Modern medicine continues to provide numerous marvels that extend the lives of humans and vastly improve their quality of life. The replacement of faulty organs by transplantation has been one of medicine’s unachievable goals for millennia. The dream of health experts has been to replace or restore severely damaged tissues or organs. Successful transplantation needs a number of critical procedures, including surgical asepsis, the development of surgical techniques for vascular anastomosis, genetic matching of donors and hosts, the use of immunosuppressive medications, and the prevention of infection in both the recipient and donor. The development of stringent antiseptic techniques adds significantly to the control of infection, while the right use of immunosuppressive medicines and tissue typing boosts the transplantation success rate.
What is Transplantation?
Transplantation is the transfer of cells, tissues, or organs from one spot within a body to another, or from one individual to another. In the latter situation, the individual who provides the transplant organ is referred to as a donor, and the individual who receives the transplant is referred to as a receiver.
- In immunology, transplantation refers to the process of moving cells, tissues, or organs from one location to another.
- Realizing that many diseases can be healed by implanting a healthy organ, tissue, or cells (a graft) from one individual (the donor) to another (the recipient) in need of a transplant drives the desire to perform transplants (the recipient or host).
- One barrier to successful transplantation has been addressed by the development of surgical procedures that enable the simple reimplantation of organs, but others remain. One is the paucity of transplantable organs.
- Accident victims and, in some cases, living donors contribute organs, but there are more patients in need of transplants than there are organs available.
- As of November 2000, an estimated 73,000 people in the United States were on the waiting list for an organ transplant, demonstrating the severity of the organ shortage.
- The majority of patients on the waiting list (70%) require a kidney, and the current average wait time for this organ is almost 800 days.
- While the paucity of organs for transplantation is a significant issue, the immune system is the most daunting obstacle to transplantation being a normal medical treatment.
- The immune system has evolved extensive and effective systems to protect the organism from attack by external agents; these processes also result in the rejection of grafts from anyone who is not genetically identical to the recipient.
- In 1908, Alexis Carrel published the first systematic research of transplantation; he swapped the kidneys of nine cats.
- Some recipients of kidneys from other cats were able to maintain urine production for up to 25 days. Although all of the cats ultimately perished, the experiment demonstrated that a transplanted organ could perform normally in the recipient.
- The first human kidney transplant, conducted by a Russian physician in 1935, failed because the blood types of the donor and recipient did not match. This incompatibility led the kidney to be rejected nearly immediately, and the patient died before renal function could be established.
- This chapter will detail the antibodies that cause the hyperacute rejection, the fast immunological response experienced in this instance.
- In 1954, the first successful human kidney transplant between identical twins was performed in Boston. Kidney, pancreas, heart, lung, liver, bone marrow, and cornea transplants among nonidentical individuals are conducted with increasing frequency and success today.
- A range of immunosuppressive medications, including medicines and particular antibodies designed to reduce the immunologic attack on grafts, can aid in the survival of transplants; nevertheless, the bulk of these agents have an overall immunosuppressive impact, and their long-term usage is detrimental.
- New strategies of generating specific tolerance to the graft without inhibiting other immune responses are now being researched, with the possibility of extending transplant longevity without compromising host immunity.
- This page discusses the processes driving graft rejection, the many techniques utilised to prolong graft survival, and the current clinical situation of transplantation.
- A Clinical Focus section investigates the use of organs from nonhuman species (xenotransplants) to circumvent the organ shortage for humans in need.
Types of Transplants
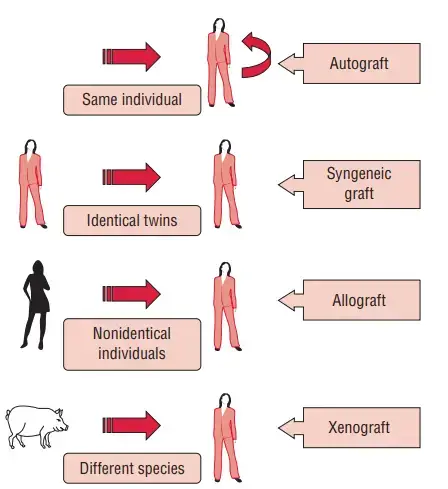
There are four primary categories of transplants. These indicate the donor’s genetic link to the recipient. The degree of immune response to a graft varies with the type of graft.
1. Autograft
- An autograft is the transplantation of an individual’s own tissue or organ from one location inside the body to another. Alternatively, the beneficiary is also the donor.
- Autograft examples include skin transplants in burn victims and bypass surgery in people with coronary artery disease.
2. Syngraft
- A syngraft is a tissue transfer between two genetically identical individuals, sometimes known as identical twins.
- In 1954, the first successful human kidney transplant was performed between identical twins.
3. Allograft
- Allograft refers to the transfer of tissue or an organ between genetically distinct individuals of the same species, i.e., from one human to another.
- This is the most prevalent form of transplantation today, and allografts have long dominated transplant research.
4. Xenograft
- A xenograft is the transfer of tissues or organs from one species to another.
- It is usually rejected by an immunocompetent recipient since it represents the most distant genetic links.
Immune reaction of the recipient to donor tissue represents a significant barrier to the effectiveness of transplantation. Rejection issues with autografts are typically low or nonexistent. Only when tissues from “others,” like in allografts and xenografts, are employed does the issue of rejection arise. Transplantation immunology is the study of the events that occur following the removal of an allograft or xenograft from a donor and its subsequent transplantation into a recipient.
Allograft Rejection
- Allografts are rejected by the allograft response mechanism. Graft rejection results from an immunological reaction developed by the recipient against the graft as a result of tissue antigen incompatibility between the donor and recipient.
