A ribonucleoprotein known as telomerase, terminal transferase adds a repeat sequence of telomeres to the 3′ end of chromosomes, the length of which varies depending on species. In most eukaryotic organisms, the chromosomal ends are capped by a repeating sequence known as a telomere. Telomeres prevent DNA damage and chromosomal fusion at the chromosome’s end. In place of telomerase, retrotransposons are used to keep the telomeres of the fruit fly Drosophila melanogaster in good shape.
In order to lengthen telomeres, the reverse transcriptase enzyme telomerase uses a template RNA molecule (such as 3′-CCCAAUCCC-5′ in Trypanosoma brucei). Gametes and most cancer cells contain functional telomerase, although most somatic cells often do not contain this enzyme or have only trace amounts of it.
What is The end-replication problem?
Eukaryotic chromosomes are linear (rod-shaped), unlike the circular chromosomes of bacteria. These terminals interfere with the DNA replication process. Since the DNA at the chromosome’s very end is not entirely replicated with each replication cycle, the chromosome gradually shortens over time.
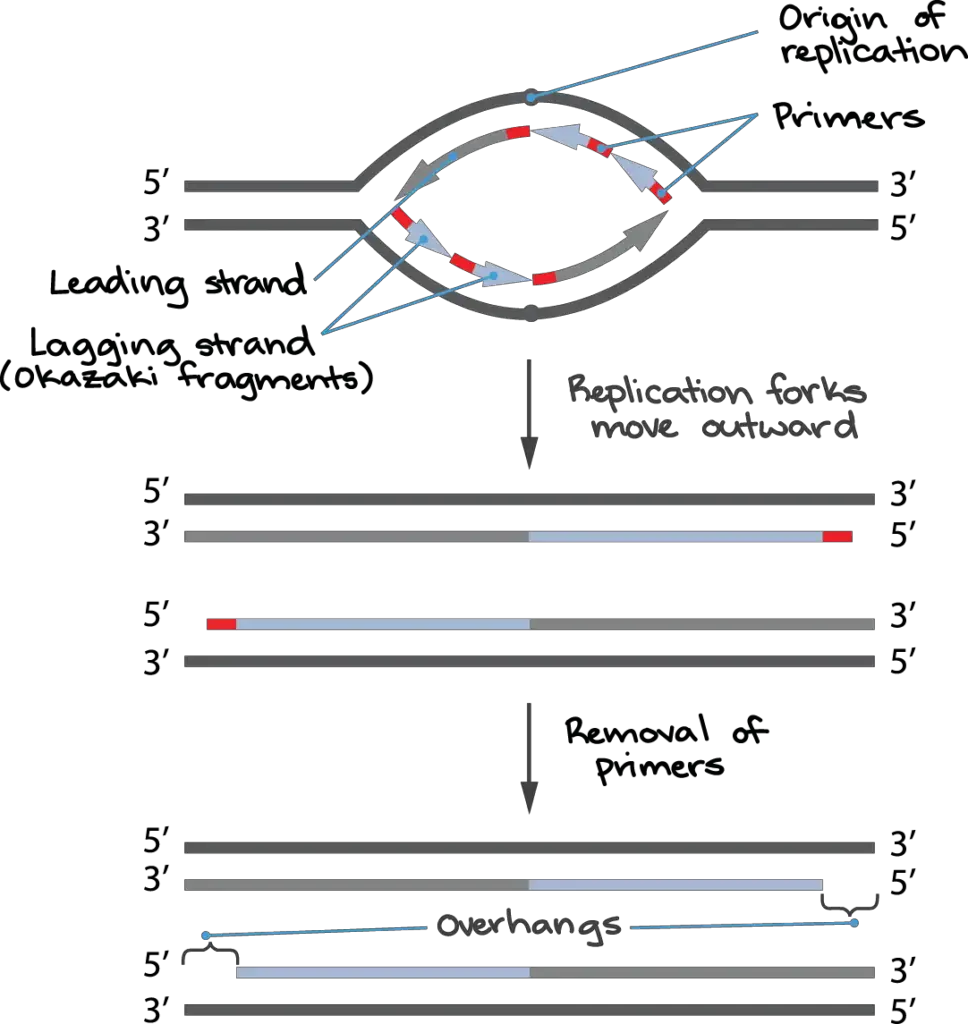
How come this is happening? The leading strand is the new DNA strand that is continually produced at a replication fork during DNA replication. The other strand, called the lagging strand, is made up of many smaller pieces called Okazaki fragments, each of which starts with its own RNA primer. (For additional information, read up on DNA replication.)
