What are Vitamins?
- Vitamins, in biological terms, are organic molecules or sets of closely associated molecules known as vitamers. These are imperative for organisms in minute quantities to ensure the appropriate functioning of metabolic processes.
- Therefore, when an organism is unable to produce these essential nutrients in adequate amounts for survival, they must be acquired through dietary means. Besides, it is noteworthy that while some species can produce certain vitamins like Vitamin C, others cannot.
- In situations where a species cannot produce a particular vitamin, it becomes essential for them. Vitamins are not singular entities; they are usually groups of molecules, termed vitamers. For instance, Vitamin E comprises eight vitamers, which include four tocopherols and four tocotrienols.
- It is crucial to understand that the classification of vitamins does not encompass other groups of essential nutrients such as minerals, essential fatty acids, and essential amino acids. Major health bodies have identified thirteen primary vitamins, including Vitamin A, B1, B2, B3, B5, B6, B7, B9, B12, C, D, E, and K. Additionally, some sources also recognize choline as a potential fourteenth vitamin.
- Delving deeper into their functions, vitamins play varied roles in biochemical processes. For example, Vitamin A is pivotal in regulating cell and tissue growth, while Vitamin D bears hormone-like properties, overseeing the mineral metabolism in bones and other organs. Then, the B complex vitamins are integral as enzyme cofactors or their precursors.
- Vitamins like C and E primarily act as antioxidants. It is vital to realize that both a deficiency and an overconsumption of vitamins can lead to clinically significant ailments. However, the likelihood of adverse effects due to excessive intake is relatively lower for water-soluble vitamins.
- Historically, the discovery of all vitamins occurred between the years 1913 and 1948. In periods when dietary intake was insufficient in providing these vitamins, individuals developed diseases linked to vitamin deficiency. However, the landscape transformed in 1935 with the commercial availability of yeast-extract vitamin B complex and semi-synthetic vitamin C tablets.
- This evolution was further cemented in the 1950s with the mass production of vitamin supplements, including multivitamins, aiming to address vitamin deficiencies in the larger population. Consequently, governments across the globe initiated the fortification of staple foods, such as flour and milk, with certain vitamins to combat deficiencies. Such measures, like the recommendation for folic acid supplementation during pregnancy, have been instrumental in minimizing health risks, such as infant neural tube defects.
Definition of Vitamins
Vitamins are organic molecules essential in small amounts for an organism’s proper metabolic function, which are typically obtained through the diet as they cannot be synthesized by the organism in sufficient quantities.
History and nomenclature of Vitamins
The history and nomenclature of vitamins have their roots in the early 20th century. At this time, it was discerned that diets consisting solely of purified carbohydrate, protein, fat, and minerals were insufficient for the growth and well-being of experimental rats, unlike natural foods like milk which supported their health. Therefore, a search began for these missing elements in the diet.
The term “accessory factors” was introduced by Hopkins to denote these unknown but essential nutrients. Later on, he identified a component from rice polishings and yeast, which proved effective against beri-beri in pigeons. Believing these accessory factors were all amines, he termed them “vitamines” derived from the Greek word “vita” meaning life. However, as further research progressed, it became clear that not all these compounds were amines. Thus, the name was shortened to “vitamin,” omitting the final “e”.
The system of classifying vitamins using letters began in 1915, introduced by McCollum and Davis. Initially, they recognized two primary vitamins: fat-soluble vitamin A and water-soluble vitamin B, which was known for its anti-beriberi properties. Soon after, another water-soluble vitamin was discovered, which had anti-scurvy properties and was termed vitamin C. Further investigations into vitamin A revealed two components: one preventing night blindness (retained the name vitamin A) and another combating rickets, named vitamin D. Subsequent discoveries included vitamin E, essential for proper rat reproduction, and vitamin K, associated with blood coagulation. The choice of the letter “K” was influenced by its relation to “koagulation.”
Regarding water-soluble vitamins, vitamin C was identified in its pure form and termed ascorbic acid. The vitamin B series, however, proved to be more intricate. It was initially thought to comprise numerous components, leading to a series of names from B1 to B12. Vitamin B1 was identified as the anti-beri-beri factor. Intense research between 1920 and 1930 resulted in the naming of vitamins B2, B3, B4, and so forth up to B12. However, it was soon realized that some of these were either mixtures of previously identified vitamins or not vitamins at all. Consequently, some members of the B-complex series were dropped. In today’s nomenclature, while vitamins B1, B2, B6, and B12 retain their numerical designations, the other B-complex vitamins are more commonly referred to by their specific names.
| Year of Discovery | Vitamins and Chemical Names | Food Source |
|---|---|---|
| 1913 | Vitamin A (Retinol) | Cod Liver Oil |
| 1910 | Vitamin B1 (Thiamine) | Rice Bran |
| 1920 | Vitamin C (Ascorbic Acid) | Citrus, Most Fresh Foods |
| 1920 | Vitamin D (Calciferol) | Cod Liver Oil |
| 1920 | Vitamin B2 (Riboflavin) | Meat, Dairy Products, Eggs |
| 1922 | Vitamin E (Tocopherol) | Wheat Germ Oil, Unrefined Vegetable Oils |
| 1929 | Vitamin K1 (Phylloquinone) | Leaf Vegetables |
| 1931 | Vitamin B5 (Pantothenic Acid) | Meat, Whole Grains, In Many Foods |
| 1934 | Vitamin B6 (Pyridoxine) | Meat, Dairy Products |
| 1936 | Vitamin B7 (Biotin) | Meat, Dairy Products, Eggs |
| 1936 | Vitamin B3 (Niacin) | Meat, Grains |
| 1941 | Vitamin B9 (Folic Acid) | Leaf Vegetables |
| 1948 | Vitamin B12 (Cobalamins) | Meat, Organs (Liver), Eggs |
Classification of Vitamins (Types of Vitamins)

Vitamins are crucial organic compounds that are indispensable for the proper functioning of the human body. Based on their solubility, vitamins can be broadly categorized into two main groups: fat-soluble and water-soluble vitamins.
1. Fat-soluble Vitamins
These vitamins are soluble in fats and oils. They include:
- Vitamin A: Essential for vision and immune function.
- Vitamin D: Crucial for bone health and calcium absorption.
- Vitamin E: An antioxidant that protects cells from damage.
- Vitamin K: Important for blood coagulation.
Being fat-soluble, these vitamins can be stored in the body’s fatty tissues and liver. Therefore, they do not need to be consumed daily, but it’s essential to maintain a regular intake to avoid deficiencies.
2. Water-soluble Vitamins
These vitamins dissolve in water and are generally not stored in the body. They are further divided into two sub-categories:
- Non B-complex:
- Vitamin C: Vital for collagen formation, wound healing, and as an antioxidant.
- B-complex: These vitamins play a crucial role in energy production and the formation of red blood cells.
- Energy-releasing:
- Thiamine (B1): Assists in energy production from carbohydrates.
- Riboflavin (B2): Essential for energy production and the metabolism of fats.
- Niacin (B3): Important for DNA repair and energy production.
- Pyridoxine (B6): Helps in the metabolism of amino acids.
- Biotin (B7): Vital for the metabolism of fats and carbohydrates.
- Pantothenic acid (B5): Essential for synthesizing coenzyme A.
- Hematopoietic:
- Folic acid (B9): Crucial for DNA synthesis and cell division.
- Vitamin B12 (cyanocobalamin): Essential for nerve function and the formation of red blood cells.
- Energy-releasing:
Since water-soluble vitamins are not stored in the body, they need to be consumed regularly in the diet. Among these, while most water-soluble vitamins function through their associated coenzymes, only one fat-soluble vitamin, Vitamin K, has been identified to operate as a coenzyme.
In summary, vitamins, whether fat-soluble or water-soluble, play vital roles in various physiological processes. It’s essential to maintain a balanced intake of these vitamins for optimal health.
| Vitamin | Susceptible to Losses | Soluble in Water | Air Exposure | Light Exposure | Heat Exposure |
|---|---|---|---|---|---|
| Vitamin A | No | Partially | Partially | Relatively Stable | |
| Vitamin C | Very Unstable | Yes | No | No | No |
| Vitamin D | No | No | No | No | No |
| Vitamin E | No | Yes | Yes | No | No |
| Vitamin K | No | No | Yes | No | No |
| Thiamine (B1) | Highly Susceptible | No | ? | > 100 °C | |
| Riboflavin (B2) | Slightly Susceptible | No | In Solution | No | |
| Niacin (B3) | Yes | No | No | No | No |
| Pantothenic Acid (B5) | Quite Stable | No | No | Yes | |
| Vitamin B6 | Yes | ? | Yes | < 160 °C | |
| Biotin (B7) | Somewhat Susceptible | ? | ? | No | |
| Folic Acid (B9) | Yes | ? (when dry) | At High Temp | ||
| Cobalamin (B12) | Yes | ? | Yes | No |
Fat soluble vitamins
- Vitamins play pivotal roles in maintaining optimal health, and among them, a specific subset, termed fat-soluble vitamins, is of particular interest. These vitamins include vitamin A, D, E, and K. As the name suggests, fat-soluble vitamins are soluble in fats and oils, as well as in fat solvents such as alcohol and acetone. This solubility trait plays a critical role in their dietary availability, absorption, and transportation, all of which are intricately linked to fat.
- A distinctive feature of these vitamins is their ability to be stored within the body, primarily in the liver and adipose (fat) tissue. This storage capability contrasts with water-soluble vitamins, which are usually excreted when in excess. Due to this storage potential, fat-soluble vitamins are not routinely excreted in urine, leading to a unique concern. Excessive consumption, especially of vitamins A and D, can result in their accumulation within the body, posing the risk of toxic effects.
- Diving deeper into their chemical nature, all fat-soluble vitamins are isoprenoid compounds. This means they are composed of one or more five-carbon units, specifically termed isoprene units. These units have a specific structure represented as CH2-C(CH3)-CH-CH2.
- Functionally, fat-soluble vitamins have a wide array of roles within the body. For instance, vitamin K holds a distinct role as a coenzyme. The diverse functions of these vitamins underscore their importance in various physiological processes, emphasizing the need for a balanced intake to ensure overall health and well-being.
Water soluble vitamins
- Water-soluble vitamins represent a diverse group of compounds that, despite their varied chemical structures, have a shared trait: their solubility in water. This characteristic makes them unique in several ways, notably in how the body handles their excess and storage.
- One significant aspect of these vitamins is their excretion pattern. Since they are water-soluble, any excess amounts are typically and readily excreted via urine. This ensures that water-soluble vitamins generally do not reach toxic levels within the body. However, the flip side of this trait is that these vitamins are not stored in large quantities, with vitamin B12 being a notable exception. Therefore, the continuous dietary intake of these vitamins is essential to prevent deficiencies.
- Furthermore, when considering vitamin deficiencies, it’s crucial to understand that they are often multifaceted. That is, deficiencies usually involve multiple vitamins rather than just one, leading to overlapping symptoms. Identifying the precise biochemical root of these symptoms can be a challenging task due to this overlap.
- A significant role of water-soluble vitamins is their involvement in the formation of coenzymes. These coenzymes are vital participants in various biochemical reactions, primarily related to energy generation or hematopoiesis (the formation of blood cellular components). This coenzymatic role provides insight into why vitamin deficiencies can lead to a range of overlapping symptoms. Common manifestations of deficiencies in vitamins involved in energy metabolism encompass dermatitis, glossitis (a condition where the tongue becomes red and swollen), cheilitis (cracking at the corners of the lips), diarrhea, mental confusion, depression, and a general feeling of discomfort or malaise.
- Moreover, specific vitamins like B1, B6, and B12 have a closer association with neurological symptoms when deficient. Recognizing the importance and functions of water-soluble vitamins underscores the necessity of maintaining a balanced diet to support overall health and well-being.
What are Vitamers?
- Vitamers, within the realm of biology and nutrition, constitute a group of chemically analogous compounds that exhibit similar vitamin activities. These compounds, often bearing subtle structural differences, play a significant role in ensuring the body’s proper functioning by fulfilling the essential vitamin requirements. It is essential to understand the concept of vitamers, as they contribute significantly to the overall understanding of vitamin-related processes in biological systems.
