What is Trypanosoma gambiense?
- Trypanosoma gambiense, a protozoan parasite, is the main causative agent of Human African Trypanosomiasis (HAT), also known as African sleeping sickness. This parasite was first identified in 1901 by Forde, with significant contributions by Sir David Bruce in linking the transmission of this disease to the tsetse fly. The infection is particularly prevalent in Central and West Africa, especially in regions near rivers and lakes where tsetse flies, the insect vectors of the disease, thrive in thick vegetation. Countries such as Nigeria and the Democratic Republic of the Congo see a high number of cases due to the prevalence of these flies.
- Human African trypanosomiasis is primarily caused by two subspecies of Trypanosoma brucei: T. brucei gambiense (TbG) and T. brucei rhodesiense (TbR). T. gambiense accounts for over 92% of reported cases and is the most common in rural, underdeveloped regions. Transmission occurs when an infected tsetse fly bites a human, introducing the parasite into the bloodstream. The disease progresses in two stages. In the first stage, symptoms such as fever, headaches, joint pain, and itching manifest within one to three weeks of infection. If untreated, the disease advances to the second stage, where neurological symptoms like confusion, poor coordination, numbness, and sleep disturbances develop. This stage is particularly dangerous, as it can lead to death without treatment.
- Diagnosis of T. gambiense infection typically involves detecting the parasite in blood smears or lymph node fluid. A lumbar puncture may also be necessary to determine the progression of the disease, especially to differentiate between the first and second stages.
- Effective management of the disease relies on early detection through blood screening, especially in high-risk populations. Treatment in the early stage is usually managed with drugs like pentamidine or suramin. Once neurological symptoms appear, more advanced treatments such as eflornithine or combinations of nifurtimox and eflornithine are employed. Fexinidazole, a newer treatment, offers the advantage of being administered orally at any stage of the disease. Though melarsoprol is an option for both types of the disease, its severe side effects limit its use to TbR infections.
- Trypanosomiasis remains a persistent health issue in sub-Saharan Africa, where approximately 70 million people are at risk across 36 countries. The Democratic Republic of the Congo bears the brunt of the disease, with over 80% of cases occurring there. Despite its severity, significant progress has been made in reducing the number of cases and deaths. For instance, while around 34,000 deaths were recorded in 1990, this number dropped to 3,500 in 2015, with further reductions in subsequent years. However, the disease is still classified as a neglected tropical disease, with periodic outbreaks such as those in the late 19th and 20th centuries highlighting its ongoing threat to public health.
- In addition to affecting humans, Trypanosoma gambiense and other related species can infect animals, particularly livestock. In such cases, the disease is referred to as Nagana, or animal trypanosomiasis, which impacts the agricultural economy in affected regions. Thus, controlling the spread of T. gambiense is vital for both human and animal health.
Classification
| Domain | Eukaryota |
| Phylum | Euglenozoa |
| Class | Kinetoplastea |
| Order | Trypanosomatida |
| Family | Trypanosomatidae |
| Genus | Trypanosoma |
History and Distribution of Trypanosoma gambiense
Trypanosoma gambiense, the parasite responsible for causing Human African Trypanosomiasis (HAT) or African sleeping sickness, has been a health concern in tropical Africa for centuries. Its history and distribution provide valuable insight into the persistent challenges in controlling the disease.
- First Discovery: The parasite was first isolated in 1901 by Forde, who discovered it in the blood of a steamboat captain along the Gambia River. This led to the naming of the species as gambiense.
- Naming of the Parasite: In 1902, Dulton officially proposed the name Trypanosoma gambiense, solidifying its identification as a distinct species among trypanosomes.
- Geographical Distribution: Trypanosoma gambiense is endemic to scattered regions in West and Central Africa. Its presence spans between 15°N and 18°S latitudes. Countries like Nigeria, Democratic Republic of the Congo, and other regions near lakes and rivers are particularly vulnerable due to the presence of the tsetse fly, the primary vector responsible for transmitting the parasite.
- Tsetse Fly Habitats: The tsetse fly thrives in low, thick vegetation near water bodies. These environmental conditions are ideal for the vector, which explains why infections tend to occur in rural, heavily vegetated regions, especially near rivers and lakes.