Primers on Okazaki fragments may usually be swapped out for DNA, and the fragments can be joined together to make a continuous strand. However, in many organisms, including humans, there is a brief region of DNA that is not covered by an Okazaki fragment when the replication fork reaches the chromosomal end; in other words, there is no way to initiate the fragment because the primer would extend past the chromosome end.
Furthermore, unlike other primers, the primer of the very last Okazaki fragment that is produced cannot be substituted with DNA.
Why is that the case?
DNA polymerases require a template to begin a new strand of DNA; they cannot initiate DNA synthesis without one. Why? Because they catalyse a polymerization reaction in which a new nucleotide joins an existing one by attaching its phosphate group to the hydroxyl group of an existing nucleotide, as depicted on the right. DNA polymerases require this hydroxyl group as a “hook” to connect nucleotides to in order to catalyse the reaction necessary to create new DNA.
Primers used to initiate Okazaki fragments are typically interchangeable. This is because DNA polymerase can use the hydroxyl at the end of the next Okazaki fragment as a starting point when replacing the primer with DNA. However, because there is no nearby Okazaki fragment at the chromosome’s terminus to supply the necessary hydroxyl, chromosome-end replication is disrupted.
These issues cause a single-stranded overhang to form at the end of each replicated eukaryotic chromosome because some of the DNA at the chromosome’s terminus is never replicated. As the process of cell division continues, the chromosome gradually shortens.
As much as 70–100 nucleotides can separate the chromosomal end from the last RNA primer on the lagging strand in human cells. This means that each round of cell division dramatically shortens the human chromosome due to the large single-stranded overhangs formed by imperfect end replication.
What happens to the leading strand end of the chromosome?
As seen in the final panel of the above diagram, the leading strand end of the chromosome is shown to be blunt (having no overhang). While the end of the leading strand is originally blunt, it undergoes processing by cellular enzymes to create an overhang of around 30 base pairs.
For reasons that will become clear shortly, it is crucial to generate this overhang in order to safeguard chromosomal ends. It also plays a role in the gradual shortening of telomeres as a result of repeated cell division.
What is Telomeres?
Eukaryotic chromosome ends are protected by DNA “caps” called telomeres, which help keep genes intact even as chromosomes age. Human and mammalian telomeres contain hundreds to thousands of copies of a short DNA sequence known as 5′-TTAGGG-3′. This sequence varies somewhat between species.
Because telomeres feature single-stranded overhangs that “look like” damaged DNA, they must be shielded from the cell’s DNA repair mechanisms. Incomplete end replication causes an overhang to form at the chromosome’s lagging strand (see figure above). Enzymes that cleave the DNA create the overhang at the leading strand end of the chromosome.
To prevent the ends of chromosomes from being damaged, telomeres generate protective loops in some organisms (including humans) by binding to complementary repetitions in the adjacent double-stranded DNA. Telomere ends are shielded and kept from activating DNA repair pathways by proteins that bind to them.
Over the course of several cell divisions, the telomere’s repeating sequence is gradually eroded, leaving behind a protective buffer for the gene-containing sections farther inside the chromosome (at least, for some period of time). Cellular ageing has been linked to telomere shortening, and the gradual loss of telomeres may explain why cells have a finite capacity for cell division.
What is Telomerase?