- To illustrate this concept further, consider the vitamers of vitamin A. Vitamin A, a fat-soluble vitamin vital for vision, immune function, and skin health, is represented by a group of vitamers. Retinol, retinal, and retinoic acid are the primary vitamers of vitamin A. Retinol is commonly found in dietary sources and serves as a storage form of vitamin A within the body. Upon ingestion, the body can convert retinol into retinal, which plays a pivotal role in the visual cycle, allowing the conversion of light stimuli into neural signals. Lastly, retinoic acid serves as the active form of vitamin A, primarily involved in gene regulation and cellular differentiation. Therefore, these vitamers of vitamin A ensure its availability and functionality in various physiological processes.
- Similarly, vitamin B6 encompasses a group of vitamers with shared vitamin activity. Pyridoxine, pyridoxal, and pyridoxamine are the principal vitamers of vitamin B6, also known as pyridoxine. Vitamin B6 is essential for various enzymatic reactions, particularly in amino acid metabolism. These vitamers serve as coenzymes that facilitate the conversion of amino acids, making them available for various metabolic pathways. Pyridoxine, found in dietary sources, can be converted into pyridoxal and pyridoxamine within the body. These vitamers, collectively or individually, contribute to the regulation of numerous biological processes, emphasizing their importance in overall health.
- In summary, vitamers are biologically relevant compounds that share qualitative vitamin activities while differing slightly in chemical structure. They are crucial for the proper functioning of the body and play essential roles in various metabolic processes. Understanding the concept of vitamers, such as those of vitamin A and vitamin B6, sheds light on the intricate mechanisms that govern the utilization of vitamins within the human body. This knowledge is indispensable in ensuring optimal health and nutrition.
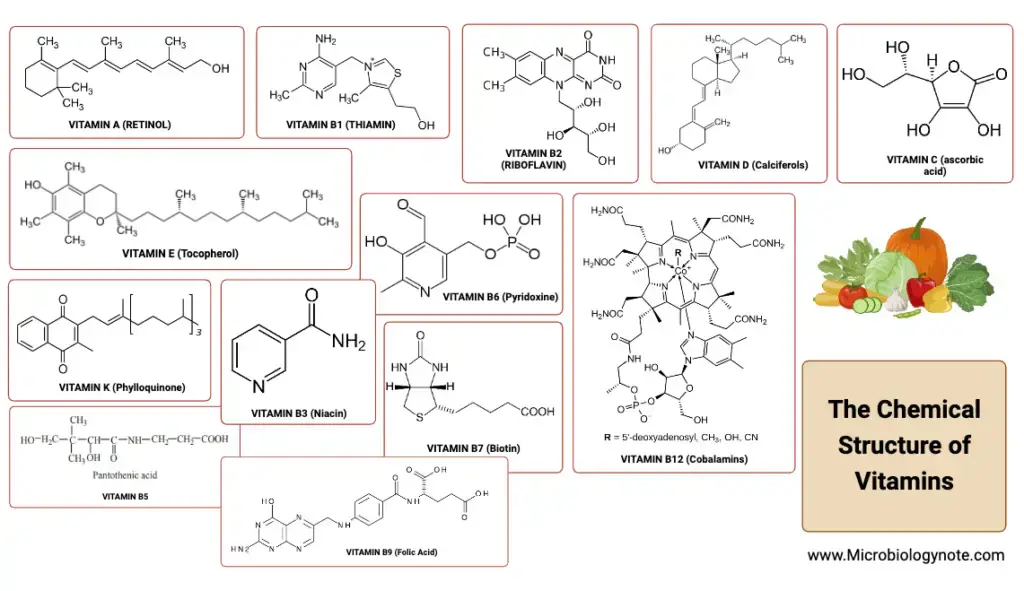
VITAMIN A
| Aspect | Description |
|---|---|
| Structure of Vitamin A | Vitamin A is a fat-soluble vitamin found in animal and plant sources. It includes forms like retinol, retinal, and retinoic acid. Retinol has a β-ionone ring, a side chain with two isoprenoid units, four double bonds, and a hydroxyl group. |
| Absorption of Vitamin A | Dietary sources: Preformed vitamin A (retinol) from animals and provitamin A (carotenoids) from plants. Digestion: In the digestive tract, retinol from animal sources is present as retinyl esters, while carotenoids are incorporated into micelles with bile salts. Intestinal Absorption: Enterocytes absorb retinyl esters and convert some carotenoids into retinol. Re-esterification: Retinol combines with fatty acids in enterocytes to form retinyl esters. |
| Transport of Vitamin A | Chylomicrons transport retinyl esters and carotenoids into the lymphatic system, releasing retinol into the bloodstream. The liver uptakes chylomicron remnants, storing or mobilizing vitamin A as needed. |
| Mobilization of Vitamin A | The liver stores vitamin A as retinyl esters in stellate cells. It can convert stored retinyl esters to retinol, which binds to retinol-binding protein (RBP) and enters the bloodstream. Tissues take up retinol-RBP complexes as needed for conversion to active forms. |
| Functions of Vitamin A | Vitamin A plays crucial roles in vision, cell growth, and differentiation, maintenance of epithelial tissues, immune function, reproduction, and as an antioxidant through carotenoids. |
| Deficiency of Vitamin A | Deficiency can result from inadequate intake, impaired absorption, reduced liver storage, or chronic alcoholism. Symptoms include night blindness, xerophthalmia, growth retardation, reproductive issues, and skin/epithelial problems if prolonged. |
Structure of Vitamin A
Vitamin A, a fat-soluble vitamin, is not present in its active form in plant-based foods. Instead, plants contain provitamins like carotenes, which can be converted into vitamin A in the body. The term “retinoids” encompasses the various forms of vitamin A, including retinol, retinal, and retinoic acid. Among these, retinol is a primary alcohol that contains a β-ionone ring. Its side chain consists of two isoprenoid units, four double bonds, and a hydroxyl group. In animal tissues, retinol exists as a retinyl ester, combined with long-chain fatty acids.
Absorption of Vitamin A
- Dietary Sources: Vitamin A is obtained from the diet in two primary forms: preformed vitamin A (retinol, found in animal sources) and provitamin A (carotenoids, found in plant sources).
- Digestion: In the digestive tract, vitamin A from animal sources is present as retinyl esters. These esters, along with carotenoids, are incorporated into micelles with the help of bile salts.
- Intestinal Absorption: The retinyl esters and carotenoids in the micelles are absorbed by the enterocytes (intestinal cells). Within these cells, retinyl esters are hydrolyzed to produce free retinol. Some of the carotenoids can be converted into retinol as well.
- Re-esterification: Once inside the enterocytes, retinol binds with fatty acids to form retinyl esters again.
Transport of Vitamin A
- Chylomicrons: The retinyl esters and a portion of carotenoids are packaged into chylomicrons, a type of lipoprotein. These chylomicrons are then released into the lymphatic system.
- Bloodstream: From the lymphatic system, the chylomicrons enter the bloodstream. As they circulate, the chylomicrons are acted upon by lipoprotein lipase, releasing retinol.
- Liver Uptake: The liver takes up the remnants of chylomicrons, which contain a significant portion of the vitamin A. Here, vitamin A can be stored or mobilized as needed.
Mobilization of Vitamin A
- Liver Storage: The liver is the primary storage site for vitamin A. Within the liver, vitamin A is stored as retinyl esters in stellate cells.
- Release into Bloodstream: When the body requires vitamin A, the liver converts stored retinyl esters back to retinol. This retinol binds to a specific transport protein called retinol-binding protein (RBP). The retinol-RBP complex is then released into the bloodstream.
- Tissue Uptake: Tissues take up the retinol-RBP complex based on their needs. Once inside the cells, retinol can be converted to its active forms (like retinoic acid) to exert its physiological effects.
Functions of Vitamin A
- Vision: Vitamin A plays a crucial role in vision. The retina of the eye contains cells called rods and cones. Rods are responsible for dim light vision, while cones are essential for bright light and color vision. The protein rhodopsin, present in rods, is a combination of 11-cis retinal and the protein opsin. When exposed to light, 11-cis-retinal isomerizes to all-trans retinal, leading to a series of biochemical changes that generate a nerve impulse. This impulse is then transmitted to the brain, allowing us to perceive visual stimuli.
- Cell Growth and Differentiation: Retinol and retinoic acid function similarly to steroid hormones. They regulate protein synthesis, influencing cell growth and differentiation.
- Epithelial Tissue Maintenance: Vitamin A ensures the health of epithelial tissues by preventing excessive keratin synthesis.
- Immune System: It is vital for a robust immune system, helping the body combat various infections.
- Reproduction: Vitamin A acts hormone-like, regulating gene expression and ensuring normal reproductive processes.
- Antioxidant Properties: Carotenoids, especially β-carotene, function as antioxidants, reducing the risk of cancers initiated by free radicals.
Deficiency of Vitamin A
Vitamin A deficiency can arise from inadequate dietary intake, impaired absorption in the intestines, reduced liver storage, or chronic alcoholism. The deficiency symptoms might not manifest immediately since the liver can supply the body’s needs for a few months. However, prolonged deficiency can lead to several health issues:
- Eye Disorders: One of the earliest signs of vitamin A deficiency is night blindness, where individuals find it challenging to see in low light. A severe deficiency can lead to xerophthalmia, characterized by dryness in the conjunctiva and cornea. If left untreated, it can progress to keratomalacia, resulting in total blindness.
- Growth Retardation: A lack of vitamin A can impede growth due to skeletal formation impairment.
- Reproductive Issues: In males, vitamin A deficiency can lead to germinal epithelium degeneration, causing sterility.
- Skin and Epithelial Cells: The skin may become rough and dry. The epithelial cells of the gastrointestinal, urinary, and respiratory tracts undergo keratinization, increasing susceptibility to bacterial infections.
VITAMIN D
| Aspect | Description |
|---|---|
| Structure and Formula | – Vitamin D is a fat-soluble vitamin that structurally resembles sterols and functions as a hormone. |
| – Two primary forms: Ergocalciferol (Vitamin D2) found in plants and Cholecalciferol (Vitamin D3) found in animals. Both are provitamins. Ergocalciferol is derived from ergosterol in plants, while Cholecalciferol is synthesized in the skin upon exposure to sunlight, converting 7-dehydrocholesterol to Cholecalciferol. | |
| – Chemical formula: C27H44O | |
| Absorption, Transport, and Storage | – Absorption: Occurs in the small intestine with bile’s aid, entering the bloodstream and binding to plasma β2-globulin. Some storage occurs in the liver and other tissues. |
| Metabolism and Biochemical Functions | – In the liver, Cholecalciferol is hydroxylated to form 25-hydroxycholecalciferol (25-OH D3), the major storage and circulatory form. Further hydroxylation in the kidneys produces 1,25-dihydroxycholecalciferol (1,25-DHCC or calcitriol), the active form. |
| – Calcitriol regulates plasma calcium and phosphate levels, enhancing their absorption in the intestine, stimulating calcium uptake in bones, and minimizing excretion in the kidneys. | |
| Functions | – Intestinal Action: Enhances calcium and phosphate absorption by inducing calcium-binding protein synthesis in intestinal cells. |
| – Bone Action: Stimulates calcium uptake in osteoblasts, aids in bone formation, and mobilizes calcium and phosphate from bones. | |
| – Renal Action: Reduces calcium and phosphate excretion in the kidneys, promoting reabsorption. | |
| Deficiency | – Vitamin D deficiency can cause rickets in children (bone deformities, soft bones, delayed teeth formation) and osteomalacia in adults (soft, demineralized bones prone to fractures). Factors include insufficient sunlight exposure, strict vegetarian diets, and medical conditions. |
- Structure: Vitamin D is a fat-soluble vitamin that structurally resembles sterols and functions similarly to a hormone. There are two primary forms of Vitamin D: Ergocalciferol (Vitamin D2) found in plants and Cholecalciferol (Vitamin D3) found in animals. Both these forms are considered provitamins. Ergocalciferol is derived from ergosterol in plants, while Cholecalciferol is synthesized in the skin upon exposure to sunlight, converting 7-dehydrocholesterol to Cholecalciferol. This synthesis has earned Vitamin D the title of the “sunshine vitamin.”
- Absorption, Transport, and Storage: Vitamin D is absorbed in the small intestine with the aid of bile. Once absorbed, it travels through the lymphatic system and enters the bloodstream, binding to plasma β2-globulin. It is then distributed throughout the body. The liver and other tissues store small amounts of Vitamin D.
