml beaker.
- Bring the items below along to the weigh room:
- 50ml beaker with NaCl(s) which you purchased previously.
- A clean 125ml Erlenmeyer flask with the number #1 on it. Check that the exterior walls of your Erlenmeyer flask is dry.
- Your sheet of report.
- A pen (not pencil).
- It is essential to weigh precisely around 0.3 grams of NaCl according to the following:
- The balance in the analytical mode is ON.
- When you have a display of 0.0000 grams, you can place the Erlenmeyer flask that is labeled #1 onto the the balance for analytical purposes. Close the balance window and press the button for TARE. ” 0.0000 grams ” will be displayed regardless of the weight that is in the Erlenmeyer flask. Write this down as the initial value on the sheet of report.
- Make sure to add NaCl(s) in the Erlenmeyer flask 1 by holding the flask in a slanting position, then tap it gently using your forefinger. This will allow tiny pieces of NaCl crystals to get into the Erlenmeyer flask that is set in the pan within the balance. Add small amounts of NaCl until the amount is approximately 0.3g. Shut the balance and windows. Keep this reading up to 1/10th milligram on your report sheet .
- After you’re done take out the Erlenmeyer flask and close the balance windows, then touch the TARE bar, then hit the off button.
- To disperse a second sample into a flask from Erlenmeyer with the label #2 Repeat the steps 2 and 3 described previously.
- For dispense the third sample, place it in a different Erlenmeyer flask, labeled #3 repeat the steps 2 and 3 described in the previous step.
- Display the three NaCl samples and your information to your instructor. Get the instructor’s signature on your report sheet.
- Clean and dry your Erlenmeyer flasks, and then store them for the next period of lab work.
References
- https://www.mt.com/in/en/home/applications/Laboratory_weighing/density-measurement.html
- https://www.slideshare.net/DrAmsavelvel/good-weighing-practices-in-qc-lab
- https://www.npl.co.uk/special-pages/guides/gpg71_mass
- https://www.cerritos.edu/chemistry/_includes/docs/Chem_111/Lab/Exp%203%20Laboratory%20Weighing%20Fall%2008.pdf
- https://www.jove.com/v/5037/measuring-mass-in-the-laboratory
- https://www.govinfo.gov/content/pkg/GOVPUB-C13-50fecb383812d067b82bca54d84af943/pdf/GOVPUB-C13-50fecb383812d067b82bca54d84af943.pdf
- https://www.wiredchemist.com/chemistry/instructional/laboratory-tutorials/weighing-techniques