The starch hydrolysis test is a biochemical test which is used to determine the ability of microorganism to hydrolyze starch. It is also referred to as amylase test. It is the process in which extracellular enzymes are produced by the bacteria to break down starch into simpler sugars. These enzymes include α-amylase and oligo-1,6-glucosidase. Starch being a complex polysaccharide composed of amylose and amylopectin cannot pass through bacterial cell membrane. Therefore the enzymes is secreted outside the cell to hydrolyze starch into smaller molecules like glucose and maltose which can be absorbed and utilized.
In this test the organism is inoculated on a medium containing starch such as starch agar and incubated for enzyme production. After incubation the plate is flooded with iodine solution. Iodine reacts with intact starch to form a dark blue or black colour. If starch is hydrolyzed around the bacterial growth a clear zone is observed surrounding the colony. This is referred to as positive starch hydrolysis test. In negative result the blue black colour extends up to the margin of growth indicating starch remains undigested. This test is commonly used in differentiation of bacterial genera such as Bacillus, Clostridium, Enterococcus and Streptococcus.
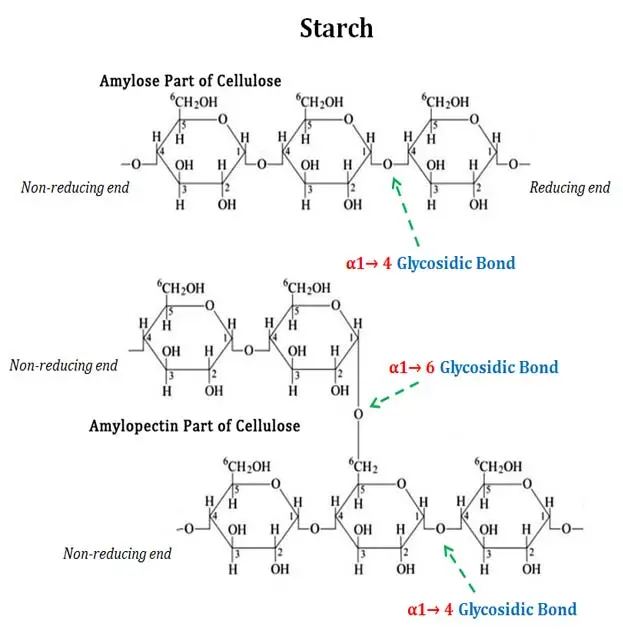
Objectives of Starch Hydrolysis Test
- To determine the ability of microorganism to produce extracellular enzymes such as α-amylase and oligo-1,6-glucosidase.
- To find out whether the organism can hydrolyse starch into simpler water soluble sugars like glucose maltose and dextrins for utilisation as source of carbon and energy.
- To differentiate bacteria on the basis of their starch hydrolysing ability especially among amylolytic and non-amylolytic organisms.
- To help in identification and differentiation of bacterial genera based on starch hydrolysis reaction.
- To differentiate starch positive organisms such as Streptococcus bovis from other bile esculin positive streptococci and enterococci which are starch negative.
- To distinguish starch hydrolysing organisms like Chryseobacterium indologenes from non-hydrolysing organisms such as Elizabethkingia meningoseptica.
- To aid in differentiation of Clostridium perfringens which is starch positive from other Clostridium species like C. difficile and C. sordellii which are starch negative.