- Metabolism and Biochemical Functions: Vitamin D undergoes metabolic transformations to become biologically active. In the liver, Cholecalciferol is hydroxylated to form 25-hydroxycholecalciferol (25-OH D3), which is the major storage and circulatory form of Vitamin D. Further hydroxylation occurs in the kidneys, producing 1,25-dihydroxycholecalciferol (1,25-DHCC), also known as calcitriol. Calcitriol is the active form of Vitamin D and plays a pivotal role in regulating plasma levels of calcium and phosphate. It acts on the intestine to increase calcium and phosphate absorption, on the bone to stimulate calcium uptake and mobilization, and on the kidney to minimize calcium and phosphate excretion.
- Functions: Vitamin D has several crucial functions:
- Intestinal Action: Calcitriol enhances the intestinal absorption of calcium and phosphate. It binds with a cytosolic receptor in intestinal cells, leading to the synthesis of a specific calcium-binding protein that facilitates calcium uptake.
- Bone Action: Calcitriol is essential for bone formation, stimulating calcium uptake in osteoblasts. It also works in tandem with the parathyroid hormone to mobilize calcium and phosphate from the bone, raising their plasma levels.
- Renal Action: In the kidneys, calcitriol reduces the excretion of calcium and phosphate, promoting their reabsorption.
- Deficiency: Vitamin D deficiency can lead to rickets in children and osteomalacia in adults. Rickets is characterized by bone deformities, soft bones, and delayed teeth formation. Osteomalacia results in demineralized, soft bones that are prone to fractures. Factors like insufficient sunlight exposure, strict vegetarian diets, and certain medical conditions can lead to Vitamin D deficiency.
VITAMIN E
| Aspect | Description |
|---|---|
| Chemical Structure and Formula | – Vitamin E, scientifically known as tocopherol, is a naturally occurring antioxidant. |
| – Chemical structure: Tocopherols and tocotrienols, with α-tocopherol being the most biologically active. | |
| – Formula: C29H50O2 (α-tocopherol) | |
| Absorption and Transport | – Absorbed in the small intestine with fats, requiring bile salts for efficient absorption. |
| – Transported in the bloodstream by incorporation into lipoproteins (VLDL and LDL). | |
| – Primarily stored in adipose tissues, liver, and muscles. | |
| Biochemical Functions | – Antioxidant properties protect against non-enzymatic oxidation of cellular components, such as unsaturated fatty acids, by oxygen and free radicals. |
| – Maintains cell membrane integrity, prevents fatty acid peroxidation, protects red blood cells, supports reproductive functions, enhances heme synthesis, aids in cellular respiration, prevents oxidation of other vitamins, facilitates amino acid absorption, supports nucleic acid synthesis, protects the liver, delays cataracts. | |
| Interaction with Selenium | – Vitamin E and selenium complement each other in neutralizing free radicals; they work synergistically. |
| Dietary Recommendations and Sources | – Recommended daily intake varies based on polyunsaturated fatty acid consumption: approximately 10 mg (15 IU) for men, 8 mg (12 IU) for women. |
| – Rich sources include vegetable oils (wheat germ, cottonseed, sunflower), as well as meat, milk, butter, eggs. | |
| Deficiency and Toxicity | – Deficiency symptoms in humans include increased erythrocyte fragility and minor neurological symptoms. In animals, it can lead to sterility and muscle degeneration. |
| – Least toxic among fat-soluble vitamins; no reported adverse effects from prolonged high intake. |
Vitamin E, scientifically termed as tocopherol, is a naturally occurring antioxidant that plays a pivotal role in many biological functions. It is especially vital for normal reproduction in numerous animals, leading to its designation as the “anti-sterility vitamin.” Interestingly, vitamin E has been described as a “vitamin in search of a disease” due to the absence of any specific deficiency disease in humans linked to it.
- Chemical Structure: Vitamin E encompasses a group of compounds known as tocopherols and tocotrienols. Among these, eight distinct tocopherols have been identified, with α-tocopherol being the most biologically active. Structurally, tocopherols are derivatives of a 6-hydroxy chromane ring combined with an isoprenoid side chain. The antioxidant properties of vitamin E primarily stem from the hydroxyl group present in the chromane ring.
- Absorption and Transport: Upon ingestion, vitamin E is absorbed in the small intestine in conjunction with fats. For efficient absorption, the presence of bile salts is imperative. Once absorbed, the liver incorporates vitamin E into lipoproteins, specifically VLDL and LDL, facilitating its transport. Notably, vitamin E reserves are predominantly found in adipose tissues, liver, and muscles.
- Biochemical Functions: The antioxidant property of vitamin E underpins most of its biochemical functions. It acts as a protective shield against the non-enzymatic oxidation of cellular components, such as unsaturated fatty acids, by molecular oxygen and free radicals. Furthermore, vitamin E:
- Ensures the structural integrity of cell membranes.
- Prevents the peroxidation of polyunsaturated fatty acids in tissues and membranes.
- Protects red blood cells from oxidative damage.
- Is integral to reproductive functions, preventing sterility.
- Enhances heme synthesis by boosting the activity of specific enzymes.
- Is essential for cellular respiration.
- Prevents the oxidation of vitamin A and carotenes.
- Facilitates proper amino acid absorption from the intestine.
- Aids in nucleic acid synthesis.
- Shields the liver from damage by toxic compounds.
- Delays the onset of cataracts in association with other vitamins.
- Has been explored for its potential in preventing chronic diseases, although clinical trials have yielded mixed results.
- Interaction with Selenium: Vitamin E’s antioxidant functions are complemented by the element selenium, present in the enzyme glutathione peroxidase. Both vitamin E and selenium work synergistically in neutralizing free radicals. Interestingly, selenium can, to some extent, compensate for vitamin E’s requirement and vice versa.
- Dietary Recommendations and Sources: The recommended daily intake of vitamin E is contingent on the consumption of polyunsaturated fatty acids. For men, approximately 10 mg (15 IU) of α-tocopherol is advised, while women are recommended 8 mg (12 IU). Rich sources of vitamin E include vegetable oils like wheat germ oil, cottonseed oil, and sunflower oil. Additionally, meat, milk, butter, and eggs also contribute to vitamin E intake.
- Deficiency and Toxicity: While vitamin E deficiency in animals can lead to sterility and muscle degeneration, in humans, it primarily results in increased erythrocyte fragility and minor neurological symptoms. Among the fat-soluble vitamins, vitamin E is the least toxic, with no reported adverse effects even after prolonged high intake.
VITAMIN K
| Aspect | Description |
|---|---|
| Chemical Structure and Formula | – Vitamin K is a fat-soluble vitamin crucial for blood clotting. |
| – Multiple forms: Vitamin K1 (phylloquinone, from plants), Vitamin K2 (menaquinone, from intestinal bacteria and animals), Vitamin K3 (menadione, synthetic). | |
| – Formula: C31H46O2 (phylloquinone) | |
| Absorption and Transport | – Absorbed with fats as chylomicrons, facilitated by bile salts. |
| – Transported with LDL and mainly stored in the liver, with smaller amounts in other tissues. | |
| Biochemical Functions | – Primary role in blood clotting, as a coenzyme for carboxylation of specific blood clotting factors (II, VII, IX, X). |
| – Facilitates carboxylation of glutamic acid residues in these proteins, transforming glutamate to γ-carboxyglutamate. | |
| – Essential for carboxylation of osteocalcin, a calcium-binding protein in bones. | |
| Dietary Requirements | – Recommended daily intake: Adults require 70-140 μg; half of this can be obtained from dietary sources. |
| Dietary Sources | – Rich sources include green vegetables (cabbage, cauliflower, spinach, alfalfa), egg yolks, meat, liver, cheese, and dairy products. |
| Deficiency Symptoms | – Rare but can occur in conditions like impaired absorption, diarrheal diseases, or prolonged antibiotic use. |
| – Symptoms include prolonged bleeding, increased blood clotting time, and, in severe cases, hemorrhage. | |
| Hypervitaminosis K | – Excessive intake can lead to hemolytic anemia and jaundice, especially in infants, due to increased red blood cell breakdown. |
| Antagonists | – Compounds like heparin, bishydroxycoumarin, salicylates, and dicumarol act as anticoagulants, opposing vitamin K’s action in blood clotting. |
Vitamin K, a fat-soluble vitamin, plays a pivotal role in the coagulation process, ensuring that blood clots effectively. The name “K” is derived from the German word “Koagulation,” emphasizing its significance in blood clotting.
- Chemical Structure: Vitamin K exists in multiple forms. Vitamin K1, or phylloquinone, is predominantly found in plants. Vitamin K2, known as menaquinone, is synthesized by intestinal bacteria and is also present in animals. Vitamin K3, or menadione, is a synthetic variant. All these forms are derivatives of naphthoquinone. They are heat stable but can be deactivated by oxidizing agents, irradiation, and extreme pH levels.
- Absorption and Transport: Vitamin K, whether obtained from dietary sources or synthesized by intestinal bacteria, is absorbed alongside fats in the form of chylomicrons. Its absorption is facilitated by bile salts. Once absorbed, it is transported with LDL and predominantly stored in the liver, with smaller amounts in other tissues.
- Biochemical Functions: Vitamin K’s primary function revolves around the blood clotting process. It facilitates the post-translational modification of specific blood clotting factors. Clotting factors II, VII, IX, and X are synthesized in the liver as inactive precursors. Vitamin K acts as a coenzyme, aiding in the carboxylation of glutamic acid residues in these proteins. This carboxylation transforms glutamate to γ-carboxyglutamate. The process is inhibited by anticoagulants like dicumarol. Additionally, vitamin K is essential for the carboxylation of osteocalcin, a calcium-binding protein in bones.
- Dietary Requirements: Although vitamin K can be synthesized in the gut, it’s recommended that half of the body’s requirement be obtained from dietary sources. The suggested daily intake for adults ranges between 70-140 μg.
- Dietary Sources: Rich sources of vitamin K include green vegetables like cabbage, cauliflower, spinach, and alfalfa. It’s also found in egg yolks, meat, liver, cheese, and other dairy products.
- Deficiency Symptoms: Vitamin K deficiency is rare due to its ample presence in diets and synthesis by intestinal bacteria. However, certain conditions like impaired absorption, diarrheal diseases, or prolonged antibiotic use can lead to deficiency. Symptoms include prolonged bleeding, increased blood clotting time, and in severe cases, hemorrhage.
- Hypervitaminosis K: Excessive intake of vitamin K can lead to hemolytic anemia and jaundice, especially in infants. This is attributed to the increased breakdown of red blood cells.
- Antagonists: Certain compounds, including heparin and bishydroxycoumarin, act as anticoagulants and oppose the action of vitamin K. Salicylates and dicumarol also exhibit antagonistic properties against vitamin K.
VITAMIN C (ASCORBIC ACID)
| Aspect | Description |
|---|---|
| Chemical Structure and Properties | – Water-soluble vitamin, ascorbic acid, resembles hexose derivatives and acts as a potent reducing agent. |
| – Can be oxidized to form dehydroascorbic acid, both forms are biologically active. The D-ascorbic acid is inactive. | |
| Biosynthesis, Absorption, and Metabolism | – Humans, primates, guinea pigs, and bats lack the ability to synthesize ascorbic acid due to the absence of the enzyme L-gulonolactone oxidase. |
| – Swiftly absorbed from the intestine, not stored in significant amounts, and excreted in the urine. | |
| Key Functions | – Collagen Formation: Essential for hydroxylation of proline and lysine in collagen synthesis. |
| – Bone Formation: Aids in the formation of the bone matrix. | |
| – Iron and Hemoglobin Metabolism: Enhances iron absorption and participates in the conversion of methemoglobin to hemoglobin. | |
| – Tryptophan and Tyrosine Metabolism: Required for serotonin synthesis and p-hydroxy phenylpyruvate oxidation. | |
| – Immune Function: Boosts antibody synthesis and enhances white blood cell phagocytosis. | |
| – Prevention of Chronic Diseases: Acts as an antioxidant, reducing the risk of cancer, cataracts, and heart ailments. | |
| Dietary Sources and RDA | – Found in citrus fruits, gooseberries, guavas, green vegetables, and potatoes. |
| – Recommended daily intake for adults: 60-70 mg, with increased amounts suggested during pregnancy and lactation. | |
| Deficiency and its Implications | – Deficiency leads to scurvy, characterized by sore gums, anemia, fragile blood vessels, and delayed wound healing. Caused by impaired collagen synthesis and reduced antioxidant protection. |
| Controversies Surrounding Megadoses | – Megadoses debated for preventing common cold; may reduce duration and severity of symptoms. Offers various health benefits as an antioxidant but can lead to kidney stone formation due to oxalate metabolites with excessive intake. |
Vitamin C, also known as ascorbic acid, is a water-soluble vitamin that plays a pivotal role in human health and disease. Its significance has been recognized for centuries, with scurvy being the first disease identified as diet-related. In the sixteenth century, the absence of fresh vegetables in the diet led to the death of approximately 10,000 mariners due to scurvy. It was James Lind, a surgeon in the English Navy, who in 1753 highlighted the importance of lime or lemon juice in preventing this disease, leading to the English Navy being colloquially termed as “Limeys.”