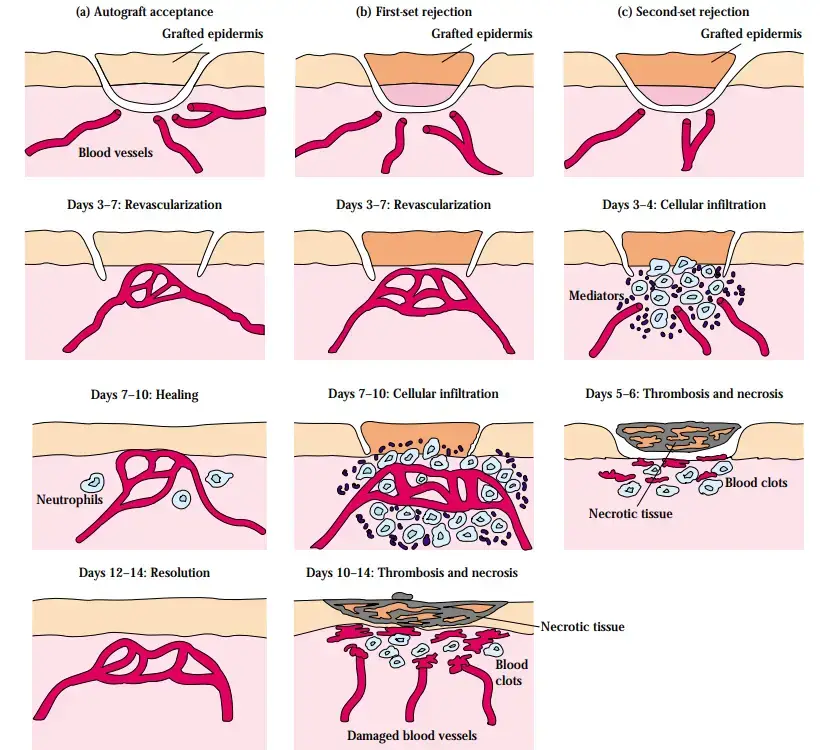
- When attempts to replace injured skin on burn victims with skin from unrelated donors were often ineffective, the problem of rejection was first identified.
- Within one to two weeks, the skin would suffer necrosis and flake off. The failure of such grafts prompted other scientists, including Peter Medawar, to investigate skin transplantation in animal models.
- These trials demonstrated that the failure of a skin graft was due to an inflammatory response, now known as rejection. Multiple experimental studies indicate that adaptive immune response is accountable for rejection.
Histocompatibility antigens
- Class II MHC (major histocompatibility complex) antigen-expressing cells play a crucial function in sensitising the recipient’s immune system.
- Following the sensitization of the recipient’s alloreactive helper T cells is their clonal proliferation.
- In turn, this results in numerous immunological and inflammatory problems. Some of these events, which ultimately result in graft rejection, are mediated by activated T cells and also by antibodies.
- Polymorphic genes inherited from both parents and expressed in a codominant manner influence whether transplanted cells are recognised as native or foreign. MHC molecules account for the vast majority of strong rejection reactions.
- T cells are the mediators of rejection reactions. Both CD4 and CD8 lymphocytes collaborate to produce an effective and robust rejection response.
- Lacking a thymus, nudized mice are incapable of initiating an allogeneic immune response. Histocompatibility is proved through the transplantation of tissues or organs from one member of the same species to another (an allograft) or from one species to another (a xenograft).
- Important for tissue transplantation, class I and class II MHC genes are among the key histocompatibility genes.
- These genes are located in the MHC region and encode antigens that must match in order for a tissue or organ graft to thrive in the recipient. These are situated on the short arm of human chromosome 6 and mouse chromosome 17.
- Antigens of minor histocompatibility are surface-expressed molecules that are not encoded by the main histocompatibility locus. They are less potent antigens for transplantation than the major histocompatibility antigens. Nevertheless, they are numerous, and their cumulative effect might significantly contribute to organ or tissue graft rejection.
- The better the compatibility between donor and recipient, the greater the likelihood of transplant survival. A six-antigen match, for instance, indicates that the donor and recipient share two HLA-A antigens, two HLA-B antigens, and two HLA-DR antigens.
- Even while antigenically different grafts may survive when a potent immunosuppressive treatment, such as cyclosporine, is given, the survival of the graft is improved by having the greatest number of antigenic matches feasible.
Mechanisms of graft rejection
There are two separate ways in which allogeneic MHC molecules are delivered for recognition by the T lymphocytes of a transplant recipient: (a) direct presentation and (b) indirect presentation.
Direct presentation
- Direct presentation is characterised by the recognition of an intact MHC molecule expressed by donor antigen-presenting cells (APCs) in the graft.
- It depends on the structural similarity between an intact allogeneic molecule and self-MHC molecules.
- A normal T-cell receptor, which is chosen to identify a self-MHC molecule and foreign peptide, reacts with an allogeneic MHC molecule and peptide to directly recognise foreign MHC molecules.
- Due to the fact that an allogeneic MHC molecule with a bonded peptide can imitate the determinant created by a self-MHC molecule and a specific foreign peptide, this is the case.
- Up to 2% of a person’s T cells are capable of identifying and responding to a single foreign MHC molecule, and this high frequency of T cells reactive with allogeneic MHC molecules is one of the reasons why allografts elicit robust immune responses in vivo.
Indirect presentation
- The “indirect presentation” entails the recognition of allogeneic MHC molecules that have been modified, but not an entire MHC molecule.
- It involves the processing of donor MHC molecules by recipient APCs and the presentation of allogeneic MHC-derived peptides in conjunction with self-MHC molecules.
- In this instance, the modified MHC molecules are recognised by T cells in the same manner as regular protein antigens. Allorecognition may come via indirect presentation by CD4 T cells.
- Due to the fact that alloantigen is primarily acquired through the endosomal vesicular pathway, it is presented by class II MHC molecules.