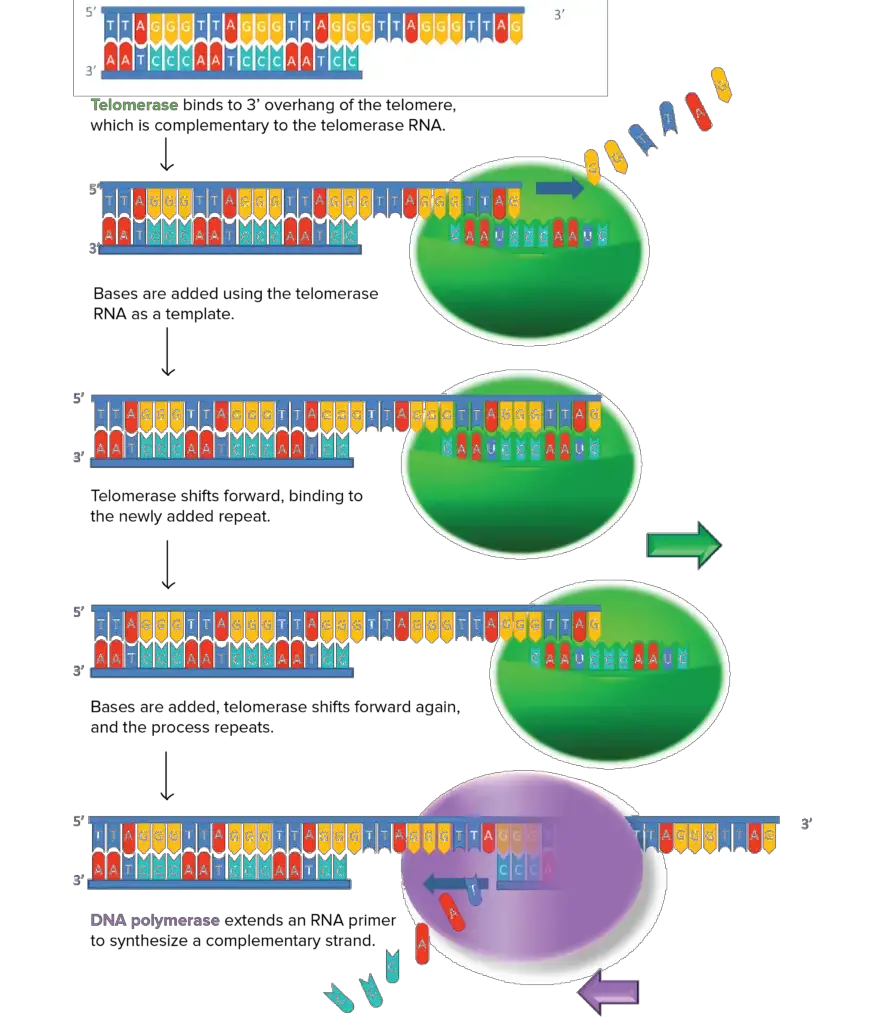
It is possible for telomere shortening to be repaired in some cells by the expression of telomerase, an enzyme that lengthens chromosome telomeres. Telomerase is a DNA polymerase that uses RNA as a template to create new DNA.
What role does telomerase play? The enzyme interacts with a specific RNA molecule that carries a sequence that is a perfect match for the telomeric repeat. Using this complementary RNA as a template, the overhanging strand of telomere DNA is extended (by adding nucleotides to it). When the overhang is sufficiently long, the usual DNA replication machinery (RNA primer and DNA polymerase) may make a complementary strand, resulting in double-stranded DNA.
An overhang will persist if the primer is not precisely located at the chromosome’s end and is therefore unable to be replaced by DNA. The telomere, however, will end up being longer in total.
Some adult stem cells and the germ cells that produce sperm and eggs have telomerase activity, but this is not the case for the vast majority of somatic cells. These cells must divide several times or, in the case of germ cells, give rise to a whole new creature, allowing the telomeric “clock” to start over.