- Chemical Structure and Properties Ascorbic acid is a hexose derivative, bearing a close resemblance to monosaccharides in its structure. Its acidic nature is attributed to the enolic hydroxyl groups, making it a potent reducing agent. The vitamin can undergo oxidation to form dehydroascorbic acid, a reaction that is reversible. Both forms are biologically active, but the D-ascorbic acid is inactive. The majority of ascorbic acid in plasma and tissues is in its reduced form.
- Biosynthesis, Absorption, and Metabolism While many animals can synthesize ascorbic acid from glucose, humans, primates, guinea pigs, and bats lack this capability due to the absence of the enzyme L-gulonolactone oxidase. Once ingested, vitamin C is swiftly absorbed from the intestine. It is not stored in significant amounts in the body and is excreted in the urine either as ascorbic acid or its metabolites.
- Key Functions
- Collagen Formation: Vitamin C is instrumental in the hydroxylation of proline and lysine during the conversion of protocollagen to collagen. This process is essential for the strength and integrity of connective tissues.
- Bone Formation: Vitamin C aids in the formation of the bone matrix.
- Iron and Hemoglobin Metabolism: It enhances iron absorption and is involved in the conversion of methemoglobin to hemoglobin.
- Tryptophan and Tyrosine Metabolism: Vitamin C is required for the synthesis of serotonin and the oxidation of p-hydroxy phenylpyruvate.
- Immune Function: It boosts the synthesis of antibodies and enhances the phagocytic action of white blood cells.
- Prevention of Chronic Diseases: As an antioxidant, vitamin C reduces the risk of diseases like cancer, cataracts, and heart ailments.
- Dietary Sources and RDA Citrus fruits, gooseberries, guavas, green vegetables, and potatoes are rich sources of vitamin C. The recommended daily intake for adults is 60-70 mg, with increased amounts suggested during pregnancy and lactation.
- Deficiency and its Implications A deficiency in vitamin C leads to scurvy, characterized by symptoms like sore gums, anemia, fragile blood vessels, and delayed wound healing. These manifestations are primarily due to impaired collagen synthesis and the antioxidant properties of the vitamin.
- Controversies Surrounding Megadoses The proposal of consuming megadoses of vitamin C, especially for preventing common cold, has been a topic of debate. While it doesn’t prevent the cold, it can reduce the duration and severity of symptoms. As an antioxidant, it offers several health benefits. However, excessive intake might lead to the formation of kidney stones due to its metabolite, oxalate.
VITAMIN B1
| Aspect | Description |
|---|---|
| Chemical Structure | – Thiamine is a water-soluble vitamin with a pyrimidine ring and a thiazole ring connected by a methylene bridge. |
| Biochemical Functions | – Thiamine pyrophosphate (TPP), the active coenzyme form, plays a crucial role in several essential reactions: |
| – Pyruvate Dehydrogenase: Converts pyruvate to acetyl CoA in carbohydrate metabolism. | |
| – α-Ketoglutarate Dehydrogenase: Important in the citric acid cycle. | |
| – Transketolase: Part of the hexose monophosphate shunt (HMP shunt). | |
| – Branched Chain α-Keto Acid Dehydrogenase: Involved in amino acid metabolism. | |
| – Neurotransmitter Synthesis: Aids in acetylcholine synthesis for nerve impulse transmission. | |
| – RBC Transketolase Activity: Essential for erythrocyte transketolase function, used as a diagnostic tool for thiamine deficiency. | |
| Recommended Dietary Allowance (RDA) | – Daily thiamine requirement varies with carbohydrate intake: 1-1.5 mg/day for adults, 0.7-1.2 mg/day for children, higher needs during pregnancy, lactation, old age, and in cases of alcoholism. |
| Dietary Sources | – Found in various dietary sources including cereals, pulses, oil seeds, nuts, yeast, pork, liver, heart, kidney, milk, and more. Concentrated in the outer layer (bran) of cereals. |
| Deficiency Symptoms | – Thiamine deficiency leads to beriberi, with early signs including loss of appetite, weakness, constipation, nausea, mental depression, peripheral neuropathy, and more. Beriberi can manifest as wet (cardiovascular) or dry (neurological) beriberi. Chronic alcoholics may develop Wernicke-Korsakoff syndrome. |
| Thiamine Deficiency Due to Thiaminase and Pyrithiamine | – Thiaminase, found in some seafood, can destroy thiamine, and pyrithiamine can interfere with thiamine function as an antimetabolite. |
| Thiamine Antagonists | – Substances like pyrithiamine and oxythiamine can act as antimetabolites and antagonize the effects of thiamine. |
Vitamin B1, also known as thiamine, is a water-soluble vitamin with a specific coenzyme called thiamine pyrophosphate (TPP), which is intimately involved in carbohydrate metabolism. Its chemistry and functions provide valuable insights into its critical role in maintaining human health.
- Chemical Structure: Thiamine consists of a pyrimidine ring and a thiazole ring, connected by a methylene bridge. It is unique among natural compounds for having a thiazole ring. Thiamine pyrophosphate (TPP), the active coenzyme form, is created by esterifying thiamine with two phosphate molecules, with ATP providing the pyrophosphate group.
- Biochemical Functions: Thiamine pyrophosphate (TPP) serves as a coenzyme in several essential reactions:
- Pyruvate Dehydrogenase: TPP facilitates the conversion of pyruvate to acetyl CoA, a crucial step in carbohydrate metabolism.
- α-Ketoglutarate Dehydrogenase: Similar to pyruvate dehydrogenase, this enzyme in the citric acid cycle requires TPP.
- Transketolase: An enzyme in the hexose monophosphate shunt (HMP shunt) relies on TPP.
- Branched Chain α-Keto Acid Dehydrogenase: This enzyme is responsible for the oxidative decarboxylation of branched-chain amino acids (valine, leucine, and isoleucine) and also depends on TPP.
- Neurotransmitter Synthesis: TPP plays a role in acetylcholine synthesis, aiding in nerve impulse transmission.
- RBC Transketolase Activity: TPP is essential for the activity of transketolase in erythrocytes, making it a diagnostic tool for assessing thiamine deficiency.
- Recommended Dietary Allowance (RDA): The daily thiamine requirement varies based on carbohydrate intake. For adults, an intake of 1-1.5 mg/day is recommended, which roughly equates to 0.5 mg per 1,000 calories of energy consumed. Children typically require 0.7-1.2 mg/day, with slightly higher needs during pregnancy, lactation, old age, and in cases of alcoholism.
- Dietary Sources: Thiamine is found in various dietary sources. Cereals, pulses, oil seeds, nuts, and yeast are excellent sources. Notably, thiamine is concentrated in the outer layer (bran) of cereals. While polishing rice removes around 80% of its thiamine content, parboiled and milled rice retains thiamine, provided cooking water is not discarded. Animal foods like pork, liver, heart, kidney, and milk also contain thiamine.
- Deficiency Symptoms: Thiamine deficiency leads to beriberi, a condition characterized by various symptoms. Early signs include loss of appetite, weakness, constipation, nausea, mental depression, peripheral neuropathy, irritability, and sensations of numbness or “pins and needles” in the legs. Beriberi can manifest in two primary forms:
- Wet Beriberi (Cardiovascular Beriberi): This type is marked by edema (swelling) of the legs, face, trunk, and serous cavities. Breathlessness, palpitations, swollen calf muscles, and heart weakness can occur, potentially leading to heart failure.
- Dry Beriberi (Neurological Beriberi): Dry beriberi primarily involves neurological symptoms such as muscle weakness, difficulty walking, dependency on support, and potential bedridden states if left untreated.
- Wernicke-Korsakoff Syndrome: This condition, seen in chronic alcoholics, results from thiamine deficiency and leads to memory loss, apathy, and characteristic eye movements.
- Thiamine Deficiency Due to Thiaminase and Pyrithiamine: Thiaminase, an enzyme found in certain seafood, can destroy thiamine by cleaving its pyrimidine and thiazole rings. Pyrithiamine, a structural analogue of thiamine, can act as an antimetabolite and interfere with thiamine function.
- Thiamine Antagonists: Substances like pyrithiamine and oxythiamine act as antimetabolites and antagonize the effects of thiamine.
VITAMIN B2
| Aspect | Description |
|---|---|
| Chemistry | – Riboflavin is a water-soluble vitamin with a three-ring structure called 6,7-dimethyl isoalloxazine attached to D-ribitol. |
| – Sensitive to light; exposure to UV rays can convert it to lumiflavin with yellow fluorescence. Structurally identical substances include lactoflavin (from milk), hepatoflavin (from the liver), and ovoflavin (from eggs). | |
| Coenzymes of Riboflavin | – Primary coenzyme forms: Flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). FMN contains ribitol linked to a phosphate group, while FAD is formed from FMN by transferring an AMP moiety from ATP. |
| Biochemical Functions | – FAD and FMN participate in redox reactions essential for energy production. They accept two hydrogen atoms (with electrons) in reversible reactions to form FMNH2 or FADH2. |
| – Flavoproteins are enzymes that use flavin coenzymes, and they are involved in carbohydrate, lipid, protein, and purine metabolism, as well as the electron transport chain. Some contain metal atoms like iron and molybdenum, making them metalloflavoproteins. | |
| Recommended Dietary Allowance (RDA) | – Adults typically require 1.2 to 1.7 mg of riboflavin per day. Pregnant and lactating women may need an additional 0.2 to 0.5 mg per day. |
| Dietary Sources | – Abundant in foods like milk, dairy products, meat, eggs, liver, kidney, cereals, fruits, vegetables, and fish. |
| Deficiency Symptoms | – Riboflavin deficiency can lead to symptoms such as cheilosis (mouth corner fissures), glossitis (smooth purplish tongue), and dermatitis. It may occur with other vitamin deficiencies and is common in chronic alcoholics. Deficiency can be assessed by measuring glutathione reductase in erythrocytes. |
| Riboflavin Antagonist | – Galactoflavin acts as an antimetabolite of riboflavin. |
Riboflavin, also known as vitamin B2, is a water-soluble vitamin that serves as a crucial coenzyme in various cellular oxidation-reduction reactions.
- Chemistry: Riboflavin consists of a heterocyclic three-ring structure called 6,7-dimethyl isoalloxazine, which is attached to D-ribitol by a nitrogen atom. Ribitol, in turn, is an open-chain form of the sugar ribose, with the aldehyde group (CHO) reduced to alcohol (CH2OH). Riboflavin is stable to heat but sensitive to light, and exposure to ultraviolet rays from sunlight can convert it to lumiflavin, which exhibits yellow fluorescence. Various substances such as lactoflavin (from milk), hepatoflavin (from the liver), and ovoflavin (from eggs) were initially thought to be different but are structurally identical to riboflavin.
- Coenzymes of riboflavin: Riboflavin primarily exists in two coenzyme forms: Flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). FMN consists of a ribitol molecule linked to a phosphate group, while FAD is formed from FMN by transferring an AMP moiety from ATP.
- Biochemical Functions: Riboflavin’s coenzymes, especially FAD and FMN, participate in numerous redox reactions crucial for energy production. These coenzymes accept two hydrogen atoms (with electrons) during reversible reactions, forming FMNH2 or FADH2. Enzymes that use flavin coenzymes, such as FMN or FAD, are referred to as flavoproteins. These coenzymes are associated with enzymes involved in carbohydrate, lipid, protein, and purine metabolism, as well as the electron transport chain. They are often bound to proteins tightly in the holoenzyme form. Many flavoproteins also contain metal atoms like iron and molybdenum, making them metalloflavoproteins.
- Recommended Dietary Allowance (RDA): For adults, the daily requirement of riboflavin ranges from 1.2 to 1.7 mg. Pregnant and lactating women are advised to have slightly higher intakes, typically an additional 0.2-0.5 mg per day.