- Some antigens of phagocytosed graft cells appear to penetrate the class I MHC antigen presentation pathway and are identified indirectly by CD8 T cells.
Stages of cell-mediated graft rejection
Two stages of cell-mediated graft rejection are possible:
- Antigen-reactive lymphocytes of the recipient proliferate in reaction to alloantigens on the graft during the sensitization phase.
- In the effector phase, the graft is destroyed by the immune system.
Sensitization phase
- During the sensitization phase, CD4 and CD8 T lymphocytes detect and proliferate in response to alloantigens produced on foreign graft cells.
- In response to major histocompatibility antigens, the donor MHC molecule and an associated peptide ligand in the cleft of the MHC molecule are recognised.
- Allogeneic class I MHC molecules contain peptides derived from proteins generated within the allogeneic cell.
- The peptides found in the groove of allogeneic class II MHC molecules are often proteins that are endocytized by the allogeneic APC.
- The recognition of alloantigens produced by graft cells stimulates robust T-cell proliferation in the host.
- In a mixed lymphocyte reaction, this proliferation can be observed in vitro. Dendritic cells and vascular endothelial cells from an allogeneic transplant stimulate the growth of host T lymphocytes.
- The majority of proliferating cells that detect class II alloantigens directly or alloantigen peptides provided by host APCs are CD4 T cells.
- It is hypothesised that this augmented population of activated TH cells plays a crucial role in eliciting the numerous effector mechanisms of allograft rejection.
Effector mechanisms in allograft rejection
Diverse effector systems are involved in allograft rejection:
- Cell-mediated reactions including delayed-type hypersensitivity and cytotoxic T lymphocyte (CTL)-mediated cytotoxicity are the most prevalent.
- Antibody plus complement lysis and antibody-dependent cell-mediated cytotoxicity are less frequent methods (ADCC).
Graft rejection involving cell-mediated reactions is distinguished by an influx of T lymphocytes and macrophages into the graft. In many instances, the infiltration resembles that of a delayed-type hypersensitive response, in which cytokines released by TD and TH cells encourage macrophage infiltration. Foreign class I alloantigens on the graft are recognised by the host’s CD8 cells, resulting in CTL-mediated death. In some instances, graft rejection is mediated by CD4 T cells that function as class II MHC-restricted cytotoxic cells.
Clinical features of graft rejection
Traditionally, rejection events are characterised as (a) hyperacute, (b) acute, and (c) chronic based on the time passed between transplantation and the rejection episode.
Hyperacute rejection
- Hyperacute rejection typically starts during the first few hours after transplantation and is mediated by preexisting antibodies against the graft’s ABO or MHC antigens.
- Antibodies against other alloantigens, such as vascular endothelial antigens, may potentially play a role in this form of rejection.
- Once antibodies bind to transplanted tissues, rejection can occur either (a) by activation of the complement system, resulting in chemotactic attraction of granulocytes and activation of inflammatory circuits, or (b) by ADCC.
- The following are pathological characteristics of hyperacute rejection:
- Consequently, thrombosis, ischemia, and necrosis develop.
- The episodes of hyperacute rejection are irreversible and always end in graft loss. Using correct cross-matching algorithms, this type of rejection can be avoided almost entirely.
- Major restriction of xenogeneic transplantation is hyperacute rejection by antibodies to all human cellular antigens (e.g., pig to human).
Acute rejection
Acute rejection typically occurs within the first several days or weeks following transplantation:
- When acute rejection occurs within the first few days after grafting, it may be the result of a subsequent immunological reaction. This shows that the patient was previously exposed to HLA antigens present in the organ donor (as a consequence of a previous transplant, pregnancy, or blood transfusions).
- When graft rejection develops within the first week after grafting, it is typically a primary (first-set) reaction. Up to 70% of transplant recipients experience at least one episode of acute rejection.
T cells largely mediate acute rejection. CD4 helper T cells are believed to play a crucial role in acute graft rejection. They release growth factors such as IL-2 and IL-4 to promote the clonal proliferation of CD8 lymphocytes and B cells. In organs that have been rejected, the cellular infiltrates are predominantly composed of monocytes and T lymphocytes with both helper and cytotoxic phenotypes, as well as smaller numbers of B lymphocytes, NK (natural killer) cells, neutrophils, and eosinophils. All of these cells have the potential to play important roles in the process of rejection. Usually, clinical suspicion is the basis for the initial diagnosis of acute rejection.
- The primary criterion for diagnosing acute rejection is the functional degradation of the transplanted organ.
- Typically, confirmation needs a biopsy of the transplanted organ.
- In rejected graft tissue, mononuclear cell infiltration is a hallmark observation.
- Another diagnostic method involves measuring cytokines (such as IL-2) in serum and urine (in the case of renal transplants).
In the majority of instances, acute rejection can be reversed by raising the dose of immunosuppressive medications or short delivering more immunosuppressants if recognised early. Delayed or chronic rejection is defined by the gradual loss of function of the transplanted organ. It appears that both immunological and non-immune systems are involved in the functional decline associated with chronic rejection.
Vascular endothelial damage is the most prevalent symptom. Increasingly, granulocytes, monocytes, and platelets attach to damaged vascular endothelium. The injured endothelium is covered by a layer of platelets and fibrin, followed by fibroblasts and smooth muscle cells that proliferate. The final outcome is a proliferative lesion in the vasculature, which develops into fibrosis and blockage.
Prevention of graft rejection
- Host immunosuppression prevents graft rejection. This is accomplished through the use of radiation, corticosteroids, and antilymphocyte serum.
- Cyclosporin A and rapamycin are also utilised; they induce immunosuppression by inhibiting T cells specifically.
