Water Molecules
- Water molecules, represented by the chemical formula H₂O, consist of two hydrogen atoms covalently bonded to one oxygen atom. The arrangement of these atoms results in a polar molecule, characterized by an uneven distribution of electrical charge. In this configuration, the hydrogen atoms acquire partial positive charges, while the oxygen atom assumes a partial negative charge. This polarity is fundamental to many of water’s unique properties and functions, influencing its interactions with other substances.
- The polar nature of water molecules plays a crucial role in their behavior. The concept of “like attracts like” highlights that polar substances, such as water, are drawn to other polar molecules, while nonpolar substances do not engage in similar interactions. For instance, oil is a nonpolar substance with an equal charge distribution, which prevents it from mixing with water. This immiscibility between oil and water can be attributed to their differing polarities, illustrating the principle that polar and nonpolar substances do not effectively attract or interact.
- In the context of water’s interactions, two specific terms are essential: hydrophilic and hydrophobic. Hydrophilic substances, often referred to as “water-loving,” possess an affinity for water, meaning they can readily interact with and dissolve in it. Conversely, hydrophobic substances, or “water-fearing” compounds, repel water and do not mix with it. A prime example of hydrophobic molecules is lipids, which contain nonpolar bonds and therefore do not interact favorably with water. Understanding these terms is vital, as they elucidate the behaviors of various biological molecules in aqueous environments.
- The significance of water cannot be overstated, as it is the most prevalent molecule in living organisms. Its unique properties, such as high surface tension, specific heat capacity, and solvent capabilities, arise from its polar nature and hydrogen bonding. Water’s ability to dissolve a wide range of substances facilitates numerous biochemical processes, including metabolic reactions and nutrient transport within cells. Thus, the study of water molecules and their interactions is foundational for understanding life at a molecular level.
- In summary, the structural and chemical properties of water molecules are integral to their role in biological systems. By appreciating the polar nature of water, the distinction between hydrophilic and hydrophobic substances, and the importance of these interactions, one can better grasp the essential functions that water fulfills in sustaining life. This knowledge is particularly relevant in fields such as biology, chemistry, and environmental science, where water’s unique characteristics underpin countless processes.
Structure of Water
The structure of water is fundamental to its unique properties and functions in various biological systems. The molecular composition, bonding characteristics, and phase transitions of water are essential concepts in understanding its role in life processes.
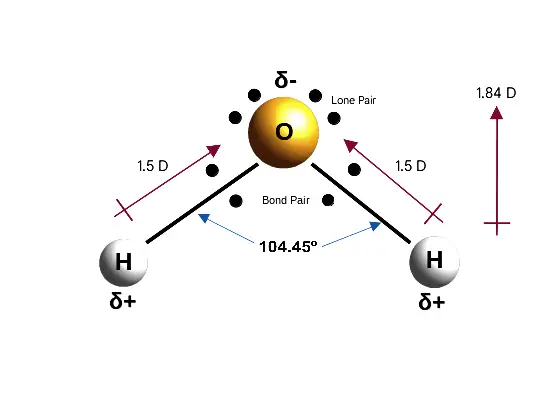
- Chemical Composition: Water is represented by the chemical formula H₂O, indicating that each molecule comprises two hydrogen atoms covalently bonded to one oxygen atom. This simple yet vital structure forms the basis of water’s characteristics.
- Bonding and Hybridization: In a water molecule, the atoms are connected through polar covalent bonds. The valence orbitals of the oxygen atom undergo sp³ hybridization, producing four sp³ hybrid orbitals arranged tetrahedrally. Two of these orbitals contain the bonded hydrogen atoms, while the other two accommodate lone pairs of electrons. This configuration results in a bent structure with an approximate bond angle of 104°, deviating from the ideal tetrahedral angle of 109° due to the repulsion exerted by the lone pairs.
- Polarity and Charge Distribution: Water is a polar molecule, primarily because oxygen is more electronegative than hydrogen. Consequently, the shared electrons are drawn closer to the oxygen atom, creating a partial negative charge on it, while the hydrogen atoms exhibit partial positive charges. This polarity is crucial for water’s interactions with other substances.