- Dietary Sources: Riboflavin is abundant in foods such as milk and dairy products, meat, eggs, liver, kidney, cereals, fruits, vegetables, and fish.
- Deficiency Symptoms: Riboflavin deficiency can lead to various symptoms, including cheilosis (fissures at the corners of the mouth), glossitis (a smooth and purplish tongue), and dermatitis. Although riboflavin deficiency is uncommon on its own, it can occur in conjunction with other vitamin deficiencies. Chronic alcoholics are particularly susceptible to B2 deficiency. The deficiency can be assessed by measuring the enzyme glutathione reductase in erythrocytes. Galactoflavin is an antimetabolite of riboflavin.
NIACIN
| Aspect | Description |
|---|---|
| Chemistry and Synthesis | – Niacin is a pyridine derivative, specifically pyridine 3-carboxylic acid. Its amide form is called niacinamide or nicotinamide. |
| Biochemical Functions | – Niacin coenzymes, NAD+ and NADP+, participate in numerous oxidation-reduction reactions by accepting hydride ions (H–) and undergoing reduction in the pyridine ring. |
| Deficiency Symptoms | – Pellagra, characterized by dermatitis, diarrhea, and dementia. Dermatitis involves skin inflammation, diarrhea leads to weight loss, and dementia results in anxiety, irritability, poor memory, and insomnia. Left untreated, it may progress to death. Common in populations with corn-based diets. |
| Therapeutic Uses | – Niacin can be used in pharmacological doses to influence various biochemical effects unrelated to its vitamin function, such as reducing lipid levels. Used in the treatment of hyperlipoproteinemia type II b. Prolonged use can have harmful side effects. |
| Recommended Dietary Allowance (RDA) | – Adults require 15 to 20 milligrams of niacin daily, while children need around 10 to 15 milligrams. The term “niacin equivalents” (NE) is used, with one NE equivalent to 1 milligram of niacin or 60 milligrams of tryptophan. Higher requirements for pregnant and lactating women. |
| Dietary Sources | – Rich sources include liver, yeast, whole grains, cereals, beans, peanuts, milk, fish, eggs, and vegetables. |
Niacin, also known as nicotinic acid, is a vital nutrient that plays a crucial role in various metabolic processes within the body.
- Chemistry and Synthesis of Coenzymes: Niacin is a pyridine derivative, specifically pyridine 3-carboxylic acid. Its amide form is called niacinamide or nicotinamide. Niacin can be synthesized within the body through the conversion of dietary nicotinamide, niacin, and the essential amino acid tryptophan. Tryptophan serves as a precursor for the synthesis of niacin coenzymes—nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP+). The conversion process involves several steps, including deamination of nicotinamide and the formation of various intermediates. Tryptophan can partially replace niacin for the synthesis of coenzymes, but both niacin and tryptophan are essential in the diet.
- Biochemical Functions: Niacin coenzymes, NAD+ and NADP+, are involved in numerous oxidation-reduction reactions in the body. They function by accepting hydride ions (H–) and undergoing reduction in the pyridine ring, which neutralizes positive charges. These coenzymes participate in reactions catalyzed by approximately 40 different enzymes classified as oxidoreductases. In these reactions, the coenzymes accept hydrogen ions (H–) from substrates, while releasing hydrogen ions (H+) into the surrounding medium. The conversion of NADH back to NAD+ and the role of NADP+ are essential for energy production and biosynthesis.
- Deficiency Symptoms: A deficiency in niacin leads to a condition known as pellagra, which is characterized by the “three Ds”: dermatitis, diarrhea, and dementia. Dermatitis involves skin inflammation, typically in areas exposed to sunlight. Diarrhea can manifest as loose stools, often with blood and mucus, leading to weight loss. Dementia is associated with the degeneration of nervous tissue, resulting in symptoms such as anxiety, irritability, poor memory, and insomnia. If left untreated, pellagra may progress to a fourth “D,” which is death. Pellagra is commonly observed in populations whose staple diet is primarily composed of corn or maize, as niacin in maize is often unavailable to the body, and corn is low in tryptophan.
- Therapeutic Uses: Niacin can be used therapeutically in pharmacological doses, typically 2-4 grams per day (200 times the recommended daily allowance), to influence various biochemical effects unrelated to its vitamin function. These effects are believed to be mediated by niacin’s impact on cyclic AMP levels. Niacin can inhibit lipolysis in adipose tissue, reduce liver triacylglycerol synthesis, and lower serum levels of low-density lipoproteins (LDL), very low-density lipoproteins (VLDL), triacylglycerol, and cholesterol. Consequently, niacin is used in the treatment of hyperlipoproteinemia type II b, which involves elevated levels of LDL and VLDL. However, it’s important to note that the prolonged use of niacin can have harmful side effects, such as depleting glycogen and fat reserves in skeletal and cardiac muscle, increasing glucose and uric acid levels in the bloodstream, and potentially causing liver damage.
- Recommended Dietary Allowance (RDA): For adults, the daily niacin requirement ranges from 15 to 20 milligrams, while children require around 10 to 15 milligrams. The term “niacin equivalents” (NE) is often used when expressing the RDA, where one NE is equivalent to 1 milligram of niacin or 60 milligrams of tryptophan. Pregnant and lactating women have higher niacin requirements due to the additional metabolic burden.
- Dietary Sources: Rich sources of niacin include liver, yeast, whole grains, cereals, beans, peanuts, milk, fish, eggs, and vegetables.
PYRIDOXINE (VITAMIN B6)
| Aspect | Description |
|---|---|
| Chemistry | – Vitamin B6 compounds are pyridine derivatives: pyridoxine (primary alcohol), pyridoxal (aldehyde), and pyridoxamine (amine). – Conversion can occur from pyridoxine, pyridoxal, and pyridoxamine to active coenzyme pyridoxal phosphate (PLP), but not the reverse. |
| Biochemical Functions | – Active coenzyme: Pyridoxal phosphate (PLP) is involved in amino acid metabolism, aiding in transamination, decarboxylation, deamination, transsulfuration, and condensation reactions. – Facilitates the synthesis of serotonin, histamine, and niacin coenzymes. – Required for heme synthesis, niacin coenzyme synthesis from tryptophan, sulfur amino acid metabolism, and more. |
| Intestinal Absorption | – PLP is needed for the absorption of amino acids from the intestine. |
| Recommended Dietary Allowance (RDA) | – For adults, the daily requirement of pyridoxine is 2-2.2 mg/day. – During pregnancy, lactation, and old age, an intake of 2.5 mg/day is recommended. – Requirements are calculated based on protein intake (<100 g/day). |
| Dietary Sources | – Rich dietary sources include animal products (egg yolk, fish, milk, meat) and vegetables (wheat, corn, cabbage, roots, and tubers). |
| Deficiency Symptoms | – Neurological symptoms: depression, irritability, nervousness, mental confusion, convulsions, peripheral neuropathy. – Hypochromic microcytic anemia due to decreased heme production. – Impaired synthesis of biogenic amines (serotonin, GABA, norepinephrine, epinephrine) can cause neurological symptoms. – In children, B6 deficiency can lead to convulsions. – Rare but may occur in specific populations or with certain drugs. |
Vitamin B6, which includes three vitamers known as pyridoxine, pyridoxal, and pyridoxamine, plays a critical role in various metabolic processes in the body.
- Chemistry: Vitamin B6 compounds are pyridine derivatives that differ from each other based on the functional group attached to the 4th carbon in the pyridine ring. Pyridoxine is a primary alcohol, pyridoxal is an aldehyde, and pyridoxamine is an amine. Pyridoxamine is primarily found in plants, while pyridoxal and pyridoxamine are present in animal foods. Pyridoxine can be converted into pyridoxal and pyridoxamine, but the reverse conversion is not possible.
- Synthesis of Coenzyme: The active form of vitamin B6 is the coenzyme pyridoxal phosphate (PLP). PLP can be synthesized from pyridoxine, pyridoxal, and pyridoxamine. In the body, vitamin B6 is excreted as 4-pyridoxic acid. The various forms of B6 and their inter-relationship are shown in Figure 7.23.
- Biochemical Functions: Pyridoxal phosphate (PLP), the coenzyme of vitamin B6, is attached to the α-amino group of lysine in enzymes. PLP is closely associated with amino acid metabolism, facilitating the synthesis of specialized products such as serotonin, histamine, and niacin coenzymes from amino acids. PLP participates in various reactions, including transamination, decarboxylation, deamination, transsulfuration, and condensation.
- Transamination: PLP is involved in transamination reactions, converting amino acids to keto acids. The resulting keto acids enter the citric acid cycle and produce energy, making B6 an energy-releasing vitamin.
- Decarboxylation: PLP-dependent decarboxylases convert certain α-amino acids into amines. For example, serotonin, histamine, and γ-aminobutyric acid (GABA) are synthesized through PLP-dependent decarboxylation.
- Heme Synthesis: PLP is required for the synthesis of δ-aminolevulinic acid (ALA), a precursor for heme synthesis.
- Niacin Coenzymes: The synthesis of niacin coenzymes (NAD+ and NADP+) from tryptophan depends on PLP. B6 deficiency can lead to increased excretion of xanthurenic acid in urine, indicating impaired niacin coenzyme synthesis.
- Sulfur Amino Acids: PLP plays a role in the metabolism of sulfur-containing amino acids, facilitating the synthesis of cysteine and taurine.
- Deamination: PLP is involved in the deamination of hydroxyl group-containing amino acids.
- Serine Synthesis: PLP is required for the synthesis of serine from glycine.
- Glycogen Phosphorylase: PLP is covalently bound to lysine residue in glycogen phosphorylase, stabilizing its structure for effective enzymatic function.
- Intestinal Absorption: PLP is needed for the absorption of amino acids from the intestine.
- Hyperoxaluria Prevention: Adequate B6 intake can help prevent hyperoxaluria and urinary stone formation.
- Recommended Dietary Allowance (RDA): The daily requirement of pyridoxine for adults is 2-2.2 mg/day. During pregnancy, lactation, and old age, an intake of 2.5 mg/day is recommended. Daily B6 requirements are calculated based on the assumption that protein intake is less than 100 g/day.
- Dietary Sources: Rich dietary sources of B6 include animal products such as egg yolk, fish, milk, and meat. Vegetables like wheat, corn, cabbage, roots, and tubers are also good sources.
- Deficiency Symptoms: Pyridoxine deficiency can lead to various neurological symptoms, including depression, irritability, nervousness, mental confusion, and, in severe cases, convulsions and peripheral neuropathy. B6 deficiency can also result in decreased heme production, leading to hypochromic microcytic anemia. Additionally, impaired synthesis of biogenic amines such as serotonin, GABA, norepinephrine, and epinephrine can cause neurological symptoms. In children, B6 deficiency can lead to convulsions due to reduced GABA production. Dietary deficiency of pyridoxine is rare but may occur in women taking oral contraceptives, alcoholics, and infants. Certain drugs, such as isoniazid and penicillamine, can deplete B6 levels and should be accompanied by pyridoxine supplementation to prevent deficiency.
BIOTIN
| Aspect | Description |
|---|---|
| Chemistry | – Biotin is a sulfur-containing monocarboxylic acid with a unique structure formed by the fusion of imidazole and thiophene rings with a valeric acid side chain. – In enzymes, biotin is covalently bound to lysine to form biocytin, the coenzyme form. |
| Biochemical Functions | – Acts as a carrier of carbon dioxide (CO2) in carboxylation reactions. – Essential for reactions involving pyruvate carboxylase, gluconeogenesis, citric acid cycle, fatty acid synthesis, propionyl CoA metabolism, and leucine metabolism. |
| Recommended Dietary Allowance (RDA) | – Adults are advised to consume a daily intake of approximately 100-300 micrograms (µg) of biotin. – Intestinal bacteria also synthesize biotin, but the extent of contribution to requirements is unclear. |
| Dietary Sources | – Found in a variety of animal and plant foods, including liver, kidney, egg yolk, milk, tomatoes, and grains. |
| Deficiency Symptoms | – Anemia, loss of appetite, nausea, dermatitis, glossitis, and muscle pain. – Severe deficiency can lead to depression, hallucinations, and more pronounced dermatitis. – Rare, but may occur due to destruction of intestinal flora or excessive raw egg consumption. |
| Antagonists | – Substances like desthiobiotin and biotin sulfonic acid act as biotin antagonists, interfering with its functions. |
Biotin is a sulfur-containing B-complex vitamin that plays a crucial role as a coenzyme in carboxylation reactions.