Immunosuppressive Agents used in Transplantation
| Agent | Mode of action |
| Azathioprine | Inhibition of nucleotide synthesis of multiple cells |
| Cyclophosphamide | Inhibition of nucleotide synthesis of multiple cells |
| Cyclosporine | Inhibition of transcription of cytokines in lymphocytes |
| Corticosteroids | Inhibition of transcription for cytokines and products involved in inflammation in multiple cells |
| Sirolimus | Inhibition of transduction induced by cytokines in T cells |
| Irradiation | DNA damage in all rapidly proliferating cells |
| Anti-CD4 and CD8 antibodies | Interference with T-cell receptor binding of CD4 and CD8 T cells |
Graft-Versus-Host Reaction
When a patient with a profound immunodeficiency (primary, secondary, or iatrogenic) receives a graft of an organ rich in immunocompetent cells, there is a substantial chance of a graft-versus-host (GVH) reaction. The likelihood of developing a GVH reaction is highest during the first two months following a transplant. GVH reactions require three essential elements, namely:
- The donor graft must contain T cells with immunocompetence.
- The host must have impaired immunity.
- The receiver should express antigens, such as MHC proteins, that the donor will recognise as foreign. Donor T cells, for example, perceive receiving cells as foreign
In the following settings, GVH reactions provide a serious problem:
- Bone marrow or thymus transplantation in newborns and children with primary immunodeficiencies.
- Transplantation of adult bone marrow
- The transplantation of organs containing a considerable quantity of lymphoid tissue, including the small intestine, lungs, and liver.
Heart and kidney transplants, which are deficient in endogenous lymphoid tissue, extremely infrequently result in a GVH reaction. In an immunocompromised, irradiated host, donor T lymphocytes become activated, proliferate, and develop into helper and effector cells, causing GVH reactions.
These activated T cells target the cells and tissues of the host, causing the manifestations of GVH illness. Donor cytotoxic T cells play a crucial function in the destruction of recipient cells. The fact that GVH reactions are prevented by the removal of donor T cells from a bone marrow graft demonstrates the importance of donor T cells.
Initial proliferation of donor T cells occurs in lymphoid tissues, specifically the liver and spleen, resulting in hepatomegaly and splenomegaly. Later, as the proliferative reaction reaches its apex, the skin and intestinal walls are severely infiltrated, causing severe skin rashes or exfoliative dermatitis and severe diarrhoea.
Many GVH reactions culminate in lethal infections and death. All immunosuppressive medications used for the prevention and treatment of rejection have been utilised to treat the GVH reaction. Thalidomide, a tranquillizer that gained recognition for its teratogenic consequences, has been utilised well for the treatment of persistent GVH that is refractory to conventional immunosuppressants.
Immune Tolerance to Allografts
In some cases, an allograft can be accepted without immunosuppressive treatments. In the case of alloantigen-free tissues, such as cartilage or heart valves, there is obviously no immunologic barrier to transplantation. However, there are occasions where the projected robust response to an allograft does not materialise. There are two primary circumstances in which an allograft may be accepted. One is when cells or tissue are grafted to a so-called privileged place that is immune system-free. The second condition occurs when a state of tolerance has been biologically produced, typically from earlier exposure to the antigens of the donor in a manner that induces immunological tolerance rather than sensitization in the receiver. Below, each of these exclusions is discussed in detail.
Privileged Sites Accept Antigenic Mismatches
- In immunologically favoured sites, allografts can be implanted without provoking a rejection response. This includes the anterior chamber of the eye, cornea, uterus, testes, and brain.
- In experimental settings, the cheek pouch of the Syrian hamster is a privileged location. Each of these locations is distinguished by the lack of lymphatic vessels and, in certain cases, blood vessels as well.
- As a result, the alloantigens of the graft are unable to sensitise the recipient’s lymphocytes, and the graft has a higher probability of being accepted even when HLA antigens are mismatched.
- The advantageous placement of the cornea has enabled exceedingly successful corneal transplants. Because the blood-brain barrier inhibits the admission or exit of many substances, including antibodies, the brain is immunologically privileged.
- In a rat model of diabetes, the successful transplantation of allogeneic pancreatic islet cells into the thymus implies that the thymus may also be an immunologically privileged location.
- Immunologically favoured locations do not stimulate an immunological response because they are successfully isolated from immune cells. This shows that it may be possible to physically isolate grafted cells.
- In one experiment, pancreatic islet cells were encapsulated in semipermeable membranes (made of an acrylic copolymer) and subsequently transplanted into diabetic mice. The islet cells were able to survive and produce insulin.
- Because the recipient’s immune cells could not pass through the barrier, the transplanted cells were not rejected.
- This unique transplant approach allowed diabetic mice to produce normal levels of insulin, and it may have applications in the treatment of human diabetes.
Early Exposure to Alloantigens Can Induce Specific Tolerance
- In contrast to nonidentical twins of other mammalian species, cow nonidentical twins preserved the ability to receive cells or tissue from their genetically unique sister throughout their entire lifetimes, as described by Ray Owen in 1945.
- Throughout the embryonic period, a shared placenta in cattle allows for the unimpeded passage of cells from one twin to the other. Although the twins may have acquired unique paternal and maternal antigens, they do not perceive their placental partner’s antigens as foreign and are able to absorb grafts from them.
- Mice experiments provided experimental evidence for the theory that tolerance comes through exposure of the growing organism to alloantigens.
- If cells from strain C are implanted into neonates of mouse strain A, the adult mice will take grafts from strain C.
- Immunocompetence and specificity of tolerance are demonstrated by the fact that injected A-strain mice reject grafts from other strains as rapidly as their untreated littermates.
- Although there are no human experimental data demonstrating this specific tolerance, anecdotal evidence implies that it may also exist in people.
- There are instances in which mismatched allografts at a single HLA locus are accepted with minimal or no immune suppression.
- In instances where the mismatched antigen is expressed by the mother but not inherited by the offspring, prenatal exposure to this antigen may have led to eventual tolerance.
