What is Simmons Citrate Agar?
- Simmons Citrate Agar is a specialized medium used in microbiology to differentiate gram-negative bacteria based on their ability to utilize citrate as a sole carbon source. It is a selective and differential medium that helps identify organisms capable of citrate utilization, making it a valuable tool in the field of microbial identification and classification.
- The development of Simmons Citrate Agar was influenced by the work of S. A. Koser, who recognized the significance of citrate utilization in distinguishing between different types of coliforms. Koser’s original citrate medium allowed the growth of Enterobacter group organisms that could use citrate as their primary carbon and energy source, while fecal coliforms like Escherichia coli were unable to grow in this medium.
- However, Koser’s citrate medium had a drawback in that heavy inoculums could lead to a false appearance of growth. To overcome this limitation, Simmons modified Koser’s formulation by incorporating agar and bromothymol blue (BTB) into the medium. This modified formulation is known as Simmons Citrate Agar.
- The addition of agar in Simmons Citrate Agar transformed the medium into a solid slant, enabling easier detection of significant growth. The solid surface also increases the amount of growth by providing more available oxygen for citrate utilization. The presence of bromothymol blue, a pH indicator, allows for the detection of pH changes in the medium resulting from citrate breakdown. A positive reaction is indicated by the color change of the BTB to an intense blue.
- Simmons Citrate Agar is widely used in the IMViC (Indole, Methyl Red, Voges-Proskauer, Citrate) tests, which are a set of biochemical tests used to differentiate members of the Enterobacteriaceae family. In the context of Simmons Citrate Agar, a positive reaction refers to the growth of organisms with an intense blue color in the slant. Organisms that show positive growth on this medium include Klebsiella, Enterobacter, Citrobacter, Providencia, Proteus, Serratia, Vibrio cholerae, Pseudomonas, Salmonella enteritidis, and members of the subgenera Salmonella II, III, and IV.
- In summary, Simmons Citrate Agar is a selective and differential medium used to determine the ability of organisms to utilize citrate as their sole carbon source. It overcomes the limitations of Koser’s original citrate medium by incorporating agar and bromothymol blue, facilitating easier detection and interpretation of results.
Composition of Simmons Citrate Agar
| Ingredients | Gms/liter |
| Magnesium sulfate | 0.200 |
| Ammonium dihydrogen phosphate | 1.000 |
| Dipotassium phosphate | 1.000 |
| Sodium citrate | 2.000 |
| Sodium chloride | 5.000 |
| Bromothymol blue | 0.080 |
| Agar | 15.000 |
Principle of Simmons Citrate Agar
The principle of Simmons Citrate Agar revolves around the ability of microorganisms to utilize citrate as a source of energy. In this medium, citrate is the sole carbon source, and ammonium dihydrogen phosphate serves as the sole nitrogen source. The presence of dipotassium phosphate acts as a buffer, sodium chloride maintains osmotic balance, and magnesium sulfate functions as a cofactor for various metabolic reactions. Bromothymol blue is used as the pH indicator, and bacteriological agar solidifies the medium.
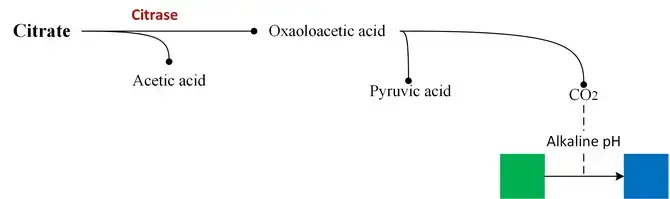
Organisms that possess the enzyme citrate-permease, capable of converting citrate to pyruvate, can grow unrestricted on Simmons Citrate Agar. As the microbe utilizes citrate, alkaline/basic byproducts accumulate. Citrate serves as an intermediate metabolite in the Krebs cycle. The breakdown of citrate by the enzyme citrase produces oxaloacetic acid and acetic acid. These products are further metabolized to pyruvic acid and carbon dioxide. The released carbon dioxide combines with sodium and water to form sodium carbonate, which is an alkaline compound. The increase in pH due to alkaline byproducts is indicated by a color change in the pH indicator.
The pH indicator bromothymol blue is initially green in the medium at a neutral pH. However, when the pH rises above 7.6, as a result of citrate utilization, the bromothymol blue changes to blue, indicating a positive test for citrate utilization.