- Chemistry: Biotin is a heterocyclic sulfur-containing monocarboxylic acid. Its structure is formed by the fusion of imidazole and thiophene rings with a valeric acid side chain. In enzymes, biotin is covalently bound to the α-amino group of lysine to form biocytin, which acts as the coenzyme of biotin.
- Biochemical Functions: Biotin acts as a carrier of carbon dioxide (CO2) in carboxylation reactions. The reaction catalyzed by pyruvate carboxylase, which converts pyruvate to oxaloacetate, is a well-studied example. In this enzyme, biotin is bound to the apoenzyme and linked to the α-amino group of lysine, forming the active holoenzyme. Biotin-enzyme reacts with CO2 in the presence of ATP to form a high-energy carboxybiotin-enzyme complex, which transfers CO2 to pyruvate, resulting in the production of oxaloacetate.
- Biotin’s coenzyme role extends to various metabolic reactions:
- Gluconeogenesis and Citric Acid Cycle: Biotin-dependent pyruvate carboxylase is essential for converting pyruvate to oxaloacetate, a critical step in gluconeogenesis (glucose synthesis from non-carbohydrate sources). Oxaloacetate produced is also required for the continuous operation of the citric acid cycle.
- Fatty Acid Synthesis: The initial step in fatty acid synthesis involves a carboxylation reaction that depends on biotin.
- Propionyl CoA Metabolism: Propionyl CoA, generated during the metabolism of specific amino acids and odd-chain fatty acids, relies on biotin for further metabolism.
- Leucine Metabolism: Biotin plays a role in the metabolism of leucine, specifically in the conversion of β-methylcrotonyl CoA to β-methylglutaconyl CoA.
- Recommended Dietary Allowance (RDA): Adults are advised to consume a daily intake of approximately 100-300 micrograms (µg) of biotin. Biotin is also synthesized by intestinal bacteria, although the extent to which this contributes to the body’s requirements remains unclear.
- Dietary Sources: Biotin is found in a wide range of both animal and plant foods. Rich sources include liver, kidney, egg yolk, milk, tomatoes, and grains.
- Deficiency Symptoms: Biotin deficiency can lead to various symptoms, including anemia, loss of appetite, nausea, dermatitis, glossitis, and muscle pain. Severe deficiency may result in depression, hallucinations, and more pronounced dermatitis. Biotin deficiency is rare due to its presence in many foods and its synthesis by intestinal bacteria. However, two primary causes of deficiency are:
- Destruction of Intestinal Flora: Prolonged use of drugs like sulfonamides can disrupt intestinal flora, affecting biotin production.
- High Raw Egg Consumption: Raw egg white contains avidin, a glycoprotein that tightly binds to biotin, preventing its absorption from the intestine. It takes a significant daily intake of about 20 raw eggs to induce biotin deficiency symptoms in humans. Occasional consumption of raw eggs is generally not problematic.
- Antagonists: Substances such as desthiobiotin and biotin sulfonic acid act as biotin antagonists, interfering with its functions.
PANTOTHENIC ACID
| Aspect | Description |
|---|---|
| Chemistry | – Pantothenic acid is composed of pantoic acid and β-alanine linked together by a peptide bond. – Coenzyme A (CoA) is synthesized from pantothenic acid and comprises pantothenic acid linked to β-mercaptoethanolamine and adenylic acid. |
| Biochemical Functions | – Coenzyme A (CoA) serves as a carrier for activated acetyl or acyl groups in over 70 metabolic pathways. – Functions include carbohydrate metabolism, lipid metabolism, amino acid metabolism, ketone body formation, detoxification, and energy production. |
| Recommended Dietary Allowance (RDA) | – The recommended daily intake of pantothenic acid for adults is about 5-10 milligrams (mg). |
| Dietary Sources | – Abundantly found in both plant and animal foods. Rich sources include eggs, liver, meat, yeast, and milk. |
| Deficiency Symptoms | – Pantothenic acid deficiency is uncommon in humans due to its widespread distribution. – Symptoms may include pain, numbness in the toes, sleeplessness, fatigue, anemia, fatty liver, and decreased steroid synthesis (in experimental animals). |
Pantothenic Acid, also known as vitamin B5 and derived from the Greek word “pantos,” meaning “everywhere,” is a widely distributed vitamin in nature. Its metabolic role as coenzyme A (CoA) is extensive.
- Chemistry and Synthesis of Coenzyme A: Pantothenic acid is composed of two components, pantoic acid, and β-alanine, linked together by a peptide bond. Coenzyme A is synthesized from pantothenate in a series of reactions. Initially, pantothenate is phosphorylated, followed by the addition of cysteine. Decarboxylation, along with the addition of AMP and a phosphate from ATP, results in the formation of Coenzyme A. Coenzyme A itself comprises pantothenic acid linked to β-mercaptoethanolamine at one end and a phosphate bridge to adenylic acid on the other end, which includes adenine and a phosphate connected to carbon-3 of ribose.
- Biochemical Functions: Coenzyme A plays a pivotal role in numerous metabolic pathways, serving as a carrier for activated acetyl or acyl groups through thioester bonds. It integrates various metabolic pathways and is essential for over 70 enzymes. Examples of these pathways include:
- Carbohydrate Metabolism: Coenzyme A participates in reactions like the conversion of pyruvate to acetyl CoA by pyruvate dehydrogenase.
- Lipid Metabolism: Coenzyme A is integral to fatty acid synthesis and the formation of triacylglycerols.
- Amino Acid Metabolism: It plays a role in the synthesis of cholesterol, vitamin D, and steroid hormones.
- Ketone Body Formation: Acetyl CoA contributes to the formation of ketone bodies and is crucial during states of fasting and energy deprivation.
- Detoxification: Coenzyme A is involved in detoxifying various metabolic products.
- Energy Production: It participates in the tricarboxylic acid (TCA) cycle, a central metabolic pathway for energy production.
- Pantothenic acid is a component of the fatty acid synthase complex and directly contributes to fatty acid formation.
- Recommended Dietary Allowance (RDA): The exact requirement of pantothenic acid for humans is not well-established, but an adult’s daily intake of about 5-10 milligrams (mg) is recommended.
- Dietary Sources: Pantothenic acid is abundantly found in both plant and animal foods. Rich sources include eggs, liver, meat, yeast, and milk.
- Deficiency Symptoms: Pantothenic acid deficiency is uncommon in humans, likely due to its widespread distribution. Symptoms may resemble those of other vitamin deficiencies, making them difficult to distinguish. Dr. Gopalan, a renowned nutritionist, linked pantothenic acid deficiency to the “burning feet syndrome,” characterized by pain, numbness in the toes, sleeplessness, and fatigue.
- In experimental animals, pantothenic acid deficiency can lead to anemia, fatty liver, and decreased steroid synthesis, but such manifestations have not been reported in humans.
FOLIC ACID
| Aspect | Description |
|---|---|
| Chemistry | – Folic acid consists of a pteridine ring, p-amino benzoic acid (PABA), and glutamic acid (with 1 to 7 residues), often referred to as pteroyl-glutamic acid (PGA). – The biologically active form is tetrahydrofolate (THF or FH4), synthesized from folic acid by dihydrofolate reductase. |
| Absorption and Storage | – Polyglutamate forms of dietary folic acid are not efficiently absorbed in the intestine. – Folates with one glutamate residue are absorbed and converted to THF. – THF is stored in the liver. The body can store 10-12 milligrams of folic acid, typically lasting for 2-3 months. In circulation, N5-methyl THF is the most abundant form. |
| Biochemical Functions | – THF plays a critical role in one-carbon metabolism, accepting or donating one-carbon units (e.g., formyl, methyl) in reactions related to amino acid and nucleotide metabolism. – Functions include purine and pyrimidine synthesis, amino acid synthesis, initiation of protein biosynthesis, and histidine metabolism. |
| Recommended Dietary Allowance (RDA) | – The daily requirement for folic acid is approximately 200 micrograms (µg). – Pregnant women are advised higher intakes during pregnancy (400 µg/day) and lactation (300 µg/day). |
| Dietary Sources | – Abundant sources include green leafy vegetables, whole grains, cereals, liver, kidney, yeast, and eggs. – Milk is a relatively poor source of folic acid. |
| Deficiency Symptoms | – Folic acid deficiency is common, particularly in pregnant women, lactating women, oral contraceptive users, and alcoholics. – Symptoms include macrocytic anemia, neural defects in the fetus, and elevated homocysteine levels associated with health complications. – Related compounds like aminopterin, methotrexate, trimethoprim, and pyrimethamine are used in cancer treatment and antibacterial drugs. |
Folic Acid, also known as folacin, derived from the Latin word “folium,” meaning “leaf,” is abundantly found in green leafy vegetables. It plays a crucial role in one-carbon metabolism and is essential for the synthesis of specific amino acids, purines, and the pyrimidine-thymine.
- Chemistry: Folic acid comprises three components: a pteridine ring, p-amino benzoic acid (PABA), and glutamic acid (with 1 to 7 residues). Typically, folic acid contains one glutamic acid residue, referred to as pteroyl-glutamic acid (PGA). The biologically active form of folic acid is tetrahydrofolate (THF or FH4), synthesized from folic acid by the enzyme dihydrofolate reductase. NADPH provides the reducing equivalents for this conversion, resulting in the addition of hydrogen atoms at positions 5, 6, 7, and 8 of THF.
- Absorption, Transport, and Storage: Dietary folic acid is often present in polyglutamate form, containing 3-7 glutamate residues, which are not absorbed effectively in the intestine. The enzyme folate conjugase in the duodenum and jejunum breaks down these residues, allowing only monoglutamate forms to be absorbed. However, inside cells, tetrahydrofolates are found as polyglutamates with 5-6 amino acid residues, which are biologically more potent. Folic acid, in its polyglutamate form, is stored to some extent in the liver. The body can store 10-12 milligrams of folic acid, typically lasting for 2-3 months. In circulation, N5-methyl tetrahydrofolate is the most abundant form.
- Biochemical Functions: Tetrahydrofolate (THF or FH4), the coenzyme of folic acid, plays an active role in one-carbon metabolism. It acts as an acceptor or donor of one-carbon units (e.g., formyl, methyl) in various reactions related to amino acid and nucleotide metabolism. These functions are vital for:
- Purine and Pyrimidine Synthesis: THF is involved in the production of purines and pyrimidines, which are essential components of DNA and RNA.
- Amino Acid Synthesis: THF is critical for the synthesis of amino acids such as glycine, serine, ethanolamine, and choline.
- Initiation of Protein Biosynthesis: It participates in the formation of N-formylmethionine, which serves as the initiator for protein biosynthesis.
- Histidine Metabolism: Folic acid plays a role in histidine metabolism, facilitating the transfer of one-carbon fragments.
- Recommended Dietary Allowance (RDA): The daily requirement for folic acid is approximately 200 micrograms (µg). Pregnant women are advised higher intakes during pregnancy (400 µg/day) and lactation (300 µg/day).
- Dietary Sources: Folic acid is widely distributed in nature, with rich sources including green leafy vegetables, whole grains, cereals, liver, kidney, yeast, and eggs. Milk is a relatively poor source of folic acid.
- Deficiency Symptoms: Folic acid deficiency is possibly the most common vitamin deficiency, primarily observed in pregnant women in both developed and developing countries. Other susceptible groups include lactating women, women using oral contraceptives, and alcoholics. Folic acid deficiency can lead to macrocytic anemia, characterized by abnormally large red blood cells (RBCs) and megaloblastic changes in bone marrow due to slowed RBC maturation. Neural defects in the fetus during pregnancy are also associated with folic acid deficiency. Additionally, elevated levels of homocysteine, resulting from functional folate deficiency, are linked to various health complications like atherosclerosis, thrombosis, and coronary heart disease. Structural analogs of folic acid, such as aminopterin and methotrexate, are used to treat cancer by inhibiting dihydrofolate reductase. Trimethoprim and pyrimethamine, related to folic acid, are used to treat bacterial infections. Sulfonamides, structural analogs of PABA, are antibacterial drugs that interfere with folic acid synthesis in bacteria.