- Intermolecular Forces in Different Phases:
- In the gaseous phase, water molecules exist independently with minimal interactions, but they still retain their polar characteristics.
- Transitioning to the liquid phase, water molecules engage in extensive hydrogen bonding, where a single water molecule can form up to four hydrogen bonds—two as a hydrogen bond donor and two as an acceptor. This leads to the formation of an intermolecular tetrahedral structure that creates a three-dimensional bonding network, contributing to water’s unique properties such as high surface tension and specific heat capacity.
- In the solid phase (ice), water molecules adopt a crystalline structure where each oxygen atom is tetrahedrally surrounded by four neighboring oxygen atoms. Here, hydrogen atoms form covalent bonds with one oxygen atom and participate in hydrogen bonds with adjacent oxygen atoms. This arrangement leads to significant open spaces in the crystal lattice, resulting in lower density compared to liquid water. Therefore, ice floats on water, an essential feature for aquatic life during freezing conditions.
- Melting and Density Changes: When ice melts, the hydrogen bonds break, allowing water molecules to come closer together, increasing the density of the liquid phase. This transition highlights the dynamic nature of water’s molecular structure and the significance of hydrogen bonding in maintaining the balance of ecosystems.
Properties of Water
| Properties | Values |
|---|---|
| Chemical formula | H₂O |
| Molar mass | 18.01528(33) g/mol |
| Odour | None |
| Density | Solid: 0.9167 g/mL at 0 °C Liquid: 0.961893 g/mL at 95 °C 0.9970474 g/mL at 25 °C 0.9998396 g/mL at 0 °C |
| Boiling point | 99.98 °C (211.96 °F; 373.13 K) |
| Melting point | 0.00 °C (32.00 °F; 273.15 K) |
| Solubility | Poorly soluble in aliphatic and aromatic hydrocarbons, and ethers. Improved solubility in amines, ketones, alcohols, carboxylates. Miscible with acetonitrile, dimethyl sulfoxide, dimethoxyethane, dimethylformamide, acetaldehyde, sulfonates, tetrahydrofuran, 1,4-dioxane, glycerol, acetone, isopropanol, propanol, ethanol, methanol. Partially miscible with bromine, ethyl acetate, diethyl ether, dichloromethane. |
| Acidity (pKa) | 13.995 |
| Vapour pressure | 3.1690 kilopascals or 0.031276 atm |
| Basicity (pKb) | 13.995 |
| Refractive index (nD) | 1.3330 (20°C) |
| Thermal conductivity | 0.6065 W/m·K |
| Viscosity | 0.890 cP |
| Structure | Crystal structure: Hexagonal Molecular shape: Bent Point group: C₂v |
| Dipole moment | 1.8546 D |
| Thermochemistry | Specific heat capacity (C): 75.375 ± 0.05 J/mol·K Std enthalpy of formation (ΔfH°298): -285.83 ± 0.040 kJ/mol Std molar entropy (S°298): 69.95 ± 0.03 J/mol·K Gibbs free energy (ΔfG°): -237.24 kJ/mol |
Physical Properties of Water
The physical properties of water are essential for understanding its behavior in various environments and its significance in biological systems. Water, with the chemical formula H₂O, exhibits several unique characteristics due to its molecular structure and the presence of hydrogen bonds.
- Transparency, Color, and Odor: Pure water is a transparent, colorless, and odorless liquid. Its ability to remain neutral allows it to readily absorb flavors and compounds dissolved within it, making it an effective medium for various biochemical reactions.
- Freezing and Boiling Points: Water possesses unusually high freezing and boiling points compared to other hydrides in the same group. These elevated temperatures result from the strong intermolecular hydrogen bonds that exist between water molecules, requiring significant energy to disrupt. This characteristic allows water to remain liquid across a wide range of environmental conditions.
- Dipole Moment: Water has a high dipole moment, which arises from the polar covalent bonds between hydrogen and oxygen. This polarity makes water an ideal solvent, facilitating the dissolution of numerous ionic and polar compounds. The dipole interactions enable water molecules to form hydration shells around solutes, which is crucial for biochemical processes.