- Due to the fact that human maternal cells do not generally pass the placental barrier, tolerance to maternal antigens that are not inherited would be an exceptional occurrence.
Immunosuppressive Therapy
A. General Immunosuppressive Therapy
- Allogeneic transplantation necessitates immunosuppression in order for the transplant to survive.
- Most immunosuppressive treatments have the drawback of being nonspecific; that is, they suppress responses to all antigens, not only those of the allograft, which increases the recipient’s risk of infection.
- Additionally, numerous immunosuppressive strategies try to inhibit the growth of activated cells.
- Nonetheless, because any quickly dividing nonimmune cells (e.g., intestinal epithelial cells or bone marrow hematopoietic stem cells) are also affected, serious or even life-threatening problems might arise.
- Long-term immunosuppressive medication increases the risk for malignancy, hypertension, and metabolic bone disease in patients.
Mitotic Inhibitors Thwart T-Cell Proliferation
- Azathioprine (Imuran), a powerful mitotic inhibitor, is frequently administered before and after transplantation to reduce T-cell proliferation in response to the graft’s alloantigens.
- Azathioprine inhibits the manufacture of inosinic acid, a precursor of the purines adenylic and guanylic acid, in cells in the S phase of the cell cycle. In the presence of azathioprine, both B-cell and T-cell proliferation are reduced.
- After treatment with azathioprine, functional immunological assays such as the MLR, CML, and skin test indicate an overall decrease in T-cell counts.
- Cyclophosphamide and methotrexate are two other mitotic inhibitors that are sometimes used in concert with other immunosuppressive medications.
- Cyclophosphamide is an alkylating chemical that inserts into the DNA helix and forms cross-links, causing the DNA chain to break. Because it is particularly efficient against rapidly dividing cells, it is occasionally administered at the time of grafting to inhibit T-cell proliferation.
- As a folic-acid antagonist, methotrexate inhibits purine production. The fact that mitotic inhibitors affect the division of all fast dividing cells and not only those implicated in the immune response against the allograft can result in harmful side effects by inhibiting the division of other functioning cells.
Corticosteroids Suppress Inflammation
- Corticosteroids, such as prednisone and dexamethasone, are potent anti-inflammatory agents that exert their effects on numerous immune response levels.
- To prevent acute episodes of graft rejection, these medications are frequently administered with a mitotic inhibitor, such as azathioprine, to transplant recipients.
Certain Fungal Metabolites Are Immunosuppressants
- Cyclosporin A (CsA), FK506 (tacrolimus), and rapamycin (sirolimus) are immunosuppressive fungal metabolites.
- Despite being chemically unrelated, CsA and FK506 have comparable effects. Both medicines impede transcription of genes encoding IL-2 and the high-affinity IL-2 receptor (IL-2R), which are required for T cell activation.
- CsA and FK506 accomplish this effect by binding to immunophilins, thereby creating a complex that inhibits the phosphatase activity of calcineurin.
- This hinders the synthesis and nuclear translocation of the cytoplasmic subunit NFATc and its subsequent assembly into the DNA-binding protein NFAT, which is required for transcription of the genes encoding a number of molecules essential for T-cell activation.
- Rapamycin has a similar molecular structure to FK506 and also binds to immunophilin. Nevertheless, the rapamycin-immunophilin combination does not reduce calcineurin activity; rather, it inhibits the proliferation and differentiation of activated TH cells during the G1 phase of the cell cycle.
- By reducing TH-cell proliferation and consequently TH-cell cytokine release, all three medicines limit the activation of effector populations involved in graft rejection, such as TH cells, TC cells, NK cells, macrophages, and B cells.
- Due to their significant immunosuppressive effects, these three drugs are indispensable in heart, liver, kidney, and bone marrow transplantation.
- It has been demonstrated that cyclosporin A increases graft survival in kidney, liver, heart, and heart-lung transplants. In a trial of 209 kidney transplants from deceased donors, the 1-year survival rate for patients receiving other immunosuppressive therapies was 64%, compared to 80% for recipients receiving cyclosporin A.
- Similar outcomes were observed with liver transplants (Figure 21-8). CsA has significant side effects, the most notable of which is renal damage, notwithstanding these outstanding results.
- It is typical for acute nephrotoxicity to proceed to chronic nephrotoxicity and drug-induced kidney failure. FK506 and rapamycin are 10–100 times more powerful as immunosuppressants than CsA and can thus be taken at lower doses with fewer adverse effects.
Total Lymphoid Irradiation Eliminates Lymphocytes
- Because lymphocytes are particularly sensitive to x-rays, x-irradiation can be utilised to remove them shortly prior to grafting in the transplant recipient.
- Before transplant surgery, the patient of total lymphoid x-irradiation undergoes several x-ray exposures to the thymus, spleen, and lymph nodes.
- The standard regimen consists of daily x-irradiation treatments of around 200 rads for several weeks, until a total of 3,400 rads have been administered.
- In this immunocompromised state, the receiver is grafted. Because the bone marrow is not bombarded with x-rays, lymphoid stem cells grow and replenish the recirculating lymphocyte population.
- These freshly generated lymphocytes look more tolerant of the graft’s antigens.
B. Specific Immunosuppressive Therapy
- A key drawback shared by all immunosuppressive treatments is their lack of specificity, which results in a more or less broad immunosuppression and an increased risk for infection in the recipient.
- Ideal would be an immunosuppressant that decreases the immune response to the graft’s alloantigens while keeping the recipient’s capacity to respond to other foreign antigens.
- Despite the fact that this objective has not yet been reached in human transplants, recent breakthroughs in animal trials suggest that it may be attainable.
- In animal trials, antibodies or soluble ligands reacting with cell-surface molecules have been used to achieve specific immunosuppression of allografts.