Starch Hydrolysis test principle
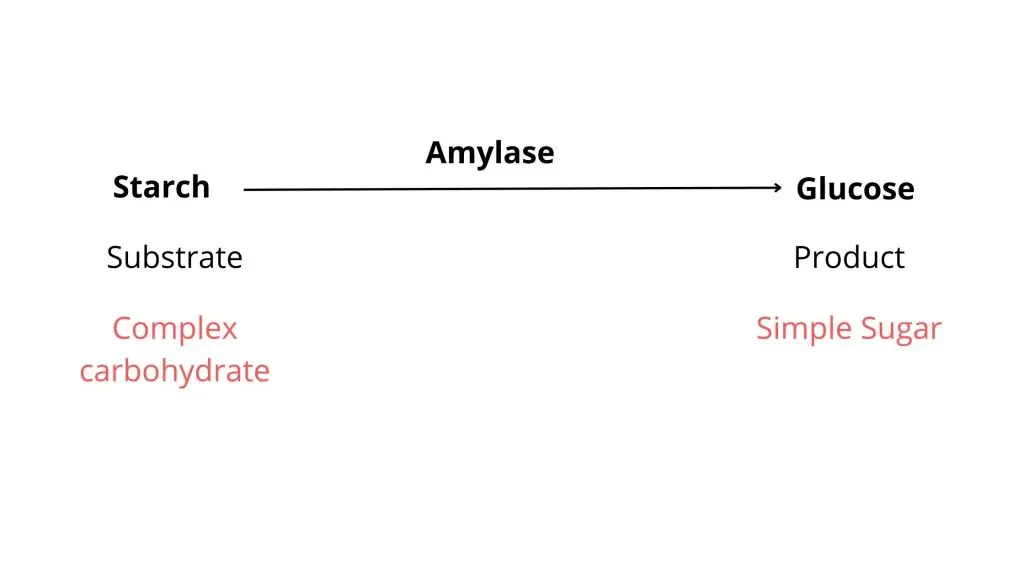
It is the process used to detect the ability of bacteria to hydrolyse starch by producing extracellular enzymes. Starch is a complex polysaccharide which is made up of amylose and amylopectin molecules. These starch molecules are large in size and cannot pass through the bacterial cell membrane. Therefore the organism secretes enzymes like α-amylase and oligo-1,6-glucosidase outside the cell which helps in breaking down starch into simpler sugars such as glucose maltose and dextrins that can be taken inside the cell and utilised for metabolism.
In this test the organism is inoculated on a medium containing starch and incubated for a specific period. After incubation iodine solution is added over the surface of the agar medium. Iodine reacts with the unhydrolysed starch to form a blue or black coloured complex. If the organism produces amylase enzyme the starch around the growth is hydrolysed and does not react with iodine resulting in a clear zone around the colony which is referred to as positive result. If the medium turns blue or black up to the margin of growth it indicates that starch is not hydrolysed and the organism is incapable of producing starch hydrolysing enzyme.
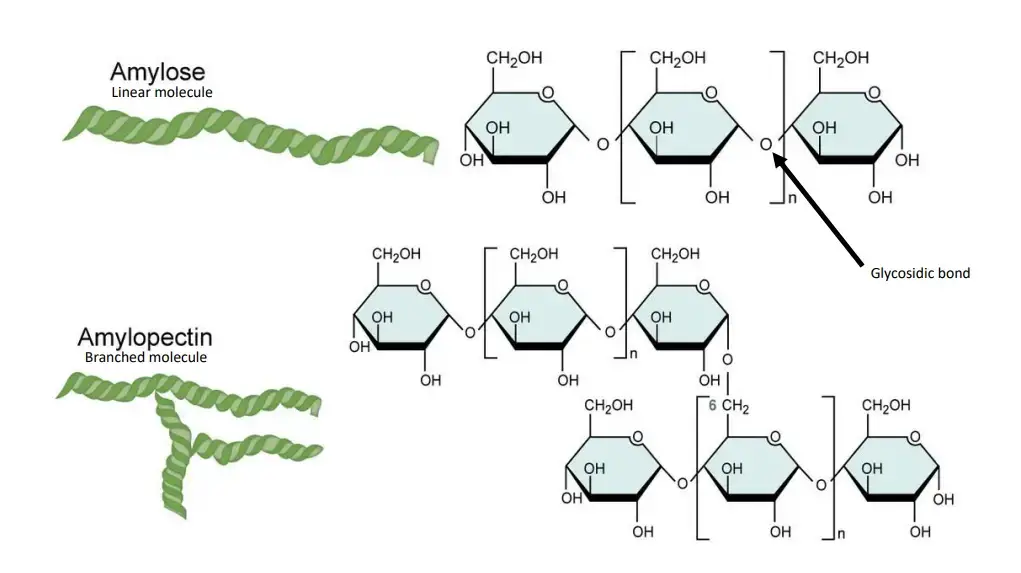
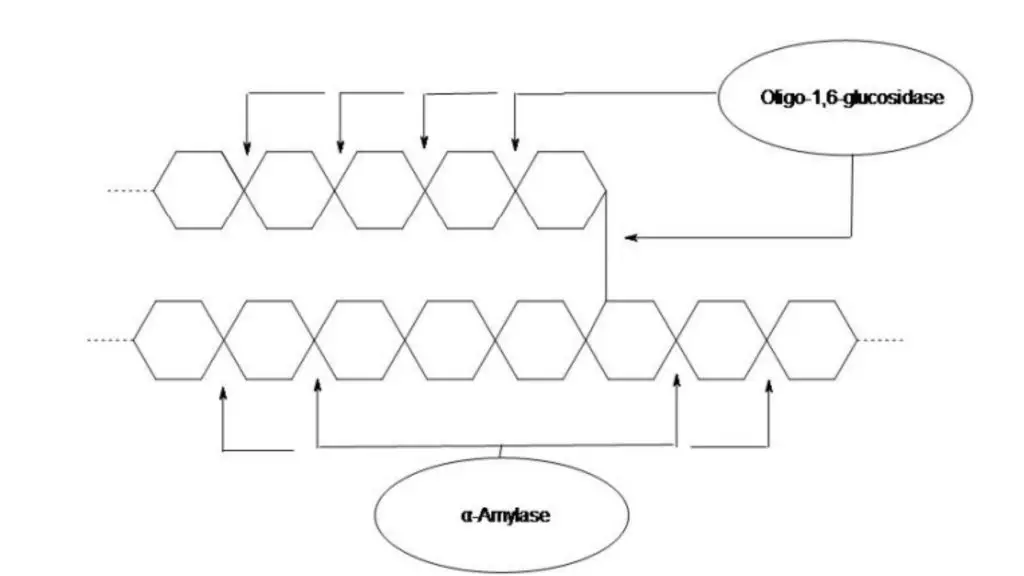
Requirement
- Starch agar medium containing soluble starch for detection of starch hydrolysis.
- Mueller Hinton agar may also be used as it contains amylose (starch).