COBALAMIN (VITAMIN B12)
| Aspect | Description |
|---|---|
| Chemistry | – Vitamin B12 (Cobalamin) has a complex structure with a corrin ring and a central cobalt atom. – The corrin ring contains four pyrrole units (A, B, C, D) with various substituents. – Cobalt at the center is bonded to the pyrrole nitrogens and holds various groups including dimethylbenzimidazole (DMB), ribose 5-phosphate, and amino-isopropanol. – Above the corrin plane, cobalt has a sixth substituent group (e.g., cyanide, hydroxyl, nitrite). |
| Absorption, Transport, and Storage | – Vitamin B12 in the diet is bound to proteins and is released by stomach enzymes. – Intrinsic factor (IF) secreted by the stomach forms a complex with B12, facilitating absorption in the ileum. – Inside mucosal cells, B12 is converted to methylcobalamin and transported bound to transcobalamin (TC-I, TC-II) in the circulation. – The liver can store approximately 4-5 milligrams of vitamin B12, lasting for several years. |
| Biochemical Functions | – Vitamin B12 participates in enzymatic reactions, with key functions in mammals including: 1. Synthesis of Methionine from Homocysteine: Using methylcobalamin. 2. Isomerization of Methylmalonyl CoA to Succinyl CoA: Using deoxyadenosyl cobalamin. – These reactions are vital for amino acid metabolism and fatty acid degradation. |
| Recommended Dietary Allowance (RDA) | – Adults require approximately 3 micrograms (µg) of vitamin B12 daily. – Children have lower requirements (0.5-1.5 µg/day), and higher intake is recommended during pregnancy and lactation (4 µg/day). |
| Dietary Sources | – Found exclusively in foods of animal origin, including liver, kidney, milk, curd, eggs, fish, pork, and chicken. – B12 is not synthesized by plants, so it is not present in plant foods. |
| Deficiency Symptoms | – Pernicious anemia is a primary condition associated with B12 deficiency, characterized by low hemoglobin, reduced red blood cells, and neurological manifestations. – Other symptoms include numbness, tingling, confusion, memory loss, and psychosis. – Neurological symptoms are attributed to methylmalonyl CoA accumulation. – A combined therapy of vitamin B12 and folic acid is used for treatment. |
| Interrelation with Folic Acid | – Deficiency of B12 or folate results in a similar type of anemia, suggesting an interrelation between the two vitamins. – B12 deficiency leads to a “folate trap” where folate is trapped as N5-methyl THF, causing megaloblastic anemia. – Combined therapy of B12 and folic acid is used to treat this condition. |
Cobalamin (Vitamin B12), often referred to as the anti-pernicious anemia vitamin, is a unique vitamin synthesized exclusively by microorganisms and not by animals or plants. It was the last vitamin to be discovered.
- Chemistry: Vitamin B12 has a highly complex structure, and its empirical formula is C63H90N14O14PCo. Its structure comprises a corrin ring with a central cobalt atom. The corrin ring is similar to the tetrapyrrole ring structure found in other compounds like heme (with Fe) and chlorophyll (with Mg).
- The corrin ring consists of four pyrrole units, two of which (A and D) are directly bound, while the other two (B and C) are connected by methene bridges. Substituents on the pyrrole rings include methyl, acetamide, and propionamide groups. Cobalt, located at the center of the corrin ring, is bonded to the four pyrrole nitrogens. Below the corrin plane, cobalt holds dimethylbenzimidazole (DMB) containing ribose 5-phosphate and amino-isopropanol. An amide group of aminoisopropanol binds with the D ring of the corrin. Above the corrin plane, cobalt has a sixth substituent group, which can be cyanide (predominant in cyanocobalamin), hydroxyl (in hydroxycobalamin), or nitrite (in nitrocobalamin).
- Absorption, Transport, and Storage: Vitamin B12 in the diet is bound to proteins and is liberated by stomach enzymes (acid hydrolases). The stomach also secretes intrinsic factor (IF), a glycoprotein with a molecular weight of around 50,000, which forms a complex with vitamin B12. This complex travels through the gut and binds to specific receptors on the surface of mucosal cells in the ileum, facilitated by Ca2+ ions. Inside the mucosal cells, B12 is converted to methylcobalamin and then transported in the circulation, primarily bound to transcobalamin (TC-I, TC-II). The liver can store about 4-5 milligrams of vitamin B12, enough to meet the body’s needs for 4-6 years.
- Biochemical Functions: Vitamin B12 is involved in various enzymatic reactions, with most of them occurring in bacteria. In mammals, there are two key reactions dependent on vitamin B12:
- Synthesis of Methionine from Homocysteine: Methylcobalamin, a form of vitamin B12, plays a crucial role in this reaction. It involves the conversion of N5-methyl tetrahydrofolate into tetrahydrofolate and is catalyzed by the enzyme homocysteine methyltransferase or methionine synthase.
- Isomerization of Methylmalonyl CoA to Succinyl CoA: This reaction is important in the degradation of odd-chain fatty acids, certain amino acids (valine, isoleucine), and pyrimidines (thymine and uracil). It is mediated by the enzyme methylmalonyl CoA mutase in the presence of vitamin B12 coenzyme, deoxyadenosyl cobalamin.
- Recommended Dietary Allowance (RDA): The daily intake of vitamin B12 needed for adults is approximately 3 micrograms (µg), with slightly lower requirements for children (0.5-1.5 µg/day) and higher needs during pregnancy and lactation (4 µg/day).
- Dietary Sources: Vitamin B12 is exclusively found in foods of animal origin, such as liver, kidney, milk, curd, eggs, fish, pork, and chicken. Curd is a good source due to its synthesis of B12 by Lactobacillus. Plants do not synthesize vitamin B12, so it is not found in plant foods. Animals acquire B12 by consuming other animals or from intestinal bacterial synthesis.
- Deficiency Symptoms: The primary condition associated with vitamin B12 deficiency is pernicious anemia, characterized by low hemoglobin levels, a decreased number of red blood cells, and neurological manifestations. The causes of pernicious anemia can include autoimmune destruction of gastric parietal cells (which produce intrinsic factor), hereditary malabsorption of B12, gastrectomy (partial or total removal of the stomach), insufficient production of intrinsic factor and/or gastric hydrochloric acid, and dietary deficiency of B12. B12 deficiency can also lead to neuronal degeneration and demyelination of the nervous system, resulting in symptoms like numbness, tingling, confusion, memory loss, and psychosis. The neurological symptoms are attributed to the accumulation of methylmalonyl CoA, which interferes with myelin sheath formation. To treat B12 deficiency, therapeutic doses of vitamin B12 (100-1000 µg) are administered intramuscularly. A combination of vitamin B12 and folic acid supplementation is often used to treat patients with megaloblastic anemias.
- Interrelation Between Folic Acid and Vitamin B12 – Folate Trap or Methyl Trap Hypothesis: Deficiency of either folate or vitamin B12 results in a similar type of anemia. This suggests a biochemical interrelation between the two vitamins. There is a common metabolic reaction between folate and vitamin B12. In B12 deficiency, increased folate levels are observed in the plasma, leading to the trapping of folate as N5-methyl THF. This folate trap, also known as the methyl trap, results in reduced nucleotide and DNA synthesis, leading to megaloblastic anemia. A combined therapy of vitamin B12 and folic acid is typically used to treat this condition.
Vitamin Like Compounds
In addition to the well-known vitamins, there exists a category of compounds known as vitamin-like compounds. These substances, though not considered essential in the diet, play significant roles in various physiological functions within the body. Here are some examples of these compounds and their functions.
1. Choline
Choline, chemically known as trimethylhydroxy ethanolamine, can be synthesized within the body from serine and is also available from dietary sources like milk, eggs, liver, and cereals.
Biochemical Functions:
- Membrane Structure and Lipid Transport: Choline, as a component of phospholipids, is crucial for maintaining membrane structure and facilitating lipid transport.
- Liver Function: Choline acts as a lipotropic factor, preventing fat accumulation in the liver. It promotes the synthesis of phospholipids, lipoproteins, and the disposal of triacylglycerols.
- One Carbon Metabolism: Choline’s three methyl groups are actively involved in one-carbon metabolism.
- Neurotransmission: Choline serves as a precursor for the synthesis of acetylcholine, an essential neurotransmitter involved in nerve impulse transmission.
While choline can be synthesized and recycled in the body, some experts consider it an essential nutrient with a recommended dietary allowance (RDA) ranging from 400 to 500 milligrams per day.
2. Inositol
Inositol, a hexahydroxy-cyclohexane also known as myo-inositol or meso-inositol, is another vitamin-like compound.
Biochemical Functions:
- Cell Membrane Component: Inositol is required for the synthesis of phosphatidylinositol, a constituent of cell membranes.
- Liver Health: Similar to choline, inositol acts as a lipotropic factor, preventing the accumulation of fat in the liver.
- Hormone Signaling: In some hormones, inositol serves as a second messenger at the membrane level, facilitating the release of calcium ions.
- Cardiac Function: Inositol is found in high concentrations in heart muscle, although its exact significance remains unclear.
- Iron and Calcium Absorption Regulation: In plants, phytin, a hexaphosphate of inositol, inhibits the absorption of iron and calcium from the intestine.
3. Lipoic Acid
Lipoic acid, also known as thioctic acid, is a sulfur-containing fatty acid existing in both oxidized and reduced forms. It is unique as it is both fat and water-soluble.
Biochemical Functions:
- Decarboxylation Reactions: Lipoic acid participates in decarboxylation reactions alongside other vitamins like thiamine, niacin, riboflavin, and pantothenic acid. These reactions are essential for converting pyruvate to acetyl CoA and α-ketoglutarate to succinyl CoA.
- Therapeutic Uses: High-dose lipoic acid administration, ranging from 100 to 600 milligrams per day, has gained attention in recent years due to its antioxidant properties. It has been used therapeutically in various conditions, including reducing free radicals in the brain, stimulating glutathione production, reducing insulin resistance, and potentially preventing stroke and myocardial infarction.
4. Para Aminobenzoic Acid (PABA)
Para aminobenzoic acid (PABA) is a structural constituent of folic acid. While it may not have established specific functions in humans, it is known to be a component of folic acid.
5. Bioflavonoids
Bioflavonoids, also known as vitamin P due to their role in maintaining capillary permeability, are now commonly referred to as bioflavonoids.
Biochemical Functions:
- Antioxidant Properties: Bioflavonoids act as antioxidants and protect ascorbic acid (vitamin C) from degradation.
- Capillary Permeability: They are believed to play a role in maintaining capillary permeability.
- Sources: Bioflavonoids are found in citrus fruits’ peel and pulp, tobacco leaves, and various vegetables.
The requirement for bioflavonoids in humans has not been firmly established.
In summary, vitamin-like compounds, while not classified as essential vitamins, serve various vital functions within the body. Choline, inositol, lipoic acid, PABA, and bioflavonoids all contribute to specific biochemical processes, making them significant components of overall nutrition and health. Additionally, their potential therapeutic applications in various health conditions highlight their importance in human well-being.
| Vitamin-Like Compound | Functions |
|---|---|
| Choline | – Component of phospholipids in cell membranes – Prevents fat accumulation in the liver – Involved in one-carbon metabolism – Precursor for acetylcholine synthesis, a neurotransmitter |
| Inositol | – Required for phosphatidylinositol synthesis in cell membranes – Acts as a lipotropic factor, preventing liver fat buildup – Functions as a second messenger for certain hormones |
| Lipoic Acid | – Involved in decarboxylation reactions in energy metabolism – Acts as a universal antioxidant, reducing free radicals – Reduces insulin resistance and low-density lipoproteins |
| Para-aminobenzoic Acid (PABA) | – Structural constituent of folic acid – Specific functions in humans not well-established, but it may play a role in certain biological processes |
| Bioflavonoids | – Act as antioxidants, protecting vitamin C – May help maintain capillary permeability and vascular health |
What are Antivitamins?
Antivitamins are substances that antagonize or oppose the actions of vitamins. These compounds typically share structural similarities with vitamins but, when ingested or administered, interfere with the absorption, utilization, or function of specific vitamins in the body. As a result, they can lead to vitamin deficiencies and related health issues. Antivitamins can be naturally occurring or synthetic and are used in some cases for therapeutic purposes. Here are a few examples of antivitamins and their corresponding vitamins:
- Dicumarol: Antagonist of Vitamin K
- Dicumarol interferes with the activation of vitamin K, which is essential for blood clotting. Consequently, it can lead to bleeding disorders.