Monoclonal Antibodies Can Suppress Graft-Rejection Responses
- Utilizing monoclonal antibodies directed against various surface molecules on immune system cells, T-cell activity in general or the activity of subpopulations of T cells has been effectively suppressed.
- Further evidence from animal model research suggests that specific monoclonals may be utilised to inhibit exclusively activated T cells.
- There is reason to expect that two types of techniques utilising antibodies to decrease rejection will find widespread therapeutic use based on their success in animal models and human studies.
- Monoclonal antibodies can be employed to deplete a recipient of a particular broad or specific cell population, or they can block co-stimulatory signals.
- In the latter instance, T cells that react to antigens present on the allograft undergo anergy induction.
- Against decrease immune cells, a monoclonal antibody to the CD3 molecule of the TCR complex is utilised.
- Such monoclonal antibodies induce a fast depletion of mature T lymphocytes in the blood upon injection.
- This depletion appears to be the result of antibody-coated T cells attaching to Fc receptors on phagocytic cells, which then phagocytose and eliminate the T cells from circulation.
- The antibody is linked with a cytotoxic agent, such as diphtheria toxin, in a further modification of this technique. The poison is internalised by the cell with which the antibody reacts, resulting in the cell’s death.
- To boost graft survival, monoclonal antibodies specific for the high-affinity IL-2 receptor are utilised in another depletion technique (anti-TAC).
- Since the high-affinity IL-2 receptor is exclusively expressed on activated T cells, exposure to antiTAC after the graft inhibits proliferation of T cells activated in response to the graft’s alloantigens.
- Monoclonal-antibody treatment, which was initially used to decrease T cells in graft recipients, has also been applied to donors’ bone marrow prior to transplantation.
- This medication is intended to reduce the immunocompetent T cells in the bone-marrow transplant; these are the cells that cause graft-versus-host disease by reacting with recipient tissues (described below).
- In all cell-depletion techniques, monoclonal antibodies with isotypes that activate the complement system are the most successful. On all activated T cells, the CD3 receptor and the high-affinity IL-2 receptor are immunosuppressive therapy targets; molecules present on specific T-cell subpopulations may also be targeted.
- A monoclonal antibody against CD4, for instance, has been demonstrated to increase transplant survival. In one trial, a single big dosage of antiCD4 was administered to monkeys right before they got a kidney transplant.
- Significantly more grafts survived in treated animals than in untreated control animals. Anti-CD4 did not diminish the number of CD4+ T cells, but rather appeared to cause T cells to enter an immunosuppressed state.
- This is a nondepleting antibody example. Other targets of monoclonal antibody therapy are the adhesion molecules on the cell surface.
- Treatment of cardiac grafts between allogeneic mice with monoclonal antibodies to the adhesion molecules ICAM-1 and LFA-1 for six days after transplantation has resulted in indefinite survival.
- However, when a single monoclonal antibody was provided, the transplanted heart was rejected. The necessity that both monoclonal antibodies be administered simultaneously likely reflects the redundancy of the adhesion molecules: LFA-1 is known to bind to ICAM-2 as well as ICAM-1, and ICAM-1 is known to bind to Mac-1 and CD43 as well as LFA-1.
- Only when all conceivable adhesin-ligand pairs are simultaneously blocked is adhesion and signal transduction through this ligand pair blocked. The fact that most monoclonal antibodies used to prolong transplant survival in humans are of mouse origin presents a practical issue.
- Numerous recipients generate an antibody response to the mouse monoclonal antibody, resulting in its fast elimination from the body.
- Through the development of human monoclonal antibodies and mouse-human chimeric antibodies, this barrier has been eliminated.
- Injecting animals with monoclonal antibodies specific for the involved cytokines, including TNF-α, IFN-γ, and IL-2, is another method for extending graft survival, given that cytokines appear to play a significant role in allograft rejection.
- It has been demonstrated that monoclonal antibodies against TNF-α extend bone-marrow transplants in mice and lower the incidence of graft-versus-host disease. In some instances, monoclonal antibodies against IFN-γ and IL-2 have been observed to extend cardiac transplants in rats.
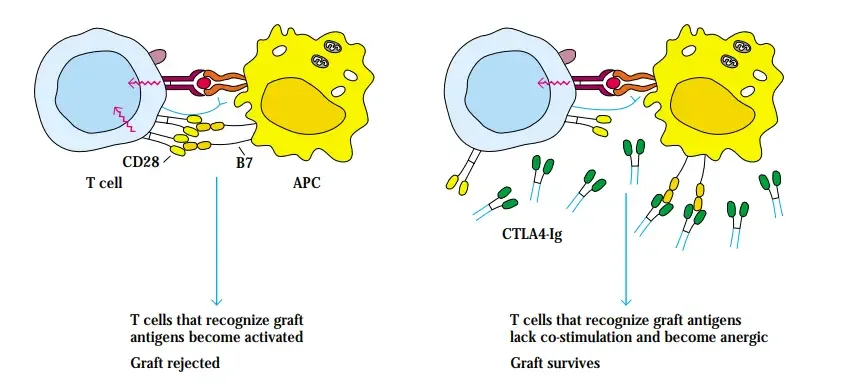
Blocking Co-Stimulatory Signals Can Induce Anergy
- TH-cell activation requires a costimulatory signal in addition to the T-cell receptor-mediated signal.
- One such signal is generated by the interaction between the B7 molecule on the membrane of antigen-presenting cells and the CD28 or CTLA-4 molecule on T cells.
- Antigen-activated T cells become anergic without a co-stimulatory signal. CD28 is found on both resting and active T cells and binds B7 with moderate affinity, whereas CTLA-4 is expressed at much lower levels and only on activated T cells, but binds B7 with 20-fold more affinity.