- Historical Significance: Although identified in the early 20th century, T. gambiense has likely been affecting populations for much longer, with evidence suggesting the disease existed in tropical Africa for centuries prior to its discovery.
- Endemic Areas: The spread of the disease is not uniform but occurs in concentrated foci. This scattered distribution of endemic areas is largely influenced by ecological conditions that favor the tsetse fly’s survival, making certain regions more prone to outbreaks than others.
- Historical Outbreaks: In recent history, three major outbreaks of sleeping sickness have been documented. The first outbreak, between 1896 and 1906, primarily affected Uganda and the Congo Basin. Subsequent outbreaks occurred in 1920 and 1970, affecting various African nations. These outbreaks highlight the cyclical nature of the disease and the difficulty in eradicating it.
- Population at Risk: Approximately 70 million people in 36 countries across sub-Saharan Africa remain at risk of infection. The Democratic Republic of the Congo is particularly affected, with more than 80% of cases occurring there.
Habitat of Trypanosoma gambiense
Trypanosoma gambiense is a parasitic protozoan that primarily infects humans and other vertebrates, thriving within various tissues and fluids of its host. Understanding its habitat is essential for comprehending its life cycle and the disease it causes, African sleeping sickness.
- Host Dependency: Trypanosoma gambiense resides within human hosts, as well as other vertebrates. It requires a living organism for survival, as it is not capable of existing outside of a host environment.
- Primary Habitat: The parasite primarily inhabits connective tissues. This is where it begins its multiplication process, proliferating rapidly before spreading to other regions of the host’s body.
- Invasion of the Lymphatic System: After initial colonization in connective tissues, the trypanosomes enter the regional lymph nodes. This phase is crucial in the spread of the infection, allowing the parasite to move beyond its initial site of multiplication.
- Circulation in Blood: Once the lymphatic system is involved, T. gambiense invades the bloodstream. The parasite circulates through the blood, which is a critical stage in its life cycle, as this facilitates further dissemination throughout the body.
- Central Nervous System Involvement: In advanced stages of the infection, T. gambiense can penetrate the central nervous system (CNS). This is the most dangerous phase of the disease, as it leads to neurological symptoms and, if untreated, can result in death.
- Transmission Through Tsetse Fly: Outside the human or vertebrate host, T. gambiense briefly inhabits the tsetse fly, which acts as its vector. The fly becomes infected by feeding on the blood of an infected individual, and the parasite continues its development inside the fly’s midgut before it is transmitted to a new host through a bite.
Hosts and Vectors of Trypanosoma gambiense
Trypanosoma gambiense, the causative agent of African sleeping sickness, has both human and animal hosts, with the tsetse fly serving as its primary vector. Understanding the interactions between these hosts and vectors is essential for grasping the transmission dynamics of this parasitic disease.
- Primary Human Host: Humans are the main reservoir for Trypanosoma brucei gambiense. The parasite thrives in the human bloodstream and tissues, where it multiplies and spreads throughout the body. Human-to-human transmission occurs through the bite of an infected tsetse fly.
- Animal Hosts: Though humans are the principal hosts, T. gambiense can also infect animals, including certain primates and ungulates. In some areas, infected animals contribute to the maintenance and spread of the parasite in the ecosystem.
- Livestock as Reservoirs: In the case of Trypanosoma brucei rhodesiense, which causes a more acute form of sleeping sickness, domestic cattle are considered the most significant animal reservoirs. These infected animals provide a source of infection for tsetse flies, which then transmit the parasite to humans.
- Tsetse Fly as Vector: The tsetse fly (Glossina spp.) is the only known vector capable of transmitting both T. gambiense and T. rhodesiense. When a tsetse fly bites an infected human or animal, the parasite enters the fly’s digestive system, where it undergoes further development before being transmitted to another host through subsequent bites.
- Transmission Cycle: The cycle begins when a tsetse fly feeds on the blood of an infected individual, picking up the parasite. Inside the fly, the parasite matures in its midgut before migrating to the salivary glands. The next time the fly feeds on a new host, the parasite is injected into the bloodstream, completing the transmission cycle.