Telomerase activity is intriguing since many cancer cells have shorter telomeres. Drugs that block telomerase as part of cancer therapy have the ability to halt their excessive division (and the growth of the cancerous tumour).
Structure of Human telomerase
- Two copies of human telomerase reverse transcriptase (TERT), telomerase RNA (TR or TERC), and dyskerin make up the human telomerase complex, as determined by the research group led by Scott Cohen at the Children’s Medical Research Institute in Sydney, Australia (DKC1).
- Telomerase subunit genes (TERT, TERC, DKC1, and TEP1) are spread out over several chromosomes.
- The protein encoded by the human TERT gene (hTERT) contains 1132 amino acids.
- It has been found that TERT polypeptide folds with and transports TERC, a non-coding RNA (451 nucleotides long).
- TERT’s’mitten’ shape makes it possible for it to add single-stranded telomere repeats by wrapping around the chromosome.
- TERT belongs to the family of enzymes known as reverse transcriptases, which synthesise dsDNA from ssRNA.
- The protein has a “right hand” ring structure like that of retroviral reverse transcriptases, viral RNA replicases, and bacteriophage B-family DNA polymerases, which is made up of four conserved domains (the RNA-Binding Domain (TRBD), fingers, palm, and thumb).
- Proteins with the TERT domain have been sequenced from a wide variety of eukaryotic organisms.
Importance of Telomerase
1. Aging
- Repeated cell division via mitosis shortens segments of DNA called telomeres, while telomerase restores these telomeres.
- Cells that divide indefinitely without telomerase are thought to achieve their “Hayflick limit” of 50 to 70 daughter cells. When cells reach this point, they become senescent and stop dividing.
- Each new generation is able to compensate for the DNA that has been lost, ensuring that the cell line can continue to divide indefinitely. Cancerous growth exhibits the same type of unchecked expansion.
- Telomerase is expressed by embryonic stem cells, allowing them to divide indefinitely and give rise to a unique individual.
- In adults, telomerase is highly expressed only in cells that need to proliferate on a regular basis, such as male sperm cells, epidermal cells, activated T cells and B cells, and some adult stem cells, but in the vast majority of cases, somatic cells do not express telomerase.
- A study in comparative biology of mammalian telomeres found that the length of telomeres in some mammalian species correlates negatively rather than positively with lifespan, leading the researchers to conclude that the relationship between telomere length and longevity is still unclear.
- In some postmitotic tissues, like the rat brain, telomeres do not shorten with age.
- Telomere lengths in human skeletal muscle do not change much between ages 23 and 74. Less than 3% of myonuclei in baboon skeletal muscle contain broken telomeres, and this fraction does not grow with age. These cells are completely differentiated postmitotic cells.
- Consequently, it appears that telomere shortening has a little role in the ageing of differentiated cells in the brain and skeletal muscle.
- Cholangiocytes and hepatocytes in the human liver do not undergo telomere shortening as one ages.
- Yet another study failed to find convincing evidence that telomere length is a relevant biomarker of normal ageing in humans with regard to key cognitive and physical capacities.
- Elevated telomerase levels in mice result in an increased cancer risk, but these animals do not survive any longer than normal, calling into doubt the efficacy of telomerase as an anti-aging therapy.
- However, overexpressing telomerase’s catalytic component in cancer-resistant mice was shown to increase their lifetime in a single research.
- According to the results of a recent scientific investigation, people who live very long lives tend to inherit a telomerase variant with enhanced activity.
Premature aging
- Syndromes of premature ageing that are linked to short telomeres include Werner syndrome, Progeria, Ataxia telangiectasia, Ataxia telangiectasia related condition, Bloom syndrome, Fanconi anaemia, and Nijmegen breakage syndrome.
- However, the mutant genes in these conditions all play important roles in repairing DNA damage, and this increased DNA damage may play a role in accelerating the ageing process (see DNA damage theory of aging). The question of whether or not a further function is involved in keeping telomere length stable is one that is currently being explored.