- CD40, which is found on the APC, and CD40 ligand (CD40L or CD154), which is found on the T cell, are a second set of co-stimulatory molecules essential for T-cell activation. D. J. Lenschow, J. A. Bluestone, and colleagues demonstrated that inhibiting the B7-mediated co-stimulatory signal with CTLA-4 after transplantation causes the host’s T cells directed against the grafted tissue to become anergic, thereby allowing the grafted tissue to survive.
- Human pancreatic islets were transplanted into mice injected with CTLA-4Ig, a soluble fusion protein composed of the extracellular domains of CTLA4 and the constant region of IgG1 heavy chain.
- The half-life of the soluble fusion protein is lengthened by include the IgG1 heavychain constant region. The xenogeneic graft survived for an extended period of time in treated mice, but was immediately rejected in untreated controls.
- The ability of the soluble version of the CTLA-4 receptor to prevent the rejection of human tissue transplants by recipient mice demonstrates that inhibiting costimulatory signals in vivo is a realistic technique.
- Allan Kirk, David Harlan, and their colleagues extended these impressive results to the transplantation of monkey kidneys with mismatched class I and class II antigens.
- After transplantation, the recipients were treated for approximately four weeks with CTLA4-Ig, a monoclonal antibody directed against CD40L, or both. Untreated control animals rejected the mismatched kidneys within 5–8 days, but animals treated with a single drug maintained the transplants for 20–98 days.
- At 150 days post-transplantation, the animals that received both reagents exhibited no signs of rejection.
- This reduction of allograft rejection did not result in systemic immunosuppression; peripheral T-cell counts remained normal and other immunological activities were present, including donor-recipient mixed lymphocyte reactivity.
- Planned human clinical trials of the procedures developed for macaques could alter clinical transplantation procedures if successful.
- The capacity to prevent allograft rejection without general immunosuppression and without the harmful side effects of suppressive medicines would let recipients to live normal lives.
Examples of Transplantation
The Most Commonly Transplanted Organ Is the Kidney
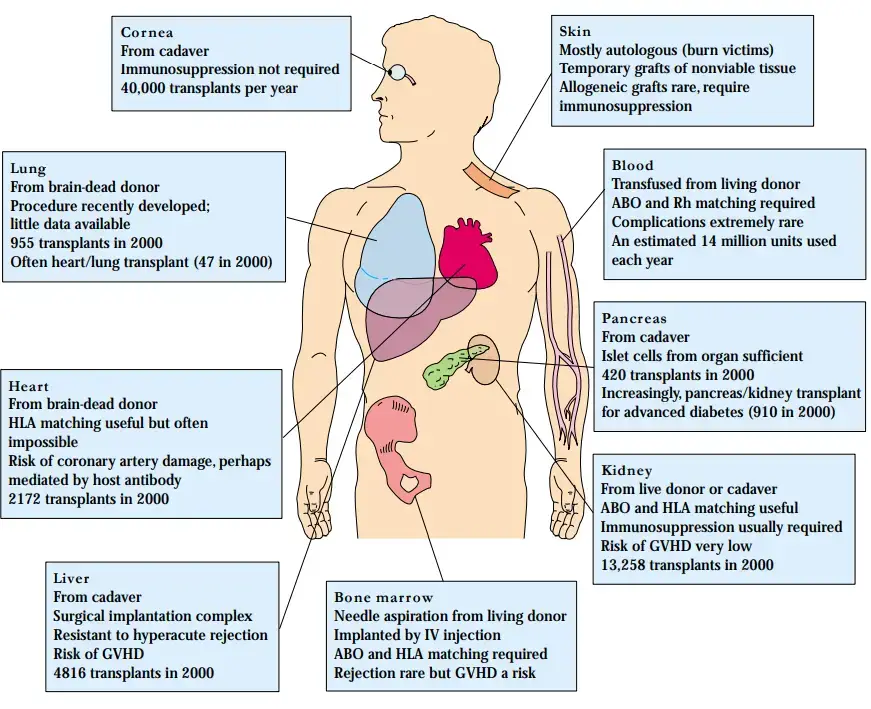
- As stated above, the kidney is the most commonly donated organ; in 2000, 13,258 kidney transplants were performed in the United States.
- Numerous clinical indications for kidney transplantation are important contributors to this figure.
- Numerous common conditions, such as diabetes and other forms of nephritis, result in kidney failure that transplantation can treat. In terms of availability, kidneys can be received not only from deceased family members or volunteers, but also from living relatives, as it is feasible to donate a kidney and live a normal life with the remaining kidney.
- In 1999, living donors provided 4,457 of the 12,483 kidneys transplanted in the United States. Kidney transplants are scientifically simpler than liver or heart transplants.
- Because several kidney transplants have been performed, detailed patient care procedures have been developed.
- Matching blood and histocompatibility groups is helpful in kidney transplantation since the organ is highly vascularized, but the kidney does not present any unique challenges that encourage rejection or graft-versus-host disease (GVHD), like bone marrow and liver do.
Xenotransplantation for the Shortage of Donor Organs
- While the immune system is a tremendous barrier to transplantation, significant progress has been made to overcome this impediment.
- However, comparable progress has not been made in overcoming the complicated problem of finding organs for individuals in need.
- Due to the lack of accessible organs, a significant proportion of patients die while awaiting a transplant. The necessity for an alternative source of donor organs has brought xenotransplantation into the spotlight.
- The larger nonhuman primates (chimpanzees and baboons) have been the primary organ donors; but, as noted in the Clinical Focus section, the use of pigs as a source of organs is currently being seriously considered.
- The first kidney transplants from chimpanzees to humans occurred in 1964. Since then, occasional attempts have been made to transplant kidney, heart, liver, and bone marrow from monkeys to humans.
- No effort has been wildly successful, but few have garnered some attention. In 1993, T. E. Starzl transplanted the livers of two baboons into patients with liver insufficiency. One patient died after 26 days, while the other died 70 days later.