- Environmental Factors: The distribution of tsetse flies, and therefore the spread of the disease, is closely linked to their natural habitat. These flies thrive in regions with dense vegetation near water bodies, where human populations and animals often reside, increasing the risk of transmission.
Morphology of Trypanosoma gambiense
The morphology of Trypanosoma gambiense is vital for understanding its identification, life cycle, and pathogenicity. This parasitic protozoan exhibits distinct morphological forms in its vertebrate hosts and insect vectors, each adapted to specific environments.
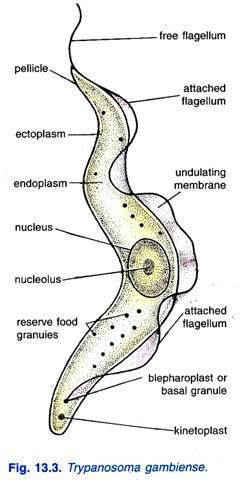
- Vertebrate Forms: In the bloodstream of vertebrate hosts, T. gambiense predominantly exists in the trypomastigote form, characterized by high pleomorphism.
- Variability: The trypomastigote form can present in three distinct shapes:
- Long Slender Form: This is the actively dividing form, typically elongated and agile, allowing for efficient movement within the bloodstream.
- Stumpy Short Form: This form is broader and may have an attenuated or absent flagellum, representing a stage in the life cycle where the parasite prepares for transmission to the tsetse fly.
- Intermediate Form: This form has characteristics between the long slender and stumpy forms, showcasing the adaptability of the parasite.
- Size: The trypomastigotes measure approximately 15 to 40 micrometers in length and 1.5 to 3.5 micrometers in width, providing a relatively large profile compared to other protozoa.
- Visual Characteristics: In fresh blood films, trypomastigotes appear as colorless, spindle-shaped bodies that exhibit rapid movement, often spiraling around red blood cells.
- Staining Properties: When stained using Giemsa or other Romanowsky stains, the cytoplasm of the trypomastigotes appears pale blue, while the nucleus exhibits a red hue. The kinetoplast, a specialized structure containing mitochondrial DNA, is identifiable as a deep red dot. Additionally, volutin granules, which store polyphosphate, stain deep blue, enhancing visibility. The undulating membrane appears pale blue, and the flagellum is typically red.
- Variability: The trypomastigote form can present in three distinct shapes:
- Insect Forms: Within the tsetse fly vector, T. gambiense undergoes morphological transformations essential for its lifecycle progression.
- Epimastigotes: This form exists in the midgut of the tsetse fly, characterized by a flagellum that is attached along the body and allows for motility within the insect.
- Metacyclic Trypomastigote: This form is adapted for transmission to the vertebrate host. It is more rounded and has undergone changes that enhance its ability to infect a new host when the tsetse fly bites.
Antigenic Variation of Trypanosoma gambiense
Antigenic variation in Trypanosoma gambiense is a crucial mechanism that enables the parasite to evade the host immune response, thereby facilitating its survival and persistence in infected individuals. This process is characterized by changes in the surface glycoproteins of the parasite, which plays a significant role in the pathogenesis of human African trypanosomiasis.
- Mechanism of Antigenic Variation: Trypanosoma gambiense employs a strategy of antigenic variation through its glycoproteins, which are vital for its interactions with the host immune system.
- Variant Surface Glycoproteins (VSGs): The surface of T. gambiense is covered with VSGs that form a protective coat. These VSGs are key to the parasite’s ability to change its surface antigens, allowing it to evade detection by the host’s immune system.
- Variant Antigenic Types (VATs): In the bloodstream of infected vertebrates, there is a cyclical fluctuation of T. gambiense, with the parasite undergoing antigenic variation approximately every 7 to 10 days. Each wave of the infection corresponds to a different VAT that expresses distinct VSGs.
- Genetic Diversity: It is estimated that a single T. gambiense parasite may possess over 1,000 different VSG genes. This extensive repertoire of genes allows for a remarkable diversity of surface antigens, making it difficult for the host’s immune system to mount an effective response against the infection.