- Heart infusion agar supplemented with about 2% starch can be used.
- Gram’s iodine or Lugol’s iodine solution for detection of unhydrolysed starch.
- Sterile petri plates for pouring and solidifying the medium.
- Inoculating loop for transfer and streaking of the organism.
- Bunsen burner to maintain aseptic conditions during inoculation.
- Incubator maintained at 35°C to 37°C for incubation of culture.
- Dropper for flooding the iodine solution over the agar surface.
- Autoclave for sterilization of media and glassware.
- Weighing machine for measuring media components.
- Test organism which should be a fresh pure culture of 18–24 hours.
- Positive control organism such as Bacillus subtilis Bacillus cereus or Streptococcus bovis.
- Negative control organism such as Escherichia coli or Staphylococcus aureus.
Starch hydrolysis test Media Preparation
1. Starch Agar
Composition (per litre)
- Beef extract – 3.0 g
- Soluble starch – 10.0 g
- Agar – 12.0 g
- Distilled water – 1000 mL
- Final pH – 7.5±0.2
Procedure
- Weigh the required quantity of ingredients and suspend in distilled water.
- Mix properly and heat with frequent agitation until the medium is completely dissolved.
- Care should be taken not to boil the medium excessively as starch may get hydrolysed.
- Sterilise the medium by autoclaving at 121°C for 15 minutes.
- Cool the medium to about 40–50°C and pour into sterile petri plates.
- Allow the plates to solidify and store for further use.