It is important to note that while citrate utilization is typically associated with members of the Enterobacteriaceae family, such as Enterobacter and Klebsiella, there have been rare cases of citrate-positive variants of Escherichia coli and citrate-negative strains of Enterobacter aerogenes found.
In summary, the principle of Simmons Citrate Agar is based on the ability of microorganisms to utilize citrate as a source of energy. The utilization of citrate leads to the accumulation of alkaline byproducts, which raise the pH of the medium. The color change of the pH indicator, bromothymol blue, from green to blue above a pH of 7.6 indicates a positive test for citrate utilization.
Preparation of Simmons Citrate Agar
To prepare Simmons Citrate Agar, follow these steps:
- Suspend 24.28 grams of Simmons Citrate Agar powder in 1000 ml of distilled water.
- Heat the mixture while stirring until it reaches a boiling point, ensuring complete dissolution of the medium.
- Thoroughly mix the solution to ensure uniform distribution of the components.
- Dispense the medium into tubes or flasks. For tubes, dispense 4.0 to 5.0 ml into 16-mm tubes.
- Sterilize the tubes or flasks containing the medium by autoclaving at 15 lbs pressure (121°C) for 15 minutes.
- After autoclaving, cool the tubes or flasks in a slanted position, allowing the medium to solidify into a slant with a shallow butt.
- Store the tubes or flasks in a refrigerator to maintain their quality and extend their shelf life. The medium can typically be stored for 6 to 8 weeks.
After preparation, the uninoculated medium will appear as a deep forest green due to the pH of the sample and the presence of the pH indicator, bromothymol blue. This color will change when the medium is inoculated with organisms capable of utilizing citrate as a carbon source.
Method of Use of Simmons Citrate Agar
To use Simmons Citrate Agar for testing the ability of an organism to utilize citrate, follow the method outlined below:
A. Inoculation of medium:
- Prepare the Simmons Citrate Agar medium as described in the preparation section or according to the manufacturer’s instructions.
- Allow the tubes to reach room temperature before inoculation.
- Use a fresh pure culture that is 16 to 18 hours old as the inoculation source.
- Take a single isolated colony from the culture and lightly streak the surface of the slant using a needle. This helps limit the amount of cell material transferred to the agar.
- Avoid using liquid cultures as the inoculum source.
- If screw caps are used, keep them loosely placed on the tube to allow for oxygen supply since citrate utilization requires oxygen.
B. Incubation conditions:
- Incubate the tubes at 35°C (+/- 2°C) for 18 to 48 hours. Some organisms may require up to 7 days of incubation due to their slower growth rate on citrate medium.
C. Interpretation of results:
- Citrate positive: If the organism has the ability to utilize citrate as a sole carbon and energy source, visible growth will appear on the slant surface. The medium will turn into an intense Prussian blue color. The alkaline carbonates and bicarbonates produced during citrate catabolism raise the pH of the medium above 7.6, leading to a color change in the pH indicator bromothymol blue from green to blue.
- Citrate negative: If there is only trace or no visible growth, and the medium remains the deep forest green color of the uninoculated agar, it indicates a citrate-negative result. Bacteria that cannot utilize citrate as the sole carbon and energy source will not grow on Simmons Citrate Agar, making the test culture indistinguishable from an uninoculated slant.
Alternative Methods to Test for Citrate Utilization: Apart from Simmons Citrate Agar, other citrate utilization media exist, such as Koser’s citrate medium and Christensen’s citrate medium.
- Koser’s citrate medium and Simmons citrate medium both contain citrate as the only carbon source. Growth in either medium indicates the ability of the test organism to use citrate as a sole carbon source. However, Koser’s medium lacks a pH indicator, which may increase the chances of misinterpretation. Simmons’ medium is commonly used in microbiology teaching labs as part of the IMViC protocol.
- Christensen’s citrate sulfide medium is an alternative citrate test medium that does not require the organism to use citrate as a sole carbon source. It contains both peptone and cysteine, allowing growth of citrate-negative bacteria as well. A positive reaction is indicated by a color change in the medium from yellow (pH 6.7) to red at more alkaline pH values. This medium can also be used to test for the generation of hydrogen sulfide (H2S) by the organism, indicated by the formation of a black precipitate due to the reaction of H2S with iron in the medium.