- Immune Evasion Strategies: Besides antigenic variation, T. gambiense employs other mechanisms to evade the host immune response.
- Immune Suppression: The parasite can induce immunosuppressive responses in the host, which dampens the effectiveness of the immune system.
- Antigenic Variation’s Role: The continuous switching of VSGs ensures that the immune system is consistently challenged, preventing it from effectively recognizing and eliminating the parasite.
Life Cycle of Trypanosoma gambiense
The life cycle of Trypanosoma brucei gambiense, the causative agent of African sleeping sickness, involves intricate interactions between the parasite, its vertebrate host (primarily humans), and its invertebrate vector, the tsetse fly (Glossina spp.). This cycle is crucial for the transmission and survival of the parasite within its environment.
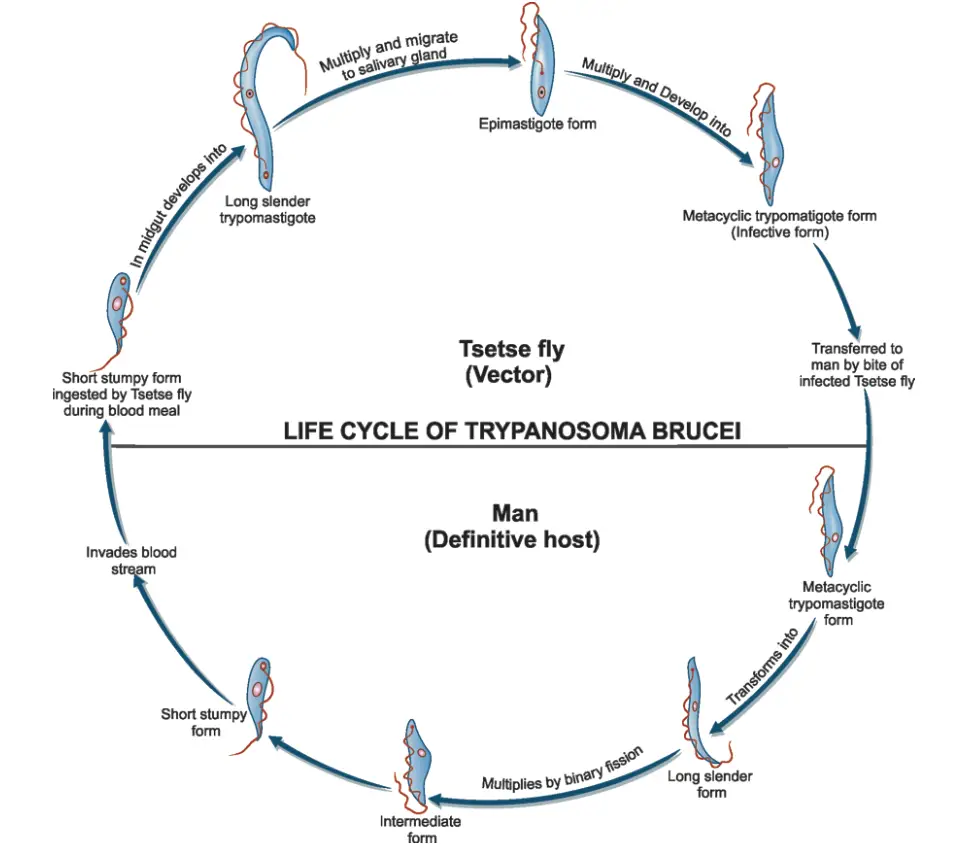
- Infection Initiation:
- During a blood meal, an infected tsetse fly injects metacyclic trypomastigotes directly into the skin tissue of the mammalian host.
- These metacyclic trypomastigotes enter the lymphatic system, where they subsequently migrate into the bloodstream.
- Development within the Vertebrate Host:
- Upon entering the bloodstream, the metacyclic trypomastigotes transform into bloodstream trypomastigotes.
- These trypomastigotes circulate throughout the body, spreading to various sites, including lymph, spinal fluid, and other body fluids.
- The parasites replicate via binary fission, a process that allows for rapid multiplication and colonization of the host.
