What is Vitamin E?
- Vitamin E, scientifically recognized as a group of eight fat-soluble compounds, encompasses four tocopherols and four tocotrienols. This vitamin plays a pivotal role in our body, primarily functioning as a fat-soluble antioxidant. Its main objective is to safeguard cell membranes from the detrimental effects of reactive oxygen species. Therefore, it is essential to understand the significance of Vitamin E in our daily diet.
- A deficiency in Vitamin E is a rare occurrence. However, when it does manifest, it is typically attributed to an underlying issue related to the digestion of dietary fat rather than a mere lack of vitamin E in one’s diet. Such a deficiency can lead to nerve-related complications.
- Besides, it’s worth noting that the recommended daily intake of Vitamin E for adults, as suggested by global health organizations, ranges between 3 to 15 mg. Then, a comprehensive analysis of over a hundred studies worldwide in 2016 revealed that the median dietary intake of alpha-tocopherol stood at 6.2 mg per day, indicating a consumption rate below the recommended levels.
- Population-based studies have shed light on the potential benefits of Vitamin E. These studies have consistently indicated that individuals who incorporated more vitamin E-rich foods in their diet or opted for vitamin E supplements had a reduced risk of developing various ailments, including cardiovascular diseases, cancer, and dementia, among others. However, it’s crucial to approach these findings with caution.
- Clinical trials, especially those that were placebo-controlled and utilized alpha-tocopherol supplements, sometimes failed to replicate these promising outcomes. In fact, some high-dose vitamin E trials even showcased adverse effects, leading to a decline in its usage in countries like the United States by 2006.
- Delving deeper into the technical aspects, both natural and synthetic tocopherols are prone to oxidation. Therefore, dietary supplements often undergo esterification, resulting in tocopheryl acetate to ensure stability. Furthermore, tocopherols and tocotrienols are available in various forms, namely α (alpha), β (beta), γ (gamma), and δ (delta).
- These forms are differentiated based on the number and position of methyl groups on their chromanol ring. Each of these vitamers boasts a chromane double ring, equipped with a hydroxyl group capable of reducing free radicals and a hydrophobic side chain that facilitates penetration into biological membranes.
- Historically, Vitamin E was discovered in 1922 and was subsequently isolated in 1935. Its first synthesis occurred in 1938. The name “tocopherol” was coined due to its essential role in ensuring successful births in rats, with the term deriving from Greek words signifying birth and to bear or carry.
- Today, alpha-tocopherol, whether extracted naturally from plant oils or synthesized as tocopheryl acetate, is widely available as a dietary supplement. It can be consumed individually or as part of a multivitamin product. Additionally, it is a common ingredient in oils and lotions designed for skin application.
- In conclusion, Vitamin E, or tocopherol, stands out as a natural antioxidant. Its significance is underscored by its essential role in normal reproduction across various animal species, leading to its alternative name – the anti-sterility vitamin.
- Despite its myriad benefits, Vitamin E remains a “vitamin in search of a disease” due to the absence of a specific deficiency disease in humans. The discovery of Vitamin E by Evans and his team in 1936, and its subsequent naming as tocopherols, further emphasizes its importance in the realm of biology and health.
Definition of Vitamin E
Vitamin E is a fat-soluble antioxidant essential for human health that protects cell membranes from damage by reactive oxygen species and plays a role in immune function, skin health, and vision. It encompasses a group of eight compounds, including four tocopherols and four tocotrienols.
Chemical structures of vitamin E

Vitamin E is a group of eight fat-soluble compounds that include four tocopherols and four tocotrienols. These compounds are essential for various physiological functions and are commonly found in various foods and supplements. Here’s a detailed breakdown of the chemical structures of vitamin E:
- Tocopherols and Tocotrienols:
- Both tocopherols and tocotrienols have a chromanol ring, but they differ in the nature of their side chains.
- Tocopherols have a saturated phytyl tail, while tocotrienols possess an unsaturated side chain.
- Subtypes:
- Each of these classes (tocopherols and tocotrienols) is further divided into four types based on the number and position of methyl groups on the chromanol ring: alpha (α-), beta (β-), gamma (γ-), and delta (δ-).
- Alpha (α-) Tocopherol and Tocotrienol:
- Fully methylated at positions R1 and R2 on the chromanol ring (R1 = R2 = CH3).
- Beta (β-) Tocopherol and Tocotrienol:
- Dimethylated with a methyl group at R1 and hydrogen at R2 (R1 = CH3, R2 = H).
- Gamma (γ-) Tocopherol and Tocotrienol:
- Dimethylated but with the methyl group at R2 and hydrogen at R1 (R1 = H, R2 = CH3).
- Delta (δ-) Tocopherol and Tocotrienol:
- Monomethylated with hydrogen at both R1 and R2 positions (R1 = R2 = H).
- Chiral Centers of α-Tocopherol:
- α-Tocopherol has three chiral centers located at position 2 on the chromanol head and at positions 40 and 80 on the phytyl tail. This leads to the possibility of eight different stereoisomers when synthesized chemically: RRR, RSR, RRS, RSS, SRR, SSR, SRS, and SSS.
- Metabolites:
- Tocopherols and tocotrienols can also be metabolized to their corresponding α-, β-, γ-, and δ-CEHCs.
In dietary sources, α-tocopherol and γ-tocopherol are the most abundant forms of vitamin E. Among all the vitamin E forms, α-tocopherol is the most studied due to its significant accumulation in tissues and its role in preventing vitamin E deficiency.
The chemical structures of vitamin E, with their chromanol rings and varying side chains, play a crucial role in their antioxidant properties and other biological functions.

Absorption, transport and storage of vitamin E.
Absorption, transport, and storage of vitamin E are essential processes that ensure the body benefits from this vital nutrient. Here’s a detailed and sequential explanation of these processes:
- Absorption in the Small Intestine:
- Vitamin E, being a fat-soluble compound, is absorbed in the small intestine along with dietary fats. This absorption process is facilitated by the presence of bile salts, which are necessary for the efficient uptake of vitamin E into the intestinal cells.
- Transport via Lipoproteins:
- Once absorbed, vitamin E is transported to the liver. In the liver, it is incorporated into specific lipoproteins, namely Very-Low-Density Lipoproteins (VLDL) and Low-Density Lipoproteins (LDL). These lipoproteins play a crucial role in carrying vitamin E through the bloodstream to various tissues and organs.
- Storage in the Body:
- The body stores vitamin E in several locations to ensure its availability when needed. The primary storage sites include:
- Adipose Tissue: This is the body’s primary storage site for vitamin E. Adipose tissue, commonly known as body fat, has the capacity to store significant amounts of this nutrient, ensuring its availability during periods of dietary deficiency.
- Liver: Besides its role in the transport of vitamin E, the liver also serves as a storage site. The liver ensures that there is a steady supply of vitamin E to meet the body’s needs.
- Muscle: Muscle tissues also store a portion of the body’s vitamin E, further emphasizing the widespread distribution and importance of this nutrient in various physiological processes.
- The body stores vitamin E in several locations to ensure its availability when needed. The primary storage sites include:
- Normal Plasma Levels:
- The concentration of tocopherol, a form of vitamin E, in the plasma is typically less than 1 mg/dl. This level is maintained through a balance of dietary intake, absorption, transport, utilization, and storage mechanisms.
Biochemical functions of vitamin E
- Antioxidant Property:
- The primary function of vitamin E revolves around its antioxidant capability. It effectively prevents the non-enzymatic oxidations of various cellular components, such as unsaturated fatty acids, by molecular oxygen and free radicals like superoxide (O2–) and hydrogen peroxide (H2O2). Furthermore, the element selenium aids in enhancing these antioxidant functions.
- Protection of Polyunsaturated Fatty Acids (PUFA):
- Being lipophilic, vitamin E associates with lipoproteins, fat deposits, and cellular membranes. It safeguards polyunsaturated fatty acids (PUFA) from detrimental peroxidation reactions. Acting as a scavenger, vitamin E undergoes oxidation (transforming into its quinone form) by free radicals, thereby sparing PUFA.
- Cell Membrane Integrity:
- Vitamin E is pivotal for maintaining the structure and integrity of cell membranes. Therefore, it is often termed a membrane antioxidant.
- Prevention of Red Blood Cell Hemolysis:
- It shields red blood cells from hemolysis caused by oxidizing agents, such as H2O2.
- Reproductive Functions:
- Vitamin E is intricately linked with reproductive processes, ensuring the prevention of sterility. It plays a vital role in preserving the germinal epithelium of gonads, which is essential for proper reproductive functionality.
- Heme Synthesis:
- It augments the synthesis of heme by boosting the activity of enzymes like delta-aminolevulinic acid (ALA) synthase and ALA dehydratase.
- Cellular Respiration:
- Vitamin E is essential for cellular respiration, particularly through the electron transport chain. It is believed to stabilize coenzyme Q.
- Protection of Other Vitamins:
- Vitamin E safeguards vitamin A and carotenes from oxidation.
- Creatine Storage:
- It ensures the proper storage of creatine in skeletal muscles.
- Amino Acid Absorption:
- Vitamin E facilitates the optimal absorption of amino acids from the intestine.
- Nucleic Acid Synthesis:
- It plays a role in the proper synthesis of nucleic acids.
- Liver Protection:
- Vitamin E shields the liver from damage caused by toxic compounds, such as carbon tetrachloride.
- Delaying Cataract Onset:
- In conjunction with vitamins A, C, and β-carotene, vitamin E helps in delaying the onset of cataracts.
- Prevention of Chronic Diseases:
- While vitamin E has been suggested for preventing chronic ailments like cancer and heart diseases, clinical trials have yielded mixed results. However, it is believed that vitamin E prevents the oxidation of LDL, which is implicated in promoting heart diseases.
Synthesis of Vitamin E
Vitamin E, a vital fat-soluble vitamin, encompasses a family of compounds known as tocochromanols. This family consists of four tocopherols and four tocotrienols. The synthesis of Vitamin E is a complex process that occurs primarily in photosynthesizing plants, algae, and cyanobacteria.
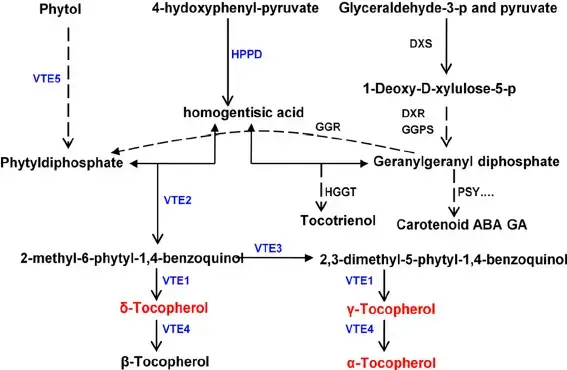
- Biosynthesis:
- The initial phase of Vitamin E synthesis begins in the plastids of plants. The closed-ring part of the molecule, known as homogentisic acid (HGA), is formed. Subsequently, the side chain is attached, with variations leading to either tocopherols or tocotrienols. The pathway for both types is identical, resulting in the formation of gamma-, alpha-, or delta- compounds. The primary function of tocochromanols in plants appears to be their antioxidant activity, protecting against ultraviolet radiation damage. Seeds, rich in lipids, contain tocochromanols to prevent lipid oxidation, ensuring seed longevity and successful germination.
- Synthesis Pathways:
- The synthesis of Vitamin E involves two primary pathways: the Shikimate pathway and the Methylerythritol Phosphate (MEP) pathway. The Shikimate pathway is responsible for generating the chromanol ring from HGA. In contrast, the MEP pathway produces the hydrophobic tail, which varies between tocopherol and tocotrienol. The specific tail’s synthesis depends on its originating molecule, with tocopherol tails emerging from the geranylgeranyl diphosphate (GGDP) group and tocotrienol tails stemming from phytyl diphosphate.
- Derivative Mechanism:
- Delving deeper into tocopherols, their derivatives are synthesized from the reaction between HGA and Phytyl-PP, producing 2-Methyl-6-phytylhydroquinone. This compound can undergo two pathways. The first pathway results in the formation of γ-Tocopherol and subsequently α-Tocopherol. The second pathway leads to the synthesis of δ-Tocopherol and then β-Tocopherol. A similar synthesis process occurs for tocotrienols, with the primary difference being the use of prenyl-PP.
- Industrial Synthesis:
- On an industrial scale, Vitamin E is synthesized as all-rac-alpha-tocopherol or dl-alpha tocopherol. This synthetic form comprises eight stereoisomers. The synthesis involves a reaction between toluene and 2,3,5-trimethyl-hydroquinone with isophytol, using iron and hydrogen chloride gas as catalysts. The synthetic form differs from the natural form extracted from plants, with the synthetic having 73.5% of the natural’s potency. For enhanced stability and longer shelf-life, manufacturers convert the phenol form of the vitamin to an ester using either acetic acid or succinic acid.
Antioxidant Function of Vitamin E

Vitamin E stands out in the realm of vitamins due to its unique non-cofactor function. Its primary biological role is as a chain-breaking antioxidant, specifically halting the cyclical progression of lipid peroxidation. All forms of vitamin E act as defenders against peroxyl radicals, safeguarding polyunsaturated fatty acids from the detrimental effects of lipid peroxidation. The methylation level of the chromanol head determines the antioxidant activity, with a-tocopherol showcasing superior antioxidant prowess compared to its less methylated counterparts like b-, g-, and d-tocopherols.
Lipid peroxidation is a process initiated by various internal and external stimuli, leading to the formation of a carbon-centered radical. This radical, when exposed to oxygen, transforms into a peroxyl radical, further propagating lipid peroxidation. The cycle continues as the peroxyl radical oxidizes an unsaturated fatty acid into a lipid hydroperoxide, regenerating the carbon-centered radical. This cycle is where vitamin E plays a crucial role. Embedded within lipid membranes, vitamin E interrupts this cycle by reacting with peroxyl radicals faster than these radicals can with unsaturated fatty acids. This action prevents the regeneration of the carbon-centered radical. However, this protective action oxidizes a-tocopherol into an a-tocopheroxyl radical, which necessitates regeneration by other antioxidants, notably vitamin C.
The interplay between vitamin C and vitamin E is evident in various pharmacokinetic studies. For instance, smokers, when compared to non-smokers, exhibited faster plasma disappearance rates of labeled a-tocopherol. This rate was inversely proportional to their plasma vitamin C levels. Further studies reinforced the idea that vitamin C plays a pivotal role in regulating vitamin E status, likely through a recycling mechanism.
Besides its antioxidant function, vitamin E, especially g-tocopherol, has the unique ability to scavenge reactive nitrogen species, forming 5-nitro-g-tocopherol. This suggests that g-tocopherol could serve as a potential biomarker for nitrative stress and may protect other biomolecules from nitration. Clinical studies have shown that individuals with coronary artery disease have a higher ratio of 5-NO2-g-tocopherol/g-tocopherol, emphasizing the role of reactive nitrogen species in cardiovascular disease.
Both a-tocopherol and g-tocopherol also possess anti-inflammatory properties. However, a-tocopherol seems to reduce inflammation only at high doses, while g-tocopherol has shown promising results in reducing thrombotic risk factors in various studies. Furthermore, both these forms of vitamin E play a role in regulating the bioavailability of nitric oxide, a vital vasodilator with anti-atherogenic properties.
In conclusion, vitamin E, particularly in its a- and g-tocopherol forms, plays a multifaceted role in human health. From acting as a potent antioxidant to regulating nitric oxide bioavailability and showcasing anti-inflammatory properties, vitamin E’s functions are diverse and essential for maintaining optimal health.
Recommended dietary allowance (RDA)
- Relation with PUFA:
- The intake of vitamin E is intrinsically linked to the consumption of polyunsaturated fatty acids. Specifically, as the intake of PUFA increases, the requirement for vitamin E also rises. This is because vitamin E plays a crucial role in preventing the oxidation of PUFAs.
- RDA for Men:
- For adult males, the RDA for vitamin E is set at 10 mg per day, which is equivalent to 15 International Units (IU).
- RDA for Women:
- For adult females, the recommended daily intake is slightly lower at 8 mg, translating to 12 IU.
- Unit Conversion:
- It’s essential to understand the conversion between milligrams and International Units when discussing vitamin E. Specifically, 1 mg of α-tocopherol is equivalent to 1.5 IU. This conversion helps in ensuring accurate dosage and understanding of dietary supplements.
- Special Considerations:
- Certain life stages, such as pregnancy and lactation, demand a higher intake of vitamin E. Therefore, a diet supplemented with vitamin E is advised for pregnant and lactating women to cater to the increased requirements during these phases.
Dietary sources of vitamin E
- Vegetable Oils:
- Among the primary sources of vitamin E are vegetable oils. Wheat germ oil stands out as a particularly rich source. Besides wheat germ oil, other oils such as cottonseed oil, peanut oil, corn oil, and sunflower oil are also commendable sources of this nutrient.
- Animal Products:
- Animal-derived products also contribute to the dietary intake of vitamin E. Meat, for instance, contains a notable amount of this vitamin. Additionally, dairy products like milk and butter, as well as eggs, are also valuable sources.
- Vegetables:
- Vegetables are not to be overlooked when considering sources of vitamin E. Their inclusion in daily diets ensures a balanced intake of this essential vitamin.
- Diverse Sources:
- It’s worth noting that soybean oil and cottonseed oil are also rich in vitamin E. These oils, along with previously mentioned sources like butter, milk, meat, and eggs, offer a diverse range of options for individuals to meet their vitamin E requirements.
Vitamin E Deficiency symptoms
- Origins of Deficiency:
- Most cases of vitamin E deficiency in humans arise from abnormalities in dietary fat absorption or metabolic issues. It’s seldom a result of a diet low in vitamin E. One notable metabolic anomaly is the mutation of genes coding for alpha-tocopherol transfer protein (α-TTP).
- Neurodegenerative Disorder:
- Individuals with the genetic defect related to α-TTP experience a progressive neurodegenerative disorder termed ataxia with vitamin E deficiency (AVED). This condition manifests even when the individual consumes standard amounts of vitamin E. To counteract the effects of the lack of α-TTP, significant amounts of alpha-tocopherol supplements are required.
- Nerve Issues:
- A deficiency in vitamin E can lead to nerve problems. This is attributed to the poor conduction of electrical impulses along nerves, stemming from alterations in the nerve membrane’s structure and function. Symptoms include ataxia, peripheral neuropathy, myopathies, retinopathy, and a weakened immune response.
- Symptoms in Animals:
- The manifestations of vitamin E deficiency differ across animal species. Common symptoms in many animals include sterility, muscle degeneration, megaloblastic anaemia, and changes in the central nervous system.
- Human Symptoms:
- In humans, severe symptoms of vitamin E deficiency are rare. However, when they do occur, they manifest as increased fragility of erythrocytes and minor neurological symptoms. Additionally, there’s an observed fragility in erythrocytes, abnormal cell membrane structures, and minor neurological signs.
- Reproductive Impact:
- In mature female rats, a deficiency of vitamin E can lead to sterility. In contrast, male rats may experience degeneration of the germinal epithelium of the testes, rendering spermatozoa non-motile.
Toxicity of vitamin E
Vitamin E, a crucial fat-soluble vitamin, plays an essential role in numerous biological functions. Among the group of fat-soluble vitamins, which includes vitamins A, D, E, and K, vitamin E stands out for its notably low toxicity levels.
- Comparison with Other Vitamins:
- When evaluating the toxicity levels of fat-soluble vitamins, vitamin E is observed to be the least toxic. This is in contrast to vitamins like A and D, which have well-documented toxic effects when consumed in excessive amounts.
- Long-Term Ingestion:
- Remarkably, even with prolonged ingestion of vitamin E, adverse effects are rarely reported. Studies have shown that the consumption of 300 mg/day of vitamin E for an extended period of 23 years did not result in any toxic effects.
- Safety Profile:
- The safety profile of vitamin E is robust, making it a relatively safe vitamin for consumption, even in higher doses. However, as with any nutrient, it’s essential to adhere to recommended dosages and consult with healthcare professionals if considering supplementation.
- Emphasis on Functions:
- Vitamin E’s primary function is as an antioxidant, protecting cells from the damaging effects of free radicals. Its low toxicity levels further emphasize its beneficial role in the body without causing harm.
References
- Bruno, R.S. (2014). Reference Module in Biomedical Sciences || Vitamin E. , (), –. doi:10.1016/b978-0-12-801238-3.00231-2
- , (2016). Meyler’s Side Effects of Drugs || Vitamin E. , (), 488–493. doi:10.1016/b978-0-444-53717-1.01640-1
- Engelking, Larry R. (2015). Textbook of Veterinary Physiological Chemistry || Vitamin E. , (), 294–298. doi:10.1016/b978-0-12-391909-0.50046-3
- Traber, M.G. (2013). Encyclopedia of Human Nutrition || Vitamin E: Metabolism and Requirements. , (), 383–389. doi:10.1016/B978-0-12-375083-9.00278-6
- Feki M, Souissi M, Mebazaa A. La vitamine E: structure, métabolisme et fonctions [Vitamin E: structure, metabolism, and functions]. Ann Med Interne (Paris). 2001 Oct;152(6):384-91. French. PMID: 11907951.
- https://lpi.oregonstate.edu/mic/vitamins/vitamin-E
- https://webbook.nist.gov/cgi/cbook.cgi?ID=59-02-9
- http://hozenkagaku.com/EnglishMDT.html