2. Mueller Hinton Agar
Composition (per litre)
- Beef extract – 2.0 g
- Acid hydrolysate of casein – 17.5 g
- Starch – 1.5 g
- Agar – 17.0 g
- Distilled water – 1000 mL
- Final pH – 7.3±0.1
Procedure
- Suspend about 38 g of Mueller Hinton agar powder in one litre of distilled water.
- Heat the medium with continuous stirring and boil gently until completely dissolved.
- Sterilise by autoclaving at 121°C for 15 minutes.
- Cool to around 45°C and pour into sterile petri dishes to uniform depth.
- Allow the medium to solidify before inoculation.
3. Starch Overlay Method (Optional)
- Dissolve 1 g agar and 1 g starch in 100 mL distilled water.
- Heat and boil until the solution becomes clear.
- Cool to about 45°C and pour a thin layer over pre solidified nutrient agar plates.
- Allow the overlay to solidify before use.
Starch hydrolysis test procedure
- Take a sterile petri plate containing starch agar or Mueller Hinton agar medium.
- Label the bottom of the plate properly with the name or number of the test organism.
- Using aseptic technique pick a small amount of the test organism from a fresh pure culture of 18–24 hours with a sterile inoculating loop.
- Inoculate the medium by making a single straight line streak or spot inoculation at the centre of the plate.
- Incubate the inoculated plates in inverted position at 35°C to 37°C for 24–48 hours.
- After incubation place the plate on a clean white background.
- Flood the surface of the agar with Gram’s iodine or Lugol’s iodine solution using a dropper.
- Allow the iodine to react with the medium for about 30 seconds to 1 minute.
- Pour off the excess iodine solution gently.
- Observe the plate immediately for the appearance of clear zone around the growth or colour development in the medium.
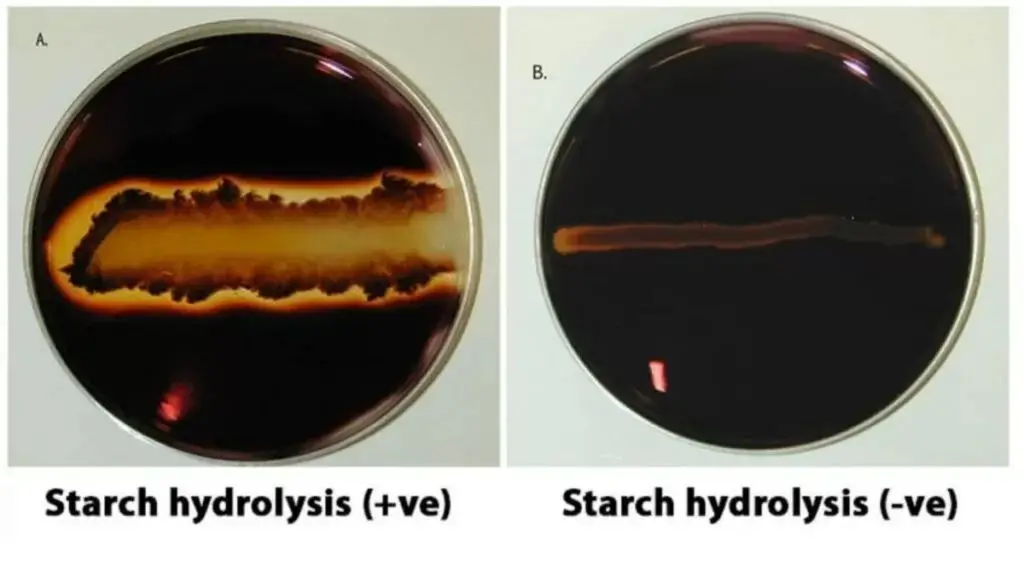
Starch Hydrolysis Test Results
Positive Result (+)– A clear colourless or pale yellow zone is observed around the bacterial growth after addition of iodine solution. It indicates that the organism produces extracellular enzymes such as α-amylase and oligo-1,6-glucosidase. These enzymes hydrolyse the starch present in the medium into smaller sugars like dextrins maltose and glucose which do not react with iodine. Hence a clear zone is formed around the colony.