- Specific Heat Capacity: Water’s high specific heat capacity is another important property. This characteristic allows water to absorb considerable heat with minimal temperature changes, thereby playing a critical role in moderating temperature fluctuations in both the environment and living organisms. For instance, this property helps regulate body temperature, ensuring that biochemical processes can occur efficiently.
- Thermal Conductivity: Water exhibits good thermal conductivity, allowing it to distribute heat evenly within its volume. This quality contributes to its effectiveness in temperature regulation, particularly in biological systems where maintaining stable internal conditions is vital.
- Surface Tension: Water has high surface tension due to the cohesive forces between water molecules. This property is significant for various biological processes, such as capillary action, which facilitates the movement of water and nutrients in plants.
- Latent Heat of Vaporization: The latent heat of vaporization of water is notably high, meaning that water can absorb substantial amounts of heat during the transition from liquid to vapor. This property is particularly important for thermoregulation in living organisms, as it enables cooling through processes such as sweating or transpiration in plants.
- Electrical Conductivity: While pure water is a poor conductor of heat and electricity, the addition of small amounts of acids or alkalis can enhance its electrical conductivity. This occurs because these substances dissociate into ions, facilitating the flow of electricity.
Chemical Properties of Water
The chemical properties of water (H₂O) are integral to its function as a solvent and its role in biological and ecological systems. Understanding these properties is crucial for comprehending how water interacts with various substances and supports life.
- Amphoteric Nature: Water is classified as amphoteric, meaning it can act both as an acid and a base. This dual capability allows it to participate in various chemical reactions. For instance, when water donates a proton (H⁺), it behaves as an acid:H2O(l)+NH3(aq)→NH4+(aq)+OH−(aq) Conversely, when it accepts a proton, it acts as a base: H2O(l)+H2S(aq)→H3O+(aq)+HS−(aq)
- Redox Reactions: Water is a participant in redox (reduction-oxidation) reactions. Electropositive elements can reduce water to form hydrogen gas, which highlights water’s role as a source of hydrogen. For example: 2H2O(l)+2Na(s)→2NaOH(aq)+H2(g) Additionally, during photosynthesis, water is oxidized to produce oxygen (O₂), showcasing its importance in metabolic processes.
- Hydrolysis Reactions: Water exhibits a strong hydrating tendency due to its high dielectric constant. This property allows water to dissolve many ionic compounds. Hydrolysis occurs when water interacts with these compounds, breaking them down into their constituent ions. For example, when sodium chloride (NaCl) is dissolved in water, it separates into sodium (Na⁺) and chloride (Cl⁻) ions.
- Universal Solvent: Water is often referred to as the universal solvent due to its ability to dissolve a vast array of substances. This capability is primarily a result of the hydrogen bonding and polarity of water molecules. Water molecules can interact with polar and ionic substances through weak electrostatic interactions. In solutions where water molecules outnumber solute molecules, hydration shells form around solutes, facilitating their dispersion throughout the solution. However, non-polar substances do not interact with water effectively, leading to precipitation.
- Specific Heat Capacity and Heat of Vaporization: Water possesses a high specific heat capacity, which allows it to absorb significant amounts of heat without experiencing drastic temperature changes. This characteristic helps regulate temperatures in both natural environments and living organisms. Water also has a high heat of vaporization, which means it can absorb considerable heat energy when transitioning from liquid to vapor. This property is essential for processes such as evaporative cooling, where organisms use the evaporation of sweat to regulate body temperature.
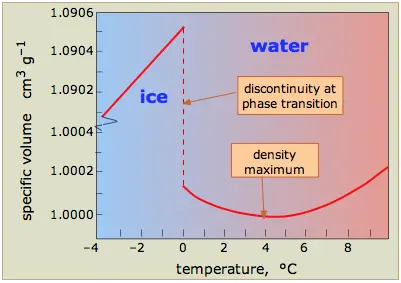
- Density and Thermal Expansion: Water’s density reaches its maximum at approximately 4°C. As water cools further towards freezing, it expands rather than contracts, leading to a decrease in density. This anomalous expansion causes ice to float on liquid water, providing insulation for aquatic ecosystems during winter months.