- In 1994, a liver from a pig was transplanted into a 26-year-old with acute hepatic failure. Before being rejected by a hyperacute rejection reaction, the liver functioned for barely thirty hours.
- In 1995, an HIV-positive man was infused with baboon bone marrow in an effort to bolster his compromised immune system using baboon immune cells that do not become infected with the virus.
- Despite the absence of any transplant-related problems, the baboon bone marrow did not appear to establish itself in the recipient.
Bone-Marrow Transplants Are Used for Leukemia, Anemia, and Immunodeficiency
- After the kidney, the most common transplant is bone marrow. Since the early 1980s, bone-marrow transplantation has been used to treat a variety of malignant and nonmalignant hematologic illnesses, including leukaemia, lymphoma, aplastic anaemia, thalassemia major, and immunodeficiency diseases, including severe combined immunodeficiency (SCID).
- The bone marrow taken via repeated needle aspiration from a living donor has erythroid, myeloid, monocytoid, megakaryocytic, and lymphocytic lineages.
- Approximately 109 cells per kilogramme of the recipient’s body weight are delivered intravenously into the graft. The first successful transplants of bone marrow were performed between identical twins.
- However, the development of the previously stated tissue-typing technologies has made it possible to find allogeneic donors with HLA antigens that are similar or almost identical to those of the recipients.
- While there is no shortage of bone marrow for transplantation, it may be difficult to find a suitable donor.
Skin Grafts Are Used to Treat Burn Victims
- The majority of human skin transplants utilise autologous tissue. In situations of severe burns, however, it is possible to employ grafts of foreign skin thawed from frozen deposits in tissue banks.
- These grafts are typically used as biologic dressings because the cellular parts are no longer alive and the graft does not grow in the new host; the grafts are left in place for a few days but are replaced frequently.
- In some situations, true allogeneic skin grafting using fresh viable donor skin has been performed, however immunosuppressive medication is required to prevent rejection.
- This is undesirable since a significant issue with burn sufferers is the increased risk of infection, which is exacerbated by immunosuppressive medication.
Heart Transplantation
- Heart transplantation is arguably the most dramatic type of transplantation; once the diseased heart has been removed, the patient must be maintained alive solely through artificial means until the transplanted heart begins to beat.
- After the heart has been removed, heart-lung machines are available to circulate and oxygenate the patient’s blood.
- The donor’s heart must be preserved so that it will begin to beat after it is transplanted into the recipient. It has been discovered that a human heart can be kept alive for a limited time in ice-cold buffer solutions that successfully short circuit the electric impulses that drive the heart’s rhythmic beating, which could otherwise harm the isolated organ.
- For a lot of years, surgical methods for implanting a heart have been available. In 1964, the first heart transplant was performed by Dr. Christian Barnard in South Africa. Since then, the one-year survival rate for heart transplantation has increased to almost 80%.
- In 2000, there were 2172 heart transplants performed in the United States and approximately 3500 performed globally. A new type of atherosclerotic disease in the coronary arteries of the transplanted organ has emerged as a concern unique to heart transplants.
- There is a potential that host antibodies cause damage to the heart’s blood arteries. Although a heart transplant may be of considerable value to individuals with various types of cardiac illness or injury, the number of accessible hearts is plainly limited.
- The normal source of these organs is brain-dead accident victims with a healthy circulatory system and heart. Due to the limited number of hearts and the urgency of the treatment, HLA compatibility is generally sought but seldom possible.
Pancreas Transplantation Offers a Cure for Diabetes Mellitus
- Diabetes mellitus is one of the most frequent diseases in the United States. This condition is caused by malfunctioning pancreatic islet cells that produce insulin.
- A pancreas transplant could give the appropriately regulated insulin levels required to normalise a diabetic individual.
- Recently, approximately 55% one-year success rates for pancreatic transplantation have been recorded. Restoring the function required to create insulin in a controlled manner does not require transplantation of the entire pancreas; islet cells alone could restore function.
- Kidney failure is a common consequence of severe diabetes, affecting around 30% of diabetics; hence, transplantation of the kidney and pancreas are necessary. In 2000, 420 pancreas transplants and 904 kidney/pancreas transplants were performed simultaneously.
- A group from the University of Wisconsin reports overcoming surgical and medical obstacles to the dual transplant and achieving survival rates of 87 percent at one year and 78 percent at five years for the 381 individuals in their research.
- Whether it is preferable to perform simultaneous kidney-pancreas transplants or separate transplants must be determined on a case-by-case basis.
Lung Transplants
- In recent years, lung transplantation, either by itself or in conjunction with heart transplantation, has been used to treat diseases such as cystic fibrosis and emphysema or acute damage to the lungs such as that caused by smoke inhalation.
- In 2000, there were 945 lung transplants and 47 heart/lung transplants. The reported one-year survival rate for lung transplants is approximately 60%.
Liver Transplants Treat Congenital Defects and Damage from Viral or Chemical Agents
- The liver is a big organ that conducts a variety of activities relating to chemical and biological material clearance and detoxification.
- Viruses such as hepatitis and chronic alcoholism can damage the liver, resulting in liver dysfunction. Exposure to toxic substances, such as in chronic alcoholism, can also damage the liver.
- The liver injury may repair itself, and the damaged tissue can regrow after the damaging factor is eliminated. If liver tissue does not regrow, the injury could be fatal.
- The majority of liver transplants are performed to treat congenital liver disorders. Because the liver is big and has a complex circulatory system, its re-implantation initially posed a technological challenge.
- The current one-year survival rate has increased to roughly 65% due to the development of techniques to overcome this key surgical obstacle.
- In 2000, 4,816 liver transplants were performed in the United States. Increasingly, a liver from a single donor may be divided and given to two recipients; typically, the smaller piece is given to a youngster and the bigger portion is given to an adult.