Negative Result (−)– The medium turns dark blue purple or black colour up to the margin of bacterial growth. It indicates that the organism does not produce starch hydrolysing enzymes. The starch remains intact in the medium and reacts with iodine to form a coloured complex resulting in no clear zone around the growth.
Partial or Intermediate Result– A reddish purple or violet coloured zone is seen around the colony. It indicates partial hydrolysis of starch where it is broken down into intermediate products known as erythrodextrins. In such cases the test may be repeated with longer incubation period for confirmation.
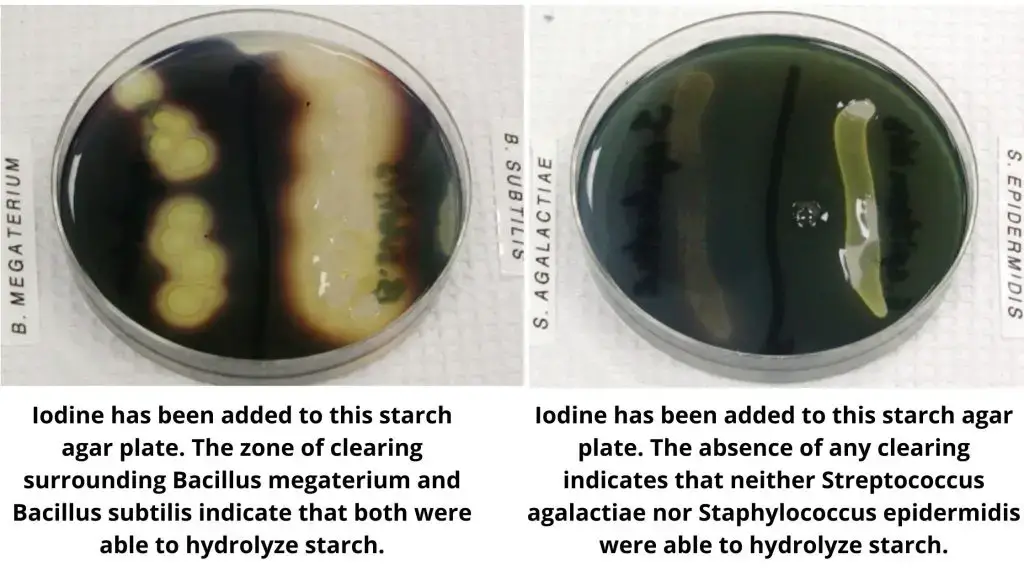
Organisms Showing Starch Hydrolysis Test Reaction
Starch Hydrolysis Positive (+)
- Bacillus subtilis
- Bacillus cereus
- Bacillus megaterium
- Clostridium perfringens
- Streptococcus bovis
- Chryseobacterium indologenes
- Pseudomonas stutzeri
Starch Hydrolysis Negative (−)
- Escherichia coli
- Staphylococcus aureus
- Staphylococcus epidermidis
- Pseudomonas aeruginosa
- Pseudomonas putida
- Enterococcus faecalis
- Streptococcus agalactiae
- Corynebacterium diphtheriae
- Clostridium difficile
- Clostridium botulinum
- Clostridium sordellii
- Elizabethkingia meningoseptica
Uses of Starch Hydrolysis Test
- It is used to determine the ability of organism to produce and secrete extracellular enzymes like α-amylase and oligo-1,6-glucosidase which helps in hydrolysis of starch into maltose glucose and dextrin.
- It is used as a biochemical test for differentiation of bacterial genera such as Bacillus Clostridium Corynebacterium Fusobacterium Enterococcus Pseudomonas and Streptococcus.
- It is used in identification of Streptococcus bovis as it shows starch positive reaction while other viridans streptococci and enterococci remains starch negative.
- It is used to differentiate Clostridium species by distinguishing starch positive Clostridium perfringens from other species like Clostridium difficile Clostridium sordellii and Clostridium botulinum.