Note: The detailed composition and preparation steps for Christensen’s citrate sulfide medium are provided in the last paragraph.
Result on Simmons Citrate Agar
Interpreting the results on Simmons Citrate Agar involves assessing the growth and color changes in the medium. The following interpretations can be made:
- Bacteria citrate positive: A positive reaction is indicated by visible growth on the slant surface accompanied by an intense blue color in the medium. Organisms that show positive growth on Simmons Citrate Agar include:
- Klebsiella
- Enterobacter
- Citrobacter
- Providencia
- Proteus
- Serratia
- Vibrio cholerae
- Pseudomonas
- Salmonella enteritidis
- Members of the subgenera Salmonella II, III, and IV
- Bacteria citrate negative: A negative reaction is evidenced by the absence of growth or only trace growth with no color change. The medium remains dark green. Organisms that typically exhibit a negative reaction on Simmons Citrate Agar include:
- Escherichia coli
- Shigella
- Yersinia
- Edwardsiella
It’s important to note that these interpretations are general guidelines, and there may be exceptions or variations depending on the specific strain or other factors.

Quality Control
Quality control measures for Simmons Citrate Agar can be assessed through various parameters. Here are some aspects of quality control for the medium:
- Appearance: The powdered medium should have a cream to yellow color and a homogeneous, free-flowing texture.
- Gelling: When prepared and solidified, the medium should form a firm gel comparable to a 1.5% Agar gel.
- Color and Clarity: The prepared medium should exhibit a forest green color and appear slightly opalescent as a gel in tubes when used as slants.
- pH: The pH of a 2.43% w/v aqueous solution of the medium should be within the range of 6.8 ± 0.2.
- pH Range: The pH of the medium should fall between 6.60 and 7.00.
- Cultural Response: After incubation at 35-37°C for 18-24 hours, the cultural characteristics of organisms inoculated on the medium should be observed and evaluated.
- Growth: The growth of the organisms should be assessed in terms of colony-forming units (CFU) and categorized as good-luxuriant, fair, or inhibited based on the level of growth observed.
- Citrate Utilization: The ability of the organisms to utilize citrate should be determined and recorded as positive or negative based on the color reaction observed.
Examples of Quality Control Results:
- Klebsiella aerogenes ATCC 13048: Inoculum of 50-100 CFU shows good-luxuriant growth and a positive reaction indicated by a blue color change.
- Escherichia coli ATCC 25922: Inoculum of ≥10³ CFU shows inhibition of growth on the medium.
- Salmonella Typhi ATCC 6539: Inoculum of 50-100 CFU shows fair to good growth with a negative reaction indicated by a green color.
These quality control measures help ensure the consistency and reliability of the Simmons Citrate Agar medium for accurate identification and differentiation of organisms based on their citrate utilization.
Uses of Simmons Citrate Agar
Simmons Citrate Agar has several uses in microbiology for the differentiation and identification of bacteria based on their citrate utilization ability. Some of its specific applications include:
- Differentiation of Enterobacteriaceae: Simmons Citrate Agar is commonly used to differentiate between members of the Enterobacteriaceae family and the aerogenes group based on their ability to utilize citrate as a sole carbon source. Organisms within the Enterobacteriaceae family that can grow on Simmons Citrate Agar, indicating citrate utilization, include Klebsiella, Enterobacter, Citrobacter, Providencia, Proteus, Serratia, Vibrio cholerae, Pseudomonas, Salmonella enteritidis, and members of Salmonella subgenus II, III, and IV.
- Differentiation of Salmonella strains: Simmons Citrate Agar can also be used to differentiate citrate-positive Salmonella strains from citrate-negative ones. For example, it can help distinguish citrate-positive Salmonella enteritidis and members of Salmonella subgenus II, III, and IV from citrate-negative Salmonella typhi, Salmonella paratyphi A, Salmonella pullorum, and Salmonella gallinarum. The ability of certain Salmonella strains to utilize citrate as a carbon source can provide valuable information for their identification and classification.
By utilizing Simmons Citrate Agar, microbiologists can gain insights into the metabolic capabilities of different bacteria, aiding in their differentiation and identification.