- Extracellular Life Stages:
- The life cycle of T. brucei gambiense is characterized by its extracellular stages within the host, which are crucial for its survival and pathogenicity.
- Transmission to the Tsetse Fly:
- The tsetse fly becomes infected with bloodstream trypomastigotes when it takes a blood meal from an infected mammalian host.
- Inside the fly’s midgut, the bloodstream trypomastigotes undergo transformation into procyclic trypomastigotes.
- Development in the Tsetse Fly:
- Procyclic trypomastigotes multiply by binary fission in the midgut.
- Following this stage, the parasites migrate out of the midgut and transform into epimastigotes.
- Epimastigotes continue to replicate through binary fission as they move towards the fly’s salivary glands.
- Cycle Duration in the Tsetse Fly:
- The entire development cycle within the tsetse fly takes approximately three weeks, during which the epimastigotes transform into infective metacyclic trypomastigotes that can be transmitted back to a mammalian host during subsequent blood meals.
- Congenital Transmission:
- Although rare, T. brucei gambiense may also be transmitted congenitally if a pregnant mother is infected, allowing the parasite to be passed to the offspring.
Laboratory Diagnosis of Trypanosoma gambiense
The diagnostic process involves several techniques, which can be categorized into microscopic examination, antibody detection, and molecular detection.
- Microscopic Examination:
- This method primarily involves the identification of trypanosomes in various body fluids.
- Samples for examination include:
- Chancre fluid
- Lymph node aspirates
- Blood
- Bone marrow
- Cerebrospinal fluid (in advanced stages of infection)
- Specific procedures include:
- Wet preparations are examined for motile trypanosomes.
- Blood smears can be prepared as thin or thick films and must be fixed and stained using Giemsa or Field stain.
- Concentration techniques enhance detection rates; these methods include:
- Centrifugation: Following blood collection, centrifuge samples to separate components, then examine the buffy coat for trypanosomes.
- Mini anion-exchange/centrifugation: This technique improves the concentration of the parasite.
- Quantitative Buffy Coat (QBC): A specialized method that provides a quantitative assessment of trypanosome levels in the blood.
- For cerebrospinal fluid, concentration involves centrifugation followed by sediment examination.
- Isolation Techniques:
- While less common, the isolation of the parasite through inoculation into rodent models (such as rats or mice) remains a sensitive diagnostic approach. However, its use is primarily limited to T. brucei rhodesiense and not typically for T. b. gambiense.
- Antibody Detection:
- Rapid diagnostic tests are employed for the screening of whole blood samples, particularly in endemic areas to control and eliminate the disease.
- Various commercially available kits utilize formats such as:
- Dipstick tests
- Card agglutination tests
- The performance of these tests may vary based on the local prevalence of the disease.
- It is important to note that in cases of T. b. rhodesiense, seroconversion occurs post-onset of clinical symptoms, thus limiting the utility of antibody detection in early diagnosis.
- Molecular Detection:
- Currently, there are no validated nucleic acid-based tests specifically for the diagnosis of African trypanosomiasis.
- The performance of available molecular diagnostic tests shows considerable variability, underscoring the need for further development and standardization.
Pathogenecity and Clinical Features of Trypanosoma gambiense
Trypanosoma brucei gambiense is the etiological agent of African trypanosomiasis, commonly referred to as West African sleeping sickness. This disease manifests in two distinct clinical stages, characterized by specific pathogenic mechanisms and clinical features. Understanding the pathogenesis and clinical manifestations of this infection is essential for effective diagnosis and treatment.
- Pathogenic Mechanism:
- The infection begins with the bite of an infected tsetse fly, which injects metacyclic trypomastigotes into the host’s skin.
- Following initial inoculation, the parasites enter the lymphatic system and bloodstream, leading to parasitemia.
- During the early stages, the parasite predominantly localizes in lymph nodes, leading to characteristic clinical symptoms.
- Initial Symptoms:
- A painless skin ulcer known as a trypanosomal chancre develops at the site of the tsetse fly bite.
- Systemic symptoms emerge, including:
- Intermittent fever
- Chills
- Rash
- Anemia
- Weight loss
- Headache
- Stage I Disease:
- This stage represents the systemic phase without central nervous system (CNS) involvement.