- It is used for differentiation of Pseudomonas species where Pseudomonas stutzeri shows ability to hydrolyze starch unlike Pseudomonas aeruginosa.
- It is used to separate Chryseobacterium indologenes which is starch positive from Elizabethkingia meningoseptica which is starch negative.
- It is used in industrial and biotechnological screening to isolate high amylase producing bacteria especially Bacillus species from soil and sediment samples.
- It is also used for general detection of starch based on iodine reaction in food samples and in plant leaves during photosynthesis experiments.
Advantages of Starch Hydrolysis Test
- It is a standard biochemical test used for broad differentiation of bacterial genera such as Bacillus Clostridium Corynebacterium Fusobacterium Enterococcus Streptococcus and Pseudomonas.
- It is important in clinical diagnosis as it helps in distinguishing Streptococcus bovis which is starch hydrolysis positive from other bile esculin positive organisms like enterococci and viridans streptococci which are starch negative.
- It is useful in identification of anaerobic bacteria by differentiating Clostridium perfringens which shows consistent positive reaction from Clostridium difficile and Clostridium botulinum which are starch negative.
- It is cost effective for laboratory use as Mueller Hinton agar already contains starch and can be used without preparation of special media.
- It helps in differentiation of closely related and similar looking pathogens such as Chryseobacterium indologenes and Elizabethkingia meningoseptica especially in hospital and neonatal settings.
- It gives clear and definite visual result in the form of clear zone around growth after iodine addition which makes interpretation easy.
- It helps in metabolic characterization of organism by determining the ability to utilize complex polysaccharides like starch as a source of carbon and energy.
Limitations of Starch Hydrolysis Test
- It is not a confirmatory test and cannot be used alone for complete identification of organism and must be supported by other biochemical or molecular tests.
- Addition of iodine reagent is toxic to bacteria and kills the organism therefore colonies cannot be subcultured or reused for further testing.
- Presence of high glucose concentration in medium may suppress amylase production due to catabolite repression leading to false negative result.
- The result is time sensitive and must be observed immediately after iodine addition as the blue black colour fades with time causing wrong interpretation.
- The test requires longer incubation period usually up to 48 hours and early reading may give false negative reaction.
- Swarming or highly motile bacteria like Proteus species may spread over agar surface making it difficult to locate clear zone accurately.
- Partial hydrolysis produces reddish violet or purple colour due to erythrodextrins which makes interpretation difficult and may require repeat testing.
- Starch agar may degrade on prolonged storage or refrigeration which can lead to false positive or unclear results.
Quality Control Organisms of Starch Hydrolysis Test
Positive Control Organisms (Starch Hydrolysis Positive)
- Bacillus cereus
- Bacillus subtilis
- Streptococcus bovis
- Clostridium perfringens (used for anaerobic testing)
- Pseudomonas stutzeri
- Chryseobacterium indologenes
Negative Control Organisms (Starch Hydrolysis Negative)
- Escherichia coli
- Staphylococcus aureus
- Enterococcus faecalis
- Staphylococcus epidermidis
- Clostridium sordellii (used for anaerobic testing)
- Serratia marcescens
- Streptococcus agalactiae
Precautions of Starch Hydrolysis Test
- The culture should be incubated for at least 48 hours before adding iodine reagent as early testing may give false negative result.
- The result should be read immediately after addition of iodine because blue black colour fades with time leading to wrong interpretation.
- Media containing high concentration of glucose should be avoided as glucose suppresses amylase production and gives false negative reaction.
- When multiple organisms are tested on same plate the streaks should be kept well separated to prevent overlapping of hydrolysis zones.
- Swarming organisms like Proteus species should not be tested on common plates and should be inoculated separately.
- Iodine reagent kills the organism therefore subculturing should be done before adding iodine if further testing is required.
- Starch agar should not be overheated or over autoclaved during preparation as heat may hydrolyze starch non enzymatically.
- Old or improperly stored starch agar plates should not be used as degradation of starch may give unreliable result.
- Appearance of reddish purple or violet zone indicates partial hydrolysis and the test should be repeated after longer incubation.