2. Cancer
- Tumor suppressor proteins p53 and Retinoblastoma protein can be inactivated in vitro to delay cellular senescence as cells approach the Hayflick limit (pRb).
- When the majority of cells in a culture die, this is known as a “crisis” in cells that have been so modified.
- Sometimes, even when faced with a crisis, a cell continues to divide. After each cell division, telomeres shorten and chromosome damage increases.
- DNA damage, including exposed chromosomal ends, is typically repaired by joining the severed strands back together. This occurs when the cell, in response to shortened telomeres, joins the ends of distant chromosomes.
- This resolves the issue of short telomeres, but it leads to numerous mutations and chromosomal abnormalities because the fused chromosomes are ripped apart at random during anaphase of cell division.
- The genome of the cell becomes increasingly unstable as this process proceeds. The cell dies by apoptosis when its chromosomes are irreparably damaged or when a second mutation occurs to activate telomerase.
- If the circumstances for cell duplication are met, certain types of cells and their progeny can become immortal (bypass the Hayflick limit) and live forever.
- When paired with unchecked cell division, the lengthy life span afforded by telomerase activity in many cancer cells is a major contributor to their ability to proliferate and form tumours.
- HeLa cells, which have been used as a model cell line in laboratories since 1951, are a type of immortal cancer cell.
3. Heart disease, diabetes and quality of life
- Blackburn also found that telomerase was active at the site of blockages in coronary artery tissue, perhaps hastening heart attacks, and that telomeres are shorter in mothers caring for extremely sick children when they say that their emotional stress is at a maximum.
- Stress has been proven to enhance telomerase activity considerably in 2009, and this effect has been studied extensively. One hour after the stress was relieved, telomerase activity in peripheral blood mononuclear cells increased by 18% throughout the sample of patients.
- A 2010 study indicated that following a three-month meditation retreat, participants’ telomerase activity was “substantially larger” than that of controls.
- Mice without telomerase developed diabetes mellitus and had reduced insulin production because they lost pancreatic insulin-producing cells.
4. Rare human diseases
- In 2005, mutations in TERT were shown to increase the risk of developing aplastic anaemia, a condition in which the bone marrow is unable to create healthy blood cells.
- Loss of the end of the short arm of chromosome 5 causes the complicated condition known as Cri du chat syndrome (CdCS). TERT resides in the deleted area, and it has been hypothesised that the loss of one copy of TERT is a cause or contributing factor of this disease.
- Some abnormalities in the telomerase subunits can lead to dyskeratosis congenita (DC), a disorder of the bone marrow.
- Nearly one-third of DC cases are X-linked recessive at the DKC1 locus, whereas roughly five percent are autosomal dominant at the TERT and TERC loci.
- Patients with DC suffer from significant bone marrow loss, which shows up in a number of ways, including aberrant skin pigmentation, leucoplakia (a white thickening of the oral mucosa), and nail dystrophy.
- Shorter telomeres and impaired telomerase activity in vitro are observed in people with TERC or DKC1 mutations compared to controls of the same age.
- A heterozygous TERT mutation was found to be the cause of autosomal dominant DC in one family. Additionally, these patients showed an elevated rate of telomere shortening and genetic anticipation (i.e., the DC phenotype worsened with each generation).
References
- Jafri, M.A., Ansari, S.A., Alqahtani, M.H. et al. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med 8, 69 (2016). https://doi.org/10.1186/s13073-016-0324-x
- Chan SR, Blackburn EH. Telomeres and telomerase. Philos Trans R Soc Lond B Biol Sci. 2004 Jan 29;359(1441):109-21. doi: 10.1098/rstb.2003.1370. PMID: 15065663; PMCID: PMC1693310.
- https://microbiologynotes.org/dna-replication-in-eukaryotes-initiation-elongation-and-termination/
- https://en.wikipedia.org/wiki/Telomerase
- https://www.khanacademy.org/science/biology/dna-as-the-genetic-material/dna-replication/a/telomeres-telomerase