- Characteristic features include:
- Hepatosplenomegaly (enlargement of the liver and spleen)
- Lymphadenopathy, particularly in the posterior cervical region, known as Winterbottom’s sign.
- Frequent development of myocarditis, especially in cases of T. brucei rhodesiense infections.
- Hematological changes, such as anemia, moderate leukocytosis, and thrombocytopenia.
- Elevated immunoglobulin levels, predominantly IgM, are consistently observed.
- Progression to Stage II Disease:
- After several months, if untreated, the disease progresses to stage II, characterized by the invasion of the central nervous system.
- Clinical manifestations of this stage include:
- Severe headaches
- Mental dullness and apathy
- Daytime sleepiness, leading to profound coma.
- Death often results from asthenia, a state of physical weakness.
- Neuropathological Changes:
- Histopathological examination reveals chronic meningoencephalitis:
- The meninges show significant infiltration of lymphocytes, plasma cells, and morula cells (atypical plasma cells with IgA).
- Perivascular cuffing is observed in brain vessels.
- Infiltration occurs in the brain and spinal cord, resulting in neuronal degeneration and microglial proliferation.
- Histopathological examination reveals chronic meningoencephalitis:
- Cerebrospinal Fluid (CSF) Abnormalities:
- Patients exhibit raised intracranial pressure.
- Pleocytosis, or an increase in the number of white blood cells in the CSF, is noted.
- Elevated total protein concentrations in the CSF further indicate CNS involvement.
FAQ
What is Trypanosoma gambiense?
Trypanosoma gambiense is a species of parasitic protozoa that causes African trypanosomiasis, also known as sleeping sickness.
How is Trypanosoma gambiense transmitted to humans?
Trypanosoma gambiense is transmitted to humans through the bite of infected tsetse flies, which are found in sub-Saharan Africa.
What are the symptoms of African trypanosomiasis?
Symptoms of African trypanosomiasis can include fever, headache, joint pain, itching, confusion, sleep disturbances, and ultimately, coma and death if left untreated.
How is African trypanosomiasis diagnosed?
Diagnosis of African trypanosomiasis typically involves identifying the parasite in blood or other bodily fluids.
How is African trypanosomiasis treated?
Treatment for African trypanosomiasis often involves medications such as pentamidine or suramin, but advanced cases may require medications such as melarsoprol or eflornithine.
How can African trypanosomiasis be prevented?
Prevention measures for African trypanosomiasis include wearing protective clothing, using insect repellents, and avoiding areas with high tsetse fly populations.
Where is Trypanosoma gambiense found?
Trypanosoma gambiense is found in sub-Saharan Africa, primarily in rural areas where tsetse flies are present.
Can animals be affected by Trypanosoma gambiense?
Yes, animals such as cattle and wild game can also be affected by Trypanosoma gambiense.
How is the spread of Trypanosoma gambiense controlled?
Control of tsetse flies through techniques such as insecticide-treated traps and aerial spraying has been effective in reducing the spread of Trypanosoma gambiense.
How many people are affected by Trypanosoma gambiense?
The World Health Organization estimates that approximately 10,000 cases of Trypanosoma gambiense occur annually, although the true number of cases may be higher due to underreporting in remote areas.
- https://www.msdmanuals.com/en-in/home/multimedia/image/life-cycle-of-trypanosoma-brucei-gambiense
- https://www.shivajicollege.ac.in/sPanel/uploads/econtent/3ca16b5dfafcb00ffe1f11bbf8a64a0c.pdf
- http://www.antimicrobe.org/Lifecycle/b54lc.asp
- https://www.biologydiscussion.com/invertebrate-zoology/protozoa/trypanosoma-gambiense-habitat-reproduction-and-life-cycle/28140
- https://www.gdcollegebegusarai.com/course_materials/july/zol96.pdf
- https://www.cdc.gov/dpdx/trypanosomiasisafrican/index.html
- https://epgp.inflibnet.ac.in/epgpdata/uploads/epgp_content/S000035ZO/P000888/M020579/ET/1498557406MorphologyTrypanosomaQuad1.pdf
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.