Limitations of Simmons Citrate Agar
Simmons Citrate Agar, despite its usefulness, has some limitations that need to be considered. These limitations include:
- pH of water: It is essential to ensure that the pH of the water used for preparing the medium falls within the range of 6.5 to 7.0. Failure to maintain the appropriate pH can affect the performance of the medium and may lead to unexpected deviations in the initial color of the medium.
- pH monitoring: The pH of the medium should be correctly monitored throughout the process. Any variations in pH can impact the results and interpretation of the test.
- Fresh preparation: Simmons Citrate Agar is recommended to be freshly prepared. If the medium is stored in dry conditions, changes in color may occur even before inoculation, particularly at the bottom of the slant. Freshly prepared medium helps ensure accurate and reliable results.
- Color change limitations: While a color change in the medium is generally indicative of positive citrate utilization, some organisms may be capable of growth on citrate without producing a noticeable color change. Therefore, growth itself should be considered a positive result, even in the absence of a color change.
- Inoculum source: It is crucial to use a pure overnight culture grown on a solid medium as the inoculum source for Simmons Citrate Agar. Using a broth suspension may not yield accurate results.
- Further identification: Simmons Citrate Agar provides preliminary information about citrate utilization, but for complete identification, it is recommended to perform additional biochemical, immunological, molecular, or mass spectrometry testing on colonies from a pure culture.
- Incubation with loose caps: Tubes should be incubated with loose caps to allow proper oxygen supply. Failure to do so may result in false-negative results.
- Light inoculum: When streaking the slant, it is important to use a light inoculum. Using a heavy inoculum may lead to false-positive results.
- Carryover contamination: When inoculating multiple biochemical tests from the same culture, it is advisable to inoculate Simmons Citrate Agar first or flame the inoculating needle before streaking this medium. This precaution is necessary to avoid the carryover of glucose or other nutrients onto the medium, which may cause false-positive results.
Considering these limitations and following the recommended procedures can help ensure accurate and reliable results when using Simmons Citrate Agar.
Precautions
Here are some comments and tips related to the use of Simmons Citrate Agar:
- Light Inoculum: It is crucial to use a light inoculum when streaking the slant with the bacterial culture. The presence of visible cell material from a heavy inoculum can lead to a false positive citrate test result. Using a light inoculum ensures more accurate interpretation of the results.
- Comparing Results: In educational settings, students can be instructed to perform the test using both light and heavy inocula and compare the results. This exercise helps them understand the significance of using a light inoculum for obtaining reliable and interpretable results.
- Multiple Identification Tests: It is important to remember that while Simmons Citrate Agar provides valuable information about citrate utilization, it is just one test in a battery of tests used for bacterial identification. Some natural isolates may exhibit weak or nonstandard test results. Therefore, it is crucial to perform multiple identification tests to obtain a comprehensive characterization of an unknown bacterium.
By keeping these comments and tips in mind, users of Simmons Citrate Agar can optimize the accuracy and reliability of their results, particularly in educational settings where students can gain a better understanding of the test’s principles and potential limitations.
FAQ
What is Simmons Citrate Agar?
Simmons Citrate Agar is a selective and differential medium used for testing the ability of microorganisms to utilize citrate as a sole carbon source.
How is Simmons Citrate Agar prepared?
Simmons Citrate Agar is prepared by dissolving the agar powder in distilled water, heating to dissolve completely, and distributing the medium into tubes or flasks. It is then sterilized by autoclaving.
What is the principle behind Simmons Citrate Agar?
The principle of Simmons Citrate Agar is based on the ability of organisms to utilize citrate as a source of energy. The breakdown of citrate leads to the production of alkaline byproducts, which raise the pH of the medium and cause a color change in the pH indicator.
What organisms can grow on Simmons Citrate Agar?
Organisms such as Klebsiella, Enterobacter, Citrobacter, Providencia, Proteus, Serratia, Vibrio cholerae, Pseudomonas, Salmonella enteritidis, and members of Salmonella subgenus II, III, and IV are capable of growing on Simmons Citrate Agar if they can utilize citrate as a carbon source.
How is the citrate utilization test interpreted?
A positive citrate utilization test is indicated by visible growth on the slant surface and a color change in the medium from green to blue due to the increased pH. A negative test shows no growth or minimal growth with no color change.
What are the limitations of Simmons Citrate Agar?
Some limitations include the need for a light inoculum, potential for misinterpretation with heavy inocula, variability in results with natural isolates, and the requirