What is Uronic Acid Pathway or Glucuronic pathway?
- Uronic Acid Pathway also Knwon as Glucuronic pathway.
- The uronic acid pathway, an intricate metabolic route within the cell’s cytoplasm, offers a distinct avenue for the transformation of glucose 6-phosphate (Glc-6-P).
- This pathway orchestrates the conversion of Glc-6-P into uridine diphosphate glucuronate (UDP-glucuronate), a pivotal participant in the synthesis of vitamin C, glycoproteins, and the detoxification of both internal and external compounds. Just like the hexose mono-phosphate shunt (HMS), the uronic acid pathway operates without generating ATP through substrate-level phosphorylation. However, it does orchestrate the production of reducing equivalents, specifically NADH, which can subsequently fuel ATP synthesis through the intricate machinery of mitochondrial oxidative phosphorylation.
- The initial stride in this pathway involves the conversion of glucose 6-phosphate into glucose 1-phosphate (Glc-1-P). Subsequently, Glc-1-P joins forces with uridine triphosphate (UTP), resulting in the formation of the dynamic nucleotide, UDP-glucose. These foundational reactions, intrinsically linked to the creation of glycogen, were meticulously detailed in a prior discourse.
- The journey then takes a transformative turn as UDP-glucose undergoes oxidation, a process catalyzed by an NAD+-dependent dehydrogenase. The outcome of this reaction yields UDP-glucuronate, necessitating the utilization of two molecules of NAD+ for this transformation. Importantly, it’s noteworthy that the term “UDP-glucuronate” is more apt at pH 7.4 due to its anionic nature, thereby underscoring the significance of utilizing this nomenclature over “UDP-glucuronic acid.”
- The liver, alongside a lesser role played by the kidneys, takes center stage in leveraging UDP-glucuronate for conjugation reactions with lipophilic endogenous compounds. For instance, notable candidates include steroid hormones, bilirubin (an end product of heme metabolism), and select lipophilic drugs and toxins that enter the body through dietary sources. In a fascinating orchestration, the liver and kidneys proceed to transform these compounds into more water-soluble, polar conjugates, paving the way for their excretion into bile and other excretory pathways.
- Through a cascade of enzymatic reactions, the uronic acid pathway furnishes a route for the conversion of specific carbohydrates, such as glucose and galactose, into uronic acid derivatives. These uronic acids, including glucuronic acid and galacturonic acid, constitute fundamental building blocks within glycosaminoglycans (GAGs), proteoglycans, and other biologically crucial compounds. Referred to variously as the uronate pathway, hexuronic acid pathway, or uronic acid pathway, this metabolic cornerstone holds the key to translating carbohydrates into uronic acids, vital intermediates integral to an array of vital metabolic processes.
Overview
The uronic acid pathway stands as a distinctive cytoplasmic thoroughfare within cellular metabolism, orchestrating intricate transformations that hold pivotal significance for various physiological processes. As we embark on an exploration of this biochemical route, we uncover its multifaceted roles and far-reaching implications for cellular health and homeostasis.
Metabolic Journey of Glc-6-P: At its core, the uronic acid pathway represents an alternate route for the metabolism of glucose 6-phosphate (Glc-6-P). In this journey, Glc-6-P embarks on a series of enzymatic conversions that lead to the synthesis of uridine diphosphate glucuronate (UDP-glucuronate). This molecular product emerges as a crucial player in diverse cellular functions, particularly hepatic conjugation reactions.
Crucial Role in Hepatic Conjugation: The formation of UDP-glucuronate through the uronic acid pathway is a cornerstone of hepatic detoxification processes. This molecule serves as a potent agent for the conjugation of lipophilic, non-polar compounds, rendering them more water-soluble and amenable to excretion from the body. This detoxification mechanism is vital for safeguarding the organism against potential harm from endogenous and exogenous substances.
Feline Quirks and Drug Metabolism: Interestingly, the uronic acid pathway sheds light on a unique aspect of feline biology. Cats, unlike many other species, face challenges in conjugating and excreting certain drugs from their bodies. This limitation is particularly notable for substances that are typically metabolized through the hepatic glucuronide conjugation mechanism. The uronic acid pathway’s involvement in drug metabolism unveils the intricacies of feline physiology.
The Vitamin C Connection: In the realm of nutrient synthesis, the uronic acid pathway unfolds a tale of diversity. Most animals, excluding primates, fish, flying mammals, songbirds, and guinea pigs, utilize this pathway for the synthesis of L-ascorbic acid, better known as vitamin C. This essential nutrient holds pivotal roles in collagen formation, immune support, and antioxidant defense, showcasing the pathway’s contributions to holistic health.
Branching Pathways and Glycoprotein Formation: L-Gulonate emerges as a pivotal branch point within the uronic acid pathway, bridging connections to the hexose monophosphate shunt through the creation of D-xylulose. As the journey continues, the uronic acid pathway contributes to the synthesis of sugar moieties found in various classes of glycoproteins. These glycoproteins, integral to cellular communication and structure, underscore the pathway’s role in maintaining cellular integrity.
Heparin and Heparan Sulfate: The uronic acid pathway extends its reach to the formation of heparin and heparan sulfate. These complex molecules, rich in uronic acid components, play vital roles in cellular interactions, particularly within the vascular system. The pathway’s involvement in their synthesis underscores its broader influence on diverse physiological processes.
In summary, the uronic acid pathway emerges as a captivating nexus within cellular metabolism, intertwining with detoxification, nutrient synthesis, drug metabolism, and the formation of essential biomolecules. Its versatile roles underscore the complexity and interconnectedness of biochemical pathways, shedding light on the intricate dance of life within the cellular realm.
Definition of Uronic Acid Pathway or Glucuronic pathway
The uronic acid pathway is a metabolic route that converts glucose and other sugars into uronic acids, essential intermediates in various biological processes including glycosaminoglycan synthesis, glycoprotein formation, and detoxification of compounds.
What is Uronic Acid?
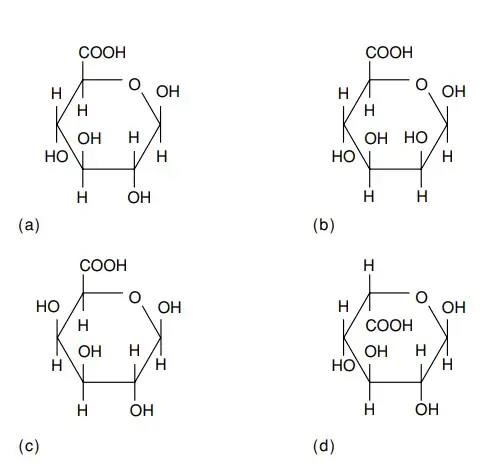
- Uronic acids emerge as a result of the oxidation of the alcohol groups present in monosaccharides. These compounds adopt the nomenclature of replacing the -ose suffix with “uronic acid.” The notable structures of frequently encountered uronic acids are illustrated in Figure. Among them, d-Mannuronic acid stands as the 2-epimer of d-glucuronic acid, whereas l-iduronic acid assumes the role of the 5-epimer of d-glucuronic acid.
- This collection of uronic acids assumes a pivotal role within specific natural heteropolysaccharides. Their significance extends to the detoxification of substances, such as pharmaceutical compounds. Notably, glucuronic acids find their place in human urine, engaging in glycosidic unions with hydroxylated compounds like menthol, borneol, and estrogens.
- This union with glucuronic acid enhances the solubility of these hydroxylated entities, rendering them more easily eliminated by the body’s mechanisms. Additionally, glucuronic acid enters into a conjugate with bilirubin, a component of bile pigment.
- Stepping into the realm of human biology, d-glucuronic acid occupies a pivotal role. It assumes membership within an array of glycosaminoglycans (GAGs), as well as forming glucuronide derivatives of hormones and drugs. The trajectory of d-glucuronic acid extends further, serving as the precursor to l-ascorbic acid, more commonly recognized as vitamin C, in various animal species.
- This biochemical journey unfolds within the uronic pathway, an alternative oxidative route for glucose, distinct from the conventional ATP-generating processes. This pathway commences with the conversion of glucose 6-phosphate into glucose 1-phosphate, which subsequently binds with uridine triphosphate to craft uridine diphosphate glucose (UDPGlc).
- A two-step oxidation process, catalyzed by the enzyme UDPGlc dehydrogenase and reliant on the nicotinamide adenine dinucleotide (NAD), transpires, culminating in the formation of UDPglucuronate.
- The narrative of uronic acids further extends to their utilization, yielding UDP glucuronate, a form of glucuronic acid that intertwines with proteoglycans or forms conjugates with steroid hormones, certain drugs, or bilirubin. In the context of bilirubin, this conjugation brings forth acylglucuronides, resulting from the attachment of two glucuronic acid molecules to the propionic acid groups.
- Such modification amplifies the water solubility of bilirubin, enabling its eventual excretion through bile and the gastrointestinal tract.
- Delving deeper into the biochemical panorama, the uronic pathway gives rise to another product of consequence: l-ascorbic acid, also known as vitamin C. Interestingly, this synthesis unfolds within mammals, excluding humans, primates, and guinea pigs. Through a series of reactions, glucuronate takes on a transformative journey to eventually yield l-ascorbic acid.
- The uronic pathway boasts its highest activity levels within the liver, kidneys, and intestines. Its paramount function revolves around generating UDPglucuronic acid, an indispensable component for detoxifying various compounds.
- This detoxification is achieved through the elimination of glucuronides in either urine or bile. The mechanism comes into play against a spectrum of substances, including carcinogens and drugs like antipyretics, hypnotics, and antimalarials.
- Remarkably, certain organs might harbor specific active uronic pathways tailored to distinct drugs. Noteworthy triggers for stimulating glucuronic acid synthesis encompass heightened consumption of substances excreted as glucuronides or the consumption of steroids and barbiturates, which induce the microsomal P-450 system.
Reaction And Key Enzymes of Uronic Acid Pathway or Glucuronic pathway
The uronic acid pathway orchestrates a series of enzymatic reactions, oxidation-reduction processes, and interconversions of diverse sugar intermediates, culminating in the efficient conversion of glucose into uronic acids. Key enzymes are central to these transformations, ensuring the smooth progression of this essential metabolic route.
- Hexokinase and Glucose-6-Phosphate Dehydrogenase: Hexokinase initiates the pathway by phosphorylating glucose, forming glucose 6-phosphate (Glc-6-P). Glucose-6-phosphate dehydrogenase then catalyzes the conversion of Glc-6-P to gluconolactone, an intermediate that sets the stage for subsequent reactions.
- Gluconolactonase and 6-Phosphogluconate Dehydrogenase: Gluconolactonase cleaves gluconolactone to form 6-phosphogluconate, a pivotal intermediate. 6-phosphogluconate dehydrogenase further drives the process by facilitating the oxidative decarboxylation of 6-phosphogluconate, generating ribulose 5-phosphate and reducing NADP+ to NADPH.
- Glucose and Galactose Dehydrogenases: These enzymes play a crucial role in catalyzing the oxidation of glucose and galactose, initiating the pathway’s conversion of sugars to uronic acids. Their actions lead to the formation of corresponding sugar acids, setting the stage for subsequent enzymatic modifications.
- Glucuronyl Transferases: Glucuronyl transferases are pivotal enzymes that transfer glucuronic acid moieties from UDP-glucuronic acid to various lipophilic compounds. This conjugation reaction renders these compounds more water-soluble and suitable for excretion. Glucuronyl transferases are essential for the detoxification of endogenous and exogenous substances in the liver and kidneys.
- Glucuronidases: On the other end of the spectrum, glucuronidases catalyze the hydrolysis of glucuronide conjugates, releasing glucuronic acid and the original lipophilic compound. This step is crucial for the reverse process, where uronic acids are converted back to their parent molecules for recycling or elimination.
In summary, the uronic acid pathway is a finely tuned sequence of enzymatic reactions, each catalyzed by specific key enzymes. Hexokinase, glucose-6-phosphate dehydrogenase, gluconolactonase, and 6-phosphogluconate dehydrogenase kickstart the pathway, while glucose and galactose dehydrogenases set the stage for subsequent transformations. Glucuronyl transferases and glucuronidases play pivotal roles in the conjugation and hydrolysis of glucuronic acid moieties, driving detoxification and metabolism. These enzymes collectively ensure the conversion of sugars into uronic acids and vice versa, contributing to essential cellular processes such as glycoprotein synthesis, vitamin C biosynthesis, and detoxification pathways.
Ascorbic Acid Synthesis
Ascorbic acid, commonly known as vitamin C, holds a pivotal role in the uronic acid pathway, contributing to vital biochemical processes within the body. This essential vitamin is intricately synthesized through a series of enzymatic reactions, with its production stemming from ribulose-5-phosphate, a key intermediate in the pathway.
- Conversion of Ribulose-5-Phosphate to UDP-Glucuronic Acid: At the heart of vitamin C biosynthesis lies the transformation of ribulose-5-phosphate. This precursor undergoes a remarkable conversion, culminating in the formation of UDP-glucuronic acid. This process sets the stage for the subsequent steps leading to the synthesis of ascorbic acid.
- Gluconolactone Oxidase: Once UDP-glucuronic acid is formed, the enzyme gluconolactone oxidase enters the scene. This enzyme catalyzes the transformation of UDP-glucuronic acid into L-gluconolactone, a crucial intermediate in the ascorbic acid biosynthesis pathway.
- Formation of Ascorbic Acid: L-gluconolactone undergoes a series of intricate biochemical processes, ultimately leading to the synthesis of ascorbic acid. This pathway involves several steps of molecular rearrangements and enzymatic reactions. As a result, L-gluconolactone is transformed into ascorbic acid, meeting the body’s essential requirement for this vital vitamin.
The production of ascorbic acid through the uronic acid pathway fulfills a fundamental physiological need. Ascorbic acid is recognized for its antioxidant properties, playing a pivotal role in various oxidation-reduction reactions within the body. Additionally, vitamin C is instrumental in collagen synthesis, immune function, and the absorption of non-heme iron from plant-based sources. Its biosynthesis through the uronic acid pathway underscores the intricate web of metabolic processes that contribute to overall health and well-being.
In summary, the uronic acid pathway serves as the conduit for the biosynthesis of ascorbic acid, a critical nutrient known for its multifaceted roles in the body. From ribulose-5-phosphate to UDP-glucuronic acid, and through the catalytic actions of enzymes like gluconolactone oxidase, the pathway orchestrates the conversion of precursors into ascorbic acid, ensuring the body’s physiological demand for this essential vitamin is met.
Steps of the Uronic Acid Pathway
The uronic acid pathway represents an alternate cytoplasmic route for the metabolism of glucose 6-phosphate (Glc-6-P), playing a pivotal role in various essential processes within the cell. This pathway converts Glc-6-P into uridine diphosphate glucuronate (UDP-glucuronate), a molecule utilized in the biosynthesis of vitamin C, glycoproteins, and hepatic detoxification of endogenous and exogenous compounds. Let’s delve into the steps of the uronic acid pathway and its significance in cellular function.
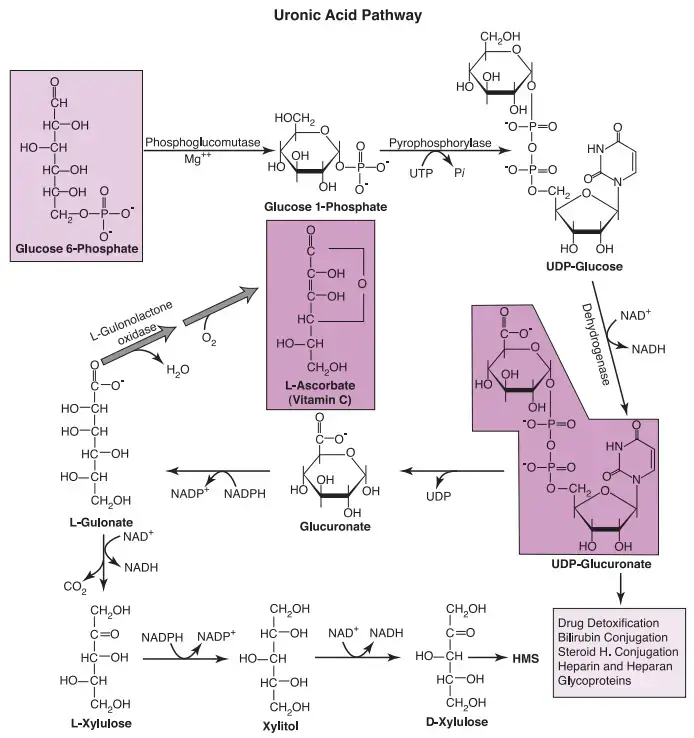
- Conversion to Glucose 1-phosphate (Glc-1-P): The pathway initiates with the conversion of Glc-6-P to glucose 1-phosphate (Glc-1-P). This reaction sets the stage for further transformations in the pathway.
- Formation of UDP-glucose: Glc-1-P then reacts with uridine triphosphate (UTP) to form the active nucleotide, UDP-glucose. This step is shared with glycogen formation and provides a crucial building block for various processes.
- Oxidation to UDP-glucuronate: UDP-glucose undergoes oxidation by an NAD+-dependent dehydrogenase, resulting in the formation of UDP-glucuronate. This compound serves as a key precursor for multiple downstream reactions.
- Conjugation Reactions: The liver and kidneys employ UDP-glucuronate for conjugation reactions with lipophilic endogenous compounds (such as steroid hormones and bilirubin) and lipophilic drugs and toxins from the diet. These conjugation reactions render the compounds more water-soluble, facilitating their excretion into bile and urine.
- Tissue-Specific Functions: UDP-glucuronate is pivotal in the biosynthesis of carbohydrate-protein complexes like glycosaminoglycans, mucopolysaccharides, and glycoproteins. These complexes contribute to the structural integrity of various tissues, including cartilage, bone, skin, and arterial walls.
- Vitamin C Biosynthesis: UDP-glucuronate serves as a precursor for L-gulonate, which is further converted to L-ascorbate (vitamin C) in most animals. However, certain species, including primates, fish, flying mammals, songbirds, and guinea pigs, lack the enzyme L-gulonolactone oxidase required for this conversion. These organisms must obtain vitamin C through their diets.
- Connection to Hexose Mono-phosphate Shunt (HMS): L-gulonate also serves as a branching point between the uronic acid pathway and the HMS. L-gulonate can be converted to L-xylulose, which, after subsequent reactions, enters the HMS.
- Physiological Impact: Deficiencies or anomalies in the uronic acid pathway can lead to disorders such as essential or idiopathic pentosuria, where excessive L-xylulose is excreted in urine. Some drugs, like barbiturates, can accelerate glucose entry into the uronic acid pathway, influencing its activity.

In summary, the uronic acid pathway is a multifaceted metabolic route with crucial roles in cellular processes. Its key functions include glycoprotein synthesis, heparin and heparan sulfate formation, production of UDP-glucuronate for detoxification, L-ascorbate biosynthesis, and minor pentose formation. While it does not directly generate ATP, it contributes to the production of reducing equivalents that can subsequently be utilized in mitochondrial oxidative phosphorylation. The uronic acid pathway showcases the intricate interplay of biochemical reactions in cellular metabolism, influencing both structural and functional aspects of the organism.
Breakdown Each Steps of the Uronic Acid Pathway or Glucuronic pathway
Step 1: Conversion to Glucose 1-phosphate (Glc-1-P)
- Enzyme: Phosphoglucomutase
- Reaction: Glucose 6-phosphate (Glc-6-P) is isomerized to glucose 1-phosphate (Glc-1-P) by the action of phosphoglucomutase.
- Addition/Reduction: Isomerization reaction involving the transfer of a phosphate group, resulting in the conversion of Glc-6-P to Glc-1-P.
Step 2: Formation of UDP-glucose
- Enzyme: UDP-glucose pyrophosphorylase
- Reaction: Glc-1-P reacts with uridine triphosphate (UTP) to form UDP-glucose and pyrophosphate.
- Addition/Reduction: Formation of a nucleotide sugar through the addition of a UTP molecule to Glc-1-P, releasing pyrophosphate.
Step 3: Oxidation to UDP-glucuronate
- Enzyme: UDP-glucose dehydrogenase
- Reaction: UDP-glucose is oxidized by NAD+ to form UDP-glucuronate, generating NADH in the process.
- Addition/Reduction: Oxidation of UDP-glucose involves the reduction of NAD+ to NADH.
Step 4: Conjugation Reactions
- Enzymes: UDP-glucuronosyltransferases (UGTs)
- Reaction: UDP-glucuronate is conjugated to lipophilic compounds, such as steroid hormones, bilirubin, and drugs, forming glucuronide conjugates.
- Addition/Reduction: Addition of UDP-glucuronate to lipophilic compounds through glycosidic bonds, rendering them more water-soluble for excretion.
Step 5: Tissue-Specific Functions
- Enzymes: Various enzymes involved in glycosaminoglycan, mucopolysaccharide, and glycoprotein biosynthesis.
- Reaction: UDP-glucuronate is utilized in the biosynthesis of carbohydrate-protein complexes, contributing to the structural integrity of various tissues.
- Addition/Reduction: Incorporation of UDP-glucuronate into complex carbohydrate-protein structures.
Step 6: Vitamin C Biosynthesis
- Enzyme: L-gulonolactone oxidase (absent in certain species)
- Reaction: UDP-glucuronate is converted to L-gulonate, which can then be further converted to L-ascorbate (vitamin C) in species possessing L-gulonolactone oxidase.
- Addition/Reduction: Reduction of UDP-glucuronate to L-gulonate and subsequent oxidation to form L-ascorbate.
Step 7: Connection to HMS
- Enzymes: Various enzymes involved in HMS and uronic acid pathway.
- Reaction: L-gulonate can be converted to L-xylulose, which enters the hexose mono-phosphate shunt (HMS) after further reactions.
- Addition/Reduction: Reduction and conversion of L-gulonate to L-xylulose.
Step 8: Physiological Impact
- Enzymes: Enzymes involved in glucose entry and metabolism.
- Reaction: Certain drugs, like barbiturates, can influence glucose entry into the uronic acid pathway, affecting its activity.
- Addition/Reduction: Alteration of glucose metabolism and its entry into the pathway.
Each step of the uronic acid pathway involves specific enzymatic reactions that result in the addition, reduction, or modification of molecules, leading to the formation of important intermediates and end products. The pathway’s complexity highlights its significance in various cellular processes, including glycoprotein synthesis, detoxification, and the biosynthesis of essential molecules like vitamin C.

Fate of L-Gulonic Acid
Within the intricate realm of cellular metabolism, L-gulonic acid, the immediate precursor of ascorbic acid (vitamin C), takes on a dynamic role with far-reaching implications for physiological processes. The destiny of L-gulonic acid unfolds through a series of interconnected pathways, each contributing to the body’s intricate biochemical orchestra.
- Production of Ascorbic Acid: One prominent fate of L-gulonic acid lies in its pivotal role as the precursor of ascorbic acid. Through enzymatic transformations and orchestrated reactions, L-gulonic acid serves as the foundational molecule from which ascorbic acid is crafted. Ascorbic acid, renowned for its antioxidant prowess and indispensable physiological functions, is synthesized from L-gulonic acid, ensuring a steady supply of this essential vitamin.
- Diverse Metabolic Pathways: L-gulonic acid’s journey extends beyond vitamin C biosynthesis. The cellular machinery can channel L-gulonic acid into diverse metabolic pathways, unleashing a cascade of reactions with far-reaching consequences. Within this intricate dance, L-gulonic acid’s fate can diverge, giving rise to other uronic acids that are integral to specialized metabolic processes.
- Synthesis of Glycosaminoglycans: One such intriguing pathway involves the transformation of L-gulonic acid into uronic acids that contribute to the synthesis of glycosaminoglycans. These intricate carbohydrate-protein complexes are vital components of connective tissues, cartilage, and various extracellular structures. By harnessing L-gulonic acid’s potential, the cell orchestrates the production of glycosaminoglycans, which play a crucial role in maintaining tissue integrity and function.
In summary, the fate of L-gulonic acid in cellular metabolism is a multi-faceted journey with significant implications for the body’s functioning. It serves as the launching pad for the synthesis of ascorbic acid, a cornerstone of physiological activities. Simultaneously, L-gulonic acid’s transformation can contribute to the intricate tapestry of metabolic pathways, yielding other uronic acids essential for specialized processes, including the formation of glycosaminoglycans. This elegant interplay underscores the sophistication of cellular metabolism, where each molecule’s destiny is meticulously orchestrated to sustain life’s intricate harmony.
Uronic Acid Pathway Regulation
The uronic acid pathway, a crucial metabolic route, operates under precise regulation to uphold cellular homeostasis and facilitate the seamless functioning of vital biochemical processes. The orchestrated activity of key enzymes within this pathway is subject to meticulous control, influenced by a myriad of factors that collectively ensure the delicate balance of metabolic equilibrium.
- Substrate Availability: A cornerstone of uronic acid pathway regulation rests upon substrate availability. The availability of glucose, the initial substrate, profoundly impacts the initiation of the pathway. Fluctuations in glucose levels trigger corresponding adjustments in enzymatic activity, orchestrating the entry and progression of substrates through the pathway.
- Hormonal Signals: Hormonal cues emanating from the intricate endocrine network play a pivotal role in fine-tuning the uronic acid pathway. Hormones such as insulin and glucagon wield influence over glucose metabolism, thereby impacting the flux of substrates within the pathway. These hormonal signals act as molecular messengers, transmitting instructions that dynamically regulate enzymatic activity.
- Gene Expression Regulation: At the core of pathway regulation lies the control of gene expression. The production of key enzymes pivotal to the uronic acid pathway is governed by intricate gene regulatory mechanisms. Transcription factors and epigenetic modifications collaboratively shape gene expression profiles, ensuring a synchronized response to cellular demands.
- Feedback Inhibition: An elegant mechanism within the regulatory framework involves feedback inhibition. Metabolites produced downstream in the uronic acid pathway can act as allosteric regulators, influencing the activity of earlier enzymes. This self-regulating loop ensures that the production of intermediates is modulated to prevent excessive accumulation or depletion.
- Cellular Signaling: Intricate signaling cascades play a role in regulating the uronic acid pathway. Cell surface receptors and intracellular messengers transduce signals that influence enzymatic activity. These signaling events serve as dynamic switches, responding to external cues and internal demands to fine-tune pathway flux.
- Metabolic Demands: The uronic acid pathway’s regulation is finely attuned to metabolic demands. Physiological requirements dictate whether substrates are preferentially channeled into ascorbic acid synthesis or directed toward the production of other uronic acids needed for specialized metabolic processes.
In summary, the regulation of the uronic acid pathway is an intricate symphony orchestrated to ensure metabolic harmony. Substrate availability, hormonal signals, gene expression regulation, feedback inhibition, cellular signaling, and metabolic demands converge to finely modulate enzymatic activity within the pathway. This orchestration guarantees that the uronic acid pathway operates in concert with cellular needs, maintaining equilibrium and enabling the seamless execution of essential biochemical processes.
Effect of Drugs on Uronic Acid Pathway
The uronic acid pathway, a central avenue of metabolic transformations, can be profoundly influenced by the administration of certain drugs. These pharmacological interventions trigger intricate cascades of enzymatic reactions, orchestrating notable effects on the uronic acid pathway and its associated metabolites.
Enhancement of Glucuronate Synthesis: The administration of specific drugs, such as barbital, aminopyrine, antipyrine, and chlorobutanol, exerts a significant impact on the uronic acid pathway’s dynamics. These drugs act as metabolic modulators, intensifying the synthesis of glucuronate from glucose. By augmenting the production of glucuronate, these drugs pave the way for an increased availability of this essential precursor for conjugation reactions.
The consequence of this heightened glucuronate synthesis is profound. Glucuronate serves as a versatile molecule crucial for the conjugation of various lipophilic compounds, rendering them more water-soluble and facilitating their efficient excretion. This detoxification process safeguards the organism against potential harm from endogenous and exogenous substances, underscoring the therapeutic potential of drugs that manipulate the uronic acid pathway.
Synthesis of Ascorbic Acid (Vitamin C): The influence of drugs on the uronic acid pathway extends beyond conjugation reactions. Remarkably, certain drugs have been observed to enhance the synthesis of ascorbic acid (vitamin C) in rats. This finding underscores the pathway’s role in not only detoxification but also in the biosynthesis of crucial biomolecules with far-reaching physiological implications.
Ascorbic acid, a potent antioxidant and enzyme cofactor, holds diverse functions ranging from collagen synthesis to immune system support. By influencing the uronic acid pathway to elevate ascorbic acid production, these drugs indirectly contribute to a cascade of cellular reactions that impact overall health and well-being.
In summary, drugs wield the power to intricately modulate the uronic acid pathway, exerting substantial effects on the synthesis of glucuronate and ascorbic acid. Their influence enhances the availability of glucuronate for conjugation reactions, reinforcing the pathway’s role in detoxification. Simultaneously, the stimulation of ascorbic acid synthesis showcases the pathway’s versatility in contributing to a broader spectrum of cellular functions. This intricate interplay underscores the potential for pharmacological interventions to shape metabolic dynamics and underscores the uronic acid pathway’s significance in maintaining cellular equilibrium.
Uronic Acid Pathway and Associated Disorders
The uronic acid pathway, a crucial metabolic thoroughfare, is not immune to the complexities of genetic defects and dysregulation. When this intricate pathway experiences disruptions, various disorders can arise, shedding light on the delicate balance required for seamless biochemical functioning. Two illustrative examples, mucopolysaccharidoses (MPS) and hereditary inclusion body myopathy (HIBM), highlight the profound consequences of uronic acid pathway disorders.
Mucopolysaccharidoses (MPS) and Hereditary Inclusion Body Myopathy (HIBM): Dysregulation or genetic anomalies within the uronic acid pathway can lead to debilitating disorders. One such instance is mucopolysaccharidoses (MPS), a group of disorders characterized by impaired glycosaminoglycan (GAG) metabolism. GAGs, synthesized within the uronic acid pathway, contribute to various vital processes. Dysfunctional GAG metabolism in MPS can lead to severe developmental abnormalities and tissue dysfunction.
Another disorder linked to the uronic acid pathway is hereditary inclusion body myopathy (HIBM). This condition is associated with impaired GAG metabolism, highlighting the pathway’s central role in maintaining proper cellular function. The interplay between GAG synthesis and cellular health underscores the significance of a well-functioning uronic acid pathway in preventing disorders such as HIBM.
Essential Pentosuria: Delving deeper into the realm of uronic acid pathway disorders, we encounter essential pentosuria, a condition that sheds light on the delicate balance within this biochemical route. Essential pentosuria arises from a deficiency in the enzyme L-xylulose reductase, which catalyzes a critical step in the pathway. This deficiency leads to the excessive excretion of L-xylulose, a sugar intermediate produced within the pathway.
Although essential pentosuria is generally considered benign and devoid of severe clinical manifestations, its presence illuminates the interplay between enzymatic activity and metabolite accumulation. Understanding the impact of this disorder on the uronic acid pathway deepens our comprehension of metabolic pathways and their regulation, highlighting the intricate connections within our biochemical landscape.
In conclusion, the uronic acid pathway’s vulnerabilities underscore its significance in sustaining proper cellular function. Disorders such as mucopolysaccharidoses, hereditary inclusion body myopathy, and essential pentosuria exemplify the far-reaching consequences of dysregulation within this intricate metabolic route. By unraveling the complexities of these disorders, we gain insight into the delicate balance required for metabolic harmony and the pivotal role the uronic acid pathway plays in maintaining cellular vitality.
Role of Uronic Acid Pathway in Animal
Within the intricate tapestry of animal metabolism, the uronic acid pathway emerges as a pivotal conductor, orchestrating a symphony of biochemical transformations that underpin a myriad of physiological processes. With its multifaceted roles, the pathway weaves together essential functions crucial for cellular health and overall organismal well-being.
- Formation of Glycosaminoglycans: Uronic acids in animals play a paramount role in the formation of glycosaminoglycans (GAGs), intricate carbohydrate-protein complexes that are essential components of connective tissues. The pathway’s contribution to GAG synthesis underscores its significance in maintaining tissue integrity, supporting mechanical resilience, and facilitating cellular communication.
- Polysaccharide Synthesis and Precursors for Ascorbic Acid: Uronic acids also serve as architectural components in the synthesis of certain polysaccharides. Moreover, they emerge as precursors for the production of ascorbic acid, a vital nutrient with far-reaching implications for biological functions. Ascorbic acid’s roles in collagen synthesis, immune support, and antioxidant defense underscore the importance of the uronic acid pathway in facilitating the availability of this essential molecule.
- Oxidation of Drugs and Xenobiotics: The uronic acid pathway extends its reach to the realm of detoxification and drug metabolism. Certain medicines and xenobiotics encountered in the body undergo oxidation within the pathway. Enzyme activities within this route facilitate the transformation of these substances into more water-soluble chemicals, hastening their elimination from the body. This process, known as drug oxidation, stands as a critical mechanism for the metabolism and detoxification of pharmacological agents and foreign compounds.
- Carbohydrate Conversion to Uronic Acids: The intricate and vital uronic acid pathway also plays a central role in the conversion of glucose and other carbohydrates into uronic acids. This enzymatic transformation serves as the foundation for various downstream processes, including the formation of glycosaminoglycans and the synthesis of ascorbic acid.
- Advancing Basic Biochemical Understanding and Medical Research: A deeper understanding of the uronic acid pathway not only enriches our knowledge of fundamental biochemical processes but also holds the key to significant advancements in medical research. The insights gained from unraveling the complexities of this pathway aid in drug discovery, medical treatment strategies, and the exploration of metabolic diseases.
In summary, the uronic acid pathway in animals weaves a complex tapestry of biochemical reactions that extend far beyond mere metabolic conversions. Its roles in glycosaminoglycan formation, polysaccharide synthesis, ascorbic acid production, drug oxidation, and carbohydrate conversion are pivotal to a spectrum of physiological functions. As a cornerstone of animal metabolism, the uronic acid pathway contributes to the intricate dance of life, facilitating both health and homeostasis in the animal kingdom.
Importance of Uronic Acid Pathway or Glucuronic pathway
- UDP-Glucuronic Acid Production: At the heart of its significance lies the production of UDP-glucuronic acid, the biologically active form of glucuronic acid. This molecule serves as a versatile building block that underpins a diverse range of biochemical reactions, contributing to the synthesis of vital compounds.
- Conjugation of Bilirubin: The uronic acid pathway plays a pivotal role in the detoxification of endogenous substances. It facilitates the conjugation of bilirubin, a breakdown product of heme metabolism, rendering it more water-soluble. This transformation prepares bilirubin for efficient excretion, preventing its accumulation and potential toxicity.
- Steroid Conjugation: As a metabolic hub, the uronic acid pathway also engages in the conjugation of steroids. By modifying these lipophilic molecules with glucuronic acid, the pathway enhances their water solubility, thereby aiding their elimination from the body. This transformation not only facilitates detoxification but also maintains hormonal balance.
- Drug Conjugation: The pathway’s contributions extend to pharmacology. The conjugation of various drugs with glucuronic acid transforms them into water-soluble derivatives. This alteration enhances their excretable nature, minimizing their accumulation and potential adverse effects. The uronic acid pathway thus serves as a natural mechanism for drug metabolism and elimination.
- Synthesis of Glycosaminoglycans (GAGs): Beyond detoxification and conjugation, the pathway contributes to the synthesis of glycosaminoglycans (GAGs), intricate carbohydrate-protein complexes. These compounds are essential components of connective tissues, cartilage, and structural matrices. The pathway’s involvement in GAG synthesis underscores its role in maintaining tissue integrity and function.
Biosynthetic functions of Uronic Acid Pathway or Glucuronic pathway
- Synthesis of Glycoprotein Sugar Moieties: The uronic acid pathway serves as a cornerstone in the assembly of sugar moieties for a diverse array of glycoproteins. By generating essential intermediates such as UDP-glucuronic acid, the pathway contributes to the glycosylation of proteins, imparting structural diversity and functional intricacies to these glycosylated biomolecules.
- Participation in Heparin and Heparan Sulfate Formation: Heparin and heparan sulfate, intricate carbohydrate-protein complexes, are critical players in various physiological processes. The uronic acid pathway’s engagement in their biosynthesis underscores its contribution to the formation of these glycosaminoglycans, which in turn support cellular adhesion, signaling, and the maintenance of extracellular matrix integrity.
- Production of UDP-Glucuronate for Conjugation Reactions: A pivotal role of the uronic acid pathway lies in generating UDP-glucuronate, a versatile precursor crucial for various conjugation reactions. This enzymatic transformation results in the attachment of glucuronic acid moieties to lipophilic compounds, facilitating their solubilization and subsequent excretion. This detoxification mechanism safeguards the organism against potential harm from endogenous and exogenous substances.
- L-Ascorbate (Vitamin C) Formation: The uronic acid pathway’s profound influence extends to the biosynthesis of L-ascorbate, commonly known as vitamin C. By navigating a series of intricate biochemical reactions, the pathway harnesses the precursor L-gluconolactone to craft this potent antioxidant and enzymatic cofactor. Vitamin C’s far-reaching roles in collagen synthesis, immune function, and oxidative stress management highlight the pathway’s pivotal contribution to overall health.
- Minor Route for Pentose Formation: Beyond its major roles, the uronic acid pathway also functions as a minor route for the formation of pentoses, exemplified by D-xylulose. These pentoses, derived from the pathway, find entry into the hexose mono-phosphate shunt, further enriching the metabolic landscape and contributing to a versatile repertoire of biochemical transformations.
Worksheet on Uronic Acid Pathway
- Definition and Overview: a. Define the uronic acid pathway. b. List the major functions of the uronic acid pathway.
- Glycosaminoglycans (GAGs): a. Explain the role of uronic acids in the formation of glycosaminoglycans. b. Name two types of glycosaminoglycans and their functions.
- Detoxification and Conjugation: a. Describe how the uronic acid pathway contributes to the detoxification of drugs and xenobiotics. b. Explain the process of conjugation and how it enhances water solubility.
- Vitamin C Synthesis: a. Elaborate on the role of the uronic acid pathway in the production of L-ascorbic acid (vitamin C). b. Mention three important functions of vitamin C in the body.
- Regulation of the Uronic Acid Pathway: a. Describe at least two factors that regulate the uronic acid pathway. b. Explain why tight regulation of this pathway is important for cellular homeostasis.
- Disorders and Dysregulation: a. Name two disorders associated with dysregulation or genetic defects in the uronic acid pathway. b. Briefly explain the consequences of these disorders on physiological processes.
- Drug Impact on the Uronic Acid Pathway: a. List four drugs that can enhance the uronic acid pathway’s activity. b. Explain how these drugs affect the synthesis of glucuronate and their implications for drug metabolism.
- Essential Pentosuria: a. Define essential pentosuria. b. Identify the enzyme deficiency responsible for essential pentosuria and its impact on the uronic acid pathway.
- Medical Research and Treatment: a. Explain how understanding the uronic acid pathway contributes to medical research. b. Discuss the potential implications of uronic acid pathway research in drug discovery and metabolic disease treatment.
- Application and Connection: a. Provide an example of how the uronic acid pathway is connected to a specific physiological process. b. Describe how the pathway’s dysfunction could lead to an imbalance in this process.
- Critical Thinking: a. Imagine a scenario where a new drug is discovered that inhibits a key enzyme in the uronic acid pathway. Predict the potential effects of this drug on various physiological processes. b. Reflect on the importance of the uronic acid pathway in maintaining overall cellular health and its role in sustaining proper physiological functions.
Bonus Challenge: Research and Presentation
Choose one of the following topics related to the uronic acid pathway, conduct research, and prepare a short presentation: a. Role of the uronic acid pathway in drug metabolism and detoxification. b. Importance of glycosaminoglycans in connective tissues and their link to the uronic acid pathway. c. Implications of vitamin C synthesis through the uronic acid pathway on human health.
Summery
Key Steps and Enzymes –
- Glucose 6-Phosphate Conversion: Glucose 6-phosphate is converted into glucose 1-phosphate (Glc-1-P) by the enzyme phosphoglucomutase.
- Formation of UDP-Glucose: Glc-1-P reacts with uridine triphosphate (UTP) to produce uridine diphosphate glucose (UDP-glucose), catalyzed by UDP-glucose pyrophosphorylase. This reaction is also involved in glycogen formation.
- Oxidation to UDP-Glucuronate: UDP-glucose is oxidized by NAD+-dependent UDP-glucose dehydrogenase to form UDP-glucuronate. This step generates reducing equivalents (NADH) for potential ATP production.
- Branching Point – L-Gulonate Formation: UDP-glucuronate can be converted to L-gulonate by a series of enzymatic reactions.
- Synthesis of Ascorbic Acid (Vitamin C): L-Gulonate serves as a precursor for the synthesis of L-ascorbic acid (vitamin C) in most animals. However, certain species, such as primates, lack the enzyme L-gulonolactone oxidase required for this conversion.
- Conjugation Reactions: UDP-glucuronate is essential for conjugation reactions in the liver and kidneys. This pathway enhances water solubility and facilitates the excretion of lipophilic compounds, drugs, and xenobiotics.
- Glycosaminoglycan (GAG) Synthesis: UDP-glucuronate is utilized in the synthesis of glycosaminoglycans, which are essential components of connective tissues and play vital roles in cellular structure and function.
- Formation of Heparin and Heparan Sulfate: The pathway contributes to the formation of heparin and heparan sulfate, molecules involved in cell communication and the regulation of various physiological processes.
Importance and Regulation:
- The uronic acid pathway has diverse roles, including detoxification, vitamin C synthesis, glycosaminoglycan formation, and the modification of biomolecules.
- Key enzymes within the pathway are regulated by factors such as substrate availability, hormonal signals, and gene expression.
Clinical Implications:
- Dysregulation or genetic defects in the uronic acid pathway can lead to disorders like mucopolysaccharidoses (MPS) and essential pentosuria.
- Certain drugs, such as phenobarbital, can enhance the uronic acid pathway’s activity, influencing drug metabolism and detoxification.
FAQ
What is the uronic acid pathway?
The uronic acid pathway is a metabolic route within cells that transforms glucose and other carbohydrates into uronic acids, which serve as precursors for various important molecules in the body.
What are the major functions of the uronic acid pathway?
The uronic acid pathway has several crucial functions, including the synthesis of glycosaminoglycans, participation in heparin formation, production of UDP-glucuronate for conjugation reactions, formation of L-ascorbic acid (vitamin C), and serving as a minor route for pentose formation.
What are glycosaminoglycans (GAGs) and how is the uronic acid pathway involved in their formation?
GAGs are complex carbohydrate-protein compounds that are essential for the structure and function of connective tissues. The uronic acid pathway contributes to the synthesis of uronic acids, which are building blocks for GAGs.
How does the uronic acid pathway contribute to the detoxification of drugs and xenobiotics?
The pathway facilitates the conjugation of glucuronic acid to lipophilic compounds, making them more water-soluble and easier to excrete from the body. This detoxification process helps eliminate drugs and xenobiotics.
What is the significance of the uronic acid pathway in vitamin C synthesis?
The uronic acid pathway plays a vital role in the synthesis of L-ascorbic acid, also known as vitamin C. This important nutrient has antioxidant properties and is involved in collagen synthesis, immune function, and other biological processes.
How is the uronic acid pathway regulated?
The uronic acid pathway is tightly regulated through factors such as substrate availability, hormonal signals, gene expression regulation, feedback inhibition, and cellular signaling. These mechanisms ensure proper functioning and balance within the pathway.
What are some disorders associated with the uronic acid pathway?
Disorders related to the uronic acid pathway include mucopolysaccharidoses (MPS) and hereditary inclusion body myopathy (HIBM), which result from dysregulation or genetic defects within the pathway.
How do certain drugs affect the uronic acid pathway?
Drugs like barbital, aminopyrine, antipyrine, and chlorobutanol can enhance the uronic acid pathway’s activity, leading to increased synthesis of glucuronate. This has implications for drug metabolism and detoxification.
What is essential pentosuria, and how does it relate to the uronic acid pathway?
Essential pentosuria is a metabolic disorder characterized by the excessive excretion of L-xylulose, a sugar intermediate produced in the uronic acid pathway. It is caused by a deficiency of the enzyme L-xylulose reductase.
How does understanding the uronic acid pathway contribute to medical research and treatment?
A deeper understanding of the uronic acid pathway enhances our knowledge of basic biochemical processes, which in turn advances medical research, drug discovery, and the development of treatments for metabolic diseases.
References
- Textbook of Biochemistry-U Satyanarayana
- Textbook of Biochemistry-DM Vasudevan
- Engelking, L. R. (2015). Uronic Acid Pathway. Textbook of Veterinary Physiological Chemistry, 179–183. doi:10.1016/b978-0-12-391909-0.50029-3
- Huffman, F. G. (2003). URONIC ACIDS. Encyclopedia of Food Sciences and Nutrition, 5890–5896. doi:10.1016/b0-12-227055-x/01221-9
- http://biocheminfo.com/2020/05/14/uronic-acid-pathway-glucuronic-pathway/
- https://www.davuniversity.org/images/files/study-material/BCH5156-3.pdf
- https://www.slideshare.net/ashokktt/uronic-acid-pathway-70775281
- https://www.slideshare.net/YESANNA/uronic-acid-pathway
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.