- Electrical Conductivity: While pure water is a poor conductor of electricity, it can undergo autoionization, resulting in the formation of hydroxide (OH⁻) and hydronium (H₃O⁺) ions. This process allows for limited conductivity in pure water, which can increase with the presence of dissolved ions from acids or bases.
- Cohesion and Adhesion: The hydrogen bonding present in water contributes to its cohesive properties, allowing water molecules to stick together. This cohesion is crucial for phenomena such as surface tension, which enables certain insects to walk on water. Water also exhibits strong adhesion, allowing it to adhere to various surfaces, facilitating capillary action. This action is vital in plants, enabling the transport of water from roots to leaves.
- Surface Tension: Water’s high surface tension, a result of cohesive forces among water molecules, allows it to resist external forces. This property is evident when water droplets form or when water slightly overfills a glass without spilling.
Hydrogen bonding in water
Hydrogen bonding in water is a fundamental chemical interaction that significantly influences the properties and behavior of this essential substance. It arises from the unique structure and polarity of water molecules, leading to a variety of biological and physical phenomena.
- Nature of Hydrogen Bonding: Hydrogen bonding occurs when a hydrogen atom covalently bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine, interacts with another electronegative atom. In water, this involves the hydrogen atoms carrying a partial positive charge due to the electronegativity of the oxygen atom, which carries a partial negative charge. Therefore, this polarity gives water its unique ability to form hydrogen bonds.
- Strength and Dynamics: While hydrogen bonds are generally weaker than covalent bonds—about one-twentieth the strength of an O-H covalent bond—they still play a crucial role in molecular interactions. The lifetime of these bonds is short, as they are continuously formed and broken, maintaining a dynamic equilibrium in liquid water. Consequently, each water molecule is usually engaged in at least one hydrogen bond with a neighboring molecule, resulting in an interconnected network.
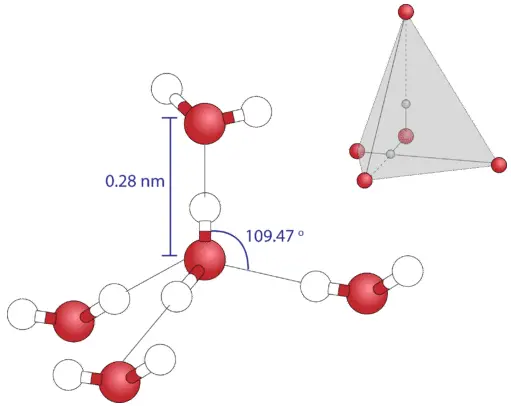
- Hydrogen Bond Formation: Each water molecule can form up to four hydrogen bonds. This occurs as the oxygen atom can form two bonds with its lone pairs, while the hydrogen atoms can each donate one bond. This tetrahedral arrangement of hydrogen bonds results in a three-dimensional bonding network that contributes to the stability of liquid water.
- Cohesion and Adhesion: The hydrogen bonding in water contributes to its cohesive properties, allowing water molecules to stick together. This cohesion is responsible for phenomena such as surface tension, which enables small objects to rest on the water’s surface. Additionally, the adhesive properties of water allow it to interact with other substances, facilitating processes such as capillary action in plants.
- Impact on Physical Properties: Hydrogen bonds in water lead to several distinctive physical properties, including high specific heat capacity and evaporative cooling. Water’s high specific heat allows it to absorb significant amounts of heat with minimal temperature change, which is vital for regulating temperatures in biological systems. The process of evaporative cooling, where water absorbs heat as it evaporates, is critical for thermoregulation in living organisms.
- Molecular Arrangement: The hydrogen bonding network results in an open structure that holds water molecules approximately 15% closer together than would be expected from van der Waals interactions alone. However, this bonding is directional, limiting the number of neighboring water molecules to approximately four, as compared to the larger numbers observed in other liquids.