- Thiaminase: Antagonist of Thiamine (Vitamin B1)
- Thiaminase breaks down thiamine, reducing its availability in the body. This can lead to thiamine deficiency and conditions like beriberi.
- Isoniazid: Antagonist of Niacin (Vitamin B3)
- Isoniazid can interfere with the conversion of tryptophan to niacin, potentially leading to niacin deficiency. Niacin is essential for various metabolic processes.
- Avidin: Antagonist of Biotin (Vitamin B7)
- Avidin, found in raw egg whites, binds to biotin and prevents its absorption in the intestine. This can result in biotin deficiency.
- Aminopterin and Methotrexate: Antagonists of Folic Acid (Vitamin B9)
- Aminopterin and methotrexate are used as chemotherapy drugs and can inhibit the action of folic acid. This can lead to folic acid deficiency and interfere with DNA synthesis.
- Trimethoprim: Antagonist of Folate (Vitamin B9)
- Trimethoprim is an antibiotic that interferes with the utilization of folate by bacteria. Prolonged use can affect the body’s folate levels.
- Sulfonilamide: Antagonist of Para-aminobenzoic Acid (PABA)
- Sulfonilamide, used as a sulfa drug, is structurally similar to PABA, a component of folic acid. It competes with PABA, potentially leading to folic acid deficiency.
Antivitamins can be found naturally in certain foods or introduced through medications or environmental exposures. They serve as examples of how substances that resemble vitamins can disrupt essential biochemical processes in the body, leading to health problems associated with vitamin deficiencies.
| Antivitamin | Corresponding Vitamin | Effect on Vitamin |
|---|---|---|
| Dicumarol | Vitamin K | Interferes with blood clotting, leading to bleeding disorders. |
| Thiaminase | Thiamine (Vitamin B1) | Breaks down thiamine, causing thiamine deficiency and conditions like beriberi. |
| Isoniazid | Niacin (Vitamin B3) | Interferes with niacin synthesis, potentially causing niacin deficiency. |
| Avidin | Biotin (Vitamin B7) | Binds to biotin, preventing its absorption, which can lead to biotin deficiency. |
| Aminopterin & Methotrexate | Folic Acid (Vitamin B9) | Inhibit folic acid action, potentially causing folic acid deficiency. |
| Trimethoprim | Folate (Vitamin B9) | Interferes with folate utilization by bacteria, affecting body’s folate levels. |
| Sulfonilamide | Para-aminobenzoic Acid (PABA) | Competes with PABA, potentially leading to folic acid deficiency. |
Deficiency of Vitamins
Vitamin deficiencies occur when the body doesn’t receive an adequate supply of essential vitamins from the diet. Deficiencies can lead to a range of health problems and symptoms. Here’s a summary of some common vitamin deficiencies and their associated symptoms:
- Vitamin A Deficiency (Hypovitaminosis A):
- Symptoms: Night blindness, dry skin, dry eyes, increased susceptibility to infections, poor wound healing.
- Severe deficiency can lead to xerophthalmia (a condition that can result in blindness) and impaired immune function.
- Vitamin B1 Deficiency (Thiamine Deficiency):
- Symptoms: Fatigue, muscle weakness, poor appetite, weight loss, beriberi (a condition characterized by muscle wasting and nerve damage), and in severe cases, Wernicke-Korsakoff syndrome (a neurological disorder).
- Vitamin B2 Deficiency (Riboflavin Deficiency):
- Symptoms: Cracks and sores at the corners of the mouth (cheilosis), inflammation and redness of the tongue (magenta tongue), dermatitis, and eye disorders.
- Vitamin B3 Deficiency (Niacin Deficiency):
- Symptoms: Pellagra, which includes the “3 Ds” – dermatitis (skin inflammation), diarrhea, and dementia. Additional symptoms include mouth sores, swollen tongue, and memory loss.
- Vitamin B5 Deficiency (Pantothenic Acid Deficiency):
- Deficiency is rare, but it can lead to fatigue, irritability, gastrointestinal disturbances, and neurological symptoms.
- Vitamin B6 Deficiency (Pyridoxine Deficiency):
- Symptoms: Anemia, depression, confusion, convulsions, and skin disorders. Severe deficiency is uncommon but can lead to neurological problems.
- Vitamin B7 Deficiency (Biotin Deficiency):
- Symptoms: Hair loss, skin rashes, neurological symptoms, and mild depression. Deficiency is rare but can occur in individuals with certain medical conditions or those who consume raw egg whites excessively.
- Vitamin B9 Deficiency (Folic Acid Deficiency):
- Symptoms: Anemia, weakness, fatigue, sore tongue, gastrointestinal problems, and in pregnant women, an increased risk of neural tube defects in the developing fetus.
- Vitamin B12 Deficiency (Cobalamin Deficiency):
- Symptoms: Pernicious anemia (a type of anemia), fatigue, weakness, pale skin, neurological symptoms (numbness, tingling, memory problems), and in severe cases, damage to the nervous system.
- Vitamin C Deficiency (Scurvy):
- Symptoms: Fatigue, weakness, swollen and bleeding gums, joint pain, and slow wound healing. Scurvy is rare but can occur in individuals with very low vitamin C intake.
- Vitamin D Deficiency:
- Symptoms: Weak or brittle bones, muscle weakness, increased susceptibility to fractures, and in severe cases, rickets in children and osteomalacia in adults.
- Vitamin E Deficiency:
- Symptoms: Peripheral neuropathy, muscle weakness, impaired coordination, and, in rare cases, hemolytic anemia.
- Vitamin K Deficiency:
- Symptoms: Increased bleeding and easy bruising due to impaired blood clotting.
| Vitamin | Deficiency | Symptoms |
|---|---|---|
| Vitamin A | Hypovitaminosis A | Night blindness, dry skin, increased infections, poor wound healing, xerophthalmia. |
| Vitamin B1 (Thiamine) | Beriberi | Fatigue, muscle weakness, poor appetite, weight loss, nerve damage. |
| Vitamin B2 (Riboflavin) | Riboflavin Deficiency | Cheilosis, magenta tongue, dermatitis, eye disorders. |
| Vitamin B3 (Niacin) | Pellagra | Dermatitis, diarrhea, dementia, mouth sores, swollen tongue, memory loss. |
| Vitamin B5 (Pantothenic Acid) | Rare, but may cause fatigue, irritability, GI issues, and neurological symptoms. | |
| Vitamin B6 (Pyridoxine) | Anemia, neurological issues | Anemia, depression, confusion, convulsions, skin disorders. |
| Vitamin B7 (Biotin) | Rare, but may cause hair loss, skin rashes, neurological symptoms. | |
| Vitamin B9 (Folic Acid) | Folate Deficiency | Anemia, weakness, fatigue, sore tongue, GI problems, neural tube defects (in pregnancy). |
| Vitamin B12 (Cobalamin) | Pernicious anemia, neurological issues | Anemia, fatigue, pale skin, numbness, tingling, memory problems. |
| Vitamin C (Ascorbic Acid) | Scurvy | Fatigue, bleeding gums, joint pain, slow wound healing, easy bruising. |
| Vitamin D | Vitamin D Deficiency | Weak or brittle bones, muscle weakness, fractures, rickets (in children), osteomalacia (in adults). |
| Vitamin E | Rare but can cause peripheral neuropathy, muscle weakness, coordination problems, anemia. | |
| Vitamin K | Impaired blood clotting | Increased bleeding, easy bruising. |
Functions of Vitamins
Vitamins are essential organic compounds that play a variety of crucial roles in the body. They are required in small amounts but are essential for maintaining good health and preventing various deficiency diseases. Here are the primary functions of various vitamins in the human body:
- Vitamin A (Retinol):
- Essential for vision, especially in low-light conditions.
- Supports the health of the skin and mucous membranes.
- Plays a role in the immune system.
- Required for proper growth and development.
- Vitamin B1 (Thiamine):
- Necessary for the metabolism of carbohydrates, particularly in energy production.
- Supports proper nerve function.
- Important for the synthesis of DNA and RNA.
- Vitamin B2 (Riboflavin):
- Essential for energy production.
- Involved in the metabolism of fats, drugs, and steroids.
- Supports healthy skin, eyes, and nerve functions.
- Vitamin B3 (Niacin):
- Plays a critical role in energy production.
- Essential for DNA repair and cell differentiation.
- Supports cardiovascular health by affecting cholesterol levels.
- Vitamin B5 (Pantothenic Acid):
- Essential for the synthesis of fatty acids.
- Required for the formation of coenzyme A (CoA) involved in various metabolic reactions.
- Supports healthy skin and hair.
- Vitamin B6 (Pyridoxine):
- Important for amino acid metabolism.
- Essential for the production of neurotransmitters like serotonin and dopamine.
- Supports the formation of hemoglobin in red blood cells.
- Vitamin B7 (Biotin):
- Plays a role in fatty acid synthesis.
- Important for the metabolism of amino acids and carbohydrates.
- Supports healthy skin, hair, and nails.
- Vitamin B9 (Folate/Folic Acid):
- Essential for DNA synthesis and repair.
- Crucial for proper cell division and growth, particularly during pregnancy.
- Helps prevent neural tube defects in developing fetuses.
- Vitamin B12 (Cobalamin):
- Required for the production of red blood cells and DNA.
- Supports proper neurological function.
- Works in conjunction with folate to prevent megaloblastic anemia.
- Vitamin C (Ascorbic Acid):
- Essential for the synthesis of collagen, a protein that forms connective tissue in the skin, bones, and blood vessels.
- Acts as an antioxidant, protecting cells from damage.
- Supports the immune system and helps the body absorb iron from plant-based foods.
- Vitamin D (Calciferol):
- Regulates calcium and phosphorus absorption, promoting healthy bones and teeth.
- Plays a role in immune system function.
- Supports cell growth and neuromuscular function.
- Vitamin E (Tocopherol):
- Acts as an antioxidant, protecting cells from oxidative damage.
- Important for immune function and skin health.
- Supports the health of blood vessels.
- Vitamin K (Phylloquinone):
- Essential for blood clotting by aiding in the synthesis of clotting factors.
- Supports bone health by regulating calcium use in bones.
- May have a role in preventing arterial calcification.
| Vitamin | Function and Roles |
|---|---|
| Vitamin A | – Essential for vision, especially in low-light conditions. – Supports the health of the skin and mucous membranes. – Plays a role in the immune system. – Required for proper growth and development. |
| Vitamin B1 | – Necessary for the metabolism of carbohydrates, particularly in energy production. – Supports proper nerve function. – Important for the synthesis of DNA and RNA. |
| Vitamin B2 | – Essential for energy production. – Involved in the metabolism of fats, drugs, and steroids. – Supports healthy skin, eyes, and nerve functions. |
| Vitamin B3 | – Plays a critical role in energy production. – Essential for DNA repair and cell differentiation. – Supports cardiovascular health by affecting cholesterol levels. |
| Vitamin B5 | – Essential for the synthesis of fatty acids. – Required for the formation of coenzyme A (CoA) involved in various metabolic reactions. – Supports healthy skin and hair. |
| Vitamin B6 | – Important for amino acid metabolism. – Essential for the production of neurotransmitters like serotonin and dopamine. – Supports the formation of hemoglobin in red blood cells. |
| Vitamin B7 | – Plays a role in fatty acid synthesis. – Important for the metabolism of amino acids and carbohydrates. – Supports healthy skin, hair, and nails. |
| Vitamin B9 | – Essential for DNA synthesis and repair. – Crucial for proper cell division and growth, particularly during pregnancy. – Helps prevent neural tube defects in developing fetuses. |
| Vitamin B12 | – Required for the production of red blood cells and DNA. – Supports proper neurological function. – Works in conjunction with folate to prevent megaloblastic anemia. |
| Vitamin C | – Essential for the synthesis of collagen, a protein that forms connective tissue in the skin, bones, and blood vessels. – Acts as an antioxidant, protecting cells from damage. – Supports the immune system and helps the body absorb iron from plant-based foods. |
| Vitamin D | – Regulates calcium and phosphorus absorption, promoting healthy bones and teeth. – Plays a role in immune system function. – Supports cell growth and neuromuscular function. |
| Vitamin E | – Acts as an antioxidant, protecting cells from oxidative damage. – Important for immune function and skin health. – Supports the health of blood vessels. |
| Vitamin K | – Essential for blood clotting by aiding in the synthesis of clotting factors. – Supports bone health by regulating calcium use in bones. – May have a role in preventing arterial calcification. |