- Ali, S. A. M. (n.d.). Carbohydrate chemistry (Disaccharides & homopolysaccharides) [Lecture notes]. Department of Biochemistry, Benha University.
- Aryal, S. (2022, August 10). Mueller Hinton Agar (MHA) – Composition, principle, uses and preparation. Microbiology Info.
- Aryal, S. (2022, August 10). Starch hydrolysis test – Principle, procedure, uses and interpretation. Microbiology Info.
- Biology Online. (2022, June 16). Iodine test. Biology Online Dictionary.
- BioTopics. (n.d.). Interaction of iodine with the amylose component of starch.
- Comprehensive technical analysis of the starch hydrolysis test: Biochemical principles, laboratory methodologies, and clinical diagnostic applications. (n.d.).
- Etherington, D., & Roberts, M. (n.d.). Dextrin. In Bookbinding and the conservation of books: A dictionary of descriptive terminology.
- Hartline, R. (2023, February 18). 1.17: Starch hydrolysis. Biology LibreTexts.
- Ho, P.-L., Ho, L.-Y., Yau, C.-Y., Tong, M.-K., & Chow, K.-H. (2017). A novel selective medium for isolation of Bacteroides fragilis from clinical specimens. Journal of Clinical Microbiology, 55(2), 384–390. https://doi.org/10.1128/JCM.01988-16
- iGEM Team Brno Czech Republic. (2021). Protocol: AmyE test (Starch hydrolysis test).
- InformationBoxTicket Lifestyles. (n.d.). Performing the starch hydrolysis test in the microbiology lab – Biochemical tests 101 [Video]. YouTube.
- Lal, A., & Cheeptham, N. (2012, November 1). Starch agar protocol. American Society for Microbiology.
- Lalucat, J., Bennasar, A., Bosch, R., García-Valdés, E., & Palleroni, N. J. (2006). Biology of Pseudomonas stutzeri. Microbiology and Molecular Biology Reviews, 70(2), 510–547. https://doi.org/10.1128/MMBR.00047-05
- Lee, W.-S. (1976). Use of Mueller-Hinton agar as amylase testing medium. Journal of Clinical Microbiology, 4(3), 312.
- Pesek, S., Lehene, M., Brânzanic, A. M. V., & Silaghi-Dumitrescu, R. (2024). The iodine/iodide/starch supramolecular complex. Molecules, 29(3), 641. https://doi.org/10.3390/molecules29030641
- Rijal, N. (n.d.). Starch hydrolysis test: Principle, procedure, results. Microbe Online.
- Schmidt, J., & John, M. (1979). Starch metabolism in Pseudomonas stutzeri: I. Studies on maltotetraose-forming amylase. Biochimica et Biophysica Acta, 566(1), 88–99. https://doi.org/10.1016/0005-2744(79)90252-3
- Sigmon, J. (2008, September 1). The starch hydrolysis test. American Society for Microbiology.
- Starch hydrolysis test. (n.d.).
- Tariq, A. L., Sudha, S., & Reyaz, A. L. (2016). Isolation and screening of Bacillus species from sediments and application in bioremediation. International Journal of Current Microbiology and Applied Sciences, 5(6), 916–924. https://doi.org/10.20546/ijcmas.2016.506.099
- Thermo Fisher Scientific. (2009). Starch hydrolysis agar [Instructions for use].
- TM Media. (2024, January 30). Mueller Hinton Agar: A comprehensive guide for microbiological applications.
- Tu, N., Vinh, D., & Thu, L. (2015). Amylase producing Bacillus megaterium T04 isolated in Rach Lang stream of Vietnam. Journal of Applied Pharmaceutical Science, 5(10), 012–015. https://doi.org/10.7324/JAPS.2015.501003
- VetBact. (2024, November 19). Pseudomonas aeruginosa.
- VUMIE. (2022, November 1). Starch hydrolysis test.
- Wikipedia contributors. (n.d.). Pseudomonas stutzeri. Wikipedia.