- Biological Significance: Hydrogen bonding is essential for many biological processes. In proteins, it contributes to the stabilization of structures and shapes critical for function. In DNA, hydrogen bonds between base pairs help maintain the integrity of the double helix, supporting its role in genetic information storage and transmission.
Functions of Water
Water plays a crucial role in various biological functions, making it an indispensable component of life. Its unique properties allow it to participate in essential processes across different biological systems. The following points detail the significant biological functions of water:
- Regulation of Bodily Processes: Water serves as a vital body fluid, essential for numerous physiological processes, including digestion, nutrient transport, and excretion. It effectively dissolves ionic and polar organic compounds, facilitating the transportation of digested products to tissues where they are needed.
- Thermoregulation: Water plays a critical role in regulating body temperature. Through the processes of sweating and evaporation, it dissipates excess heat, helping to maintain a stable internal environment. This property is particularly vital during physical activity or exposure to high temperatures.
- Medium for Metabolic Reactions: All metabolic reactions in the body occur in an aqueous environment, making water the universal solvent for biochemical processes. Its ability to dissolve a wide range of substances allows for efficient metabolic pathways, including enzymatic reactions and substrate transport.
- Habitat for Aquatic Life: Water provides essential habitats for numerous organisms. Aquatic ecosystems, such as ponds, rivers, and seas, support diverse life forms, from microscopic plankton to large marine mammals, each dependent on water for survival.
- Germination and Photosynthesis: Water is crucial for seed germination, as it activates metabolic processes necessary for seed growth. Additionally, it plays a key role in photosynthesis, the process by which plants convert light energy into chemical energy, facilitating the production of glucose and oxygen.
- Transport of Nutrients and Minerals: Water acts as a medium for the transport of essential minerals from the soil to various parts of plants. It enables the movement of nutrients through xylem vessels, ensuring that all plant cells receive the necessary elements for growth and development.
- Structural Support in Plants: Water contributes to maintaining the structural integrity of plant tissues. By exerting turgor pressure within the plant cells, it helps support plant structures, preventing wilting and ensuring upright growth.
- Formation of Biological Membranes: Water interacts with various organic compounds to contribute to the formation of biological membranes. The unique properties of water facilitate the organization of lipids and proteins, forming bilayers essential for cellular function.
- Influence on Macromolecular Structure: Water significantly impacts the fundamental components of all cells, including DNA and proteins. Hydrogen bonding between water molecules and these macromolecules regulates the folding and stability of proteins and nucleic acids, ensuring their proper function.
- Support for DNA Structure: The double helix structure of DNA is stabilized by water molecules, which surround the DNA in an organized manner. This hydration layer not only protects the DNA but also plays a vital role in the interactions necessary for replication and transcription.
Practice Flashcard
[flashcard id=”61896″]
Practice MCQ Quiz
[mcq_display ids=”61907,61902,61908,61905,61901,61904,61903,61906,61899,61900″]
- https://byjus.com/chemistry/physical-and-chemical-properties-of-water/
- https://chemistrytalk.org/properties-water-physical-chemical/
- https://library.fiveable.me/ap-bio/unit-1/elements-life/study-guide/kLZ8GN081XmAmZpivYFN
- https://www.khanacademy.org/science/ap-biology/chemistry-of-life/structure-of-water-and-hydrogen-bonding/a/hydrogen-bonding-in-water
- Hydrogen-Bonding and Water. (2022, November 14). Simon Fraser University. https://chem.libretexts.org/@go/page/3579
- http://www.esalq.usp.br/lepse/imgs/conteudo_thumb/Hydrogen-Bonding-in-Water.pdf
- https://www.khanacademy.org/science/ap-biology/chemistry-of-life/structure-of-water-and-hydrogen-bonding/e/structure-of-water-and-hydrogen-bonding-exercise
- https://inspiritvr.com/structure-of-water-and-hydrogen-bonding-study-guide/
- Structure of Water. (2022, August 9). https://chem.libretexts.org/@go/page/53837
- https://water.lsbu.ac.uk/water/water_hydrogen_bonding.html
- https://alevelbiology.co.uk/notes/water/
- https://studymind.co.uk/notes/hydrogen-bonding-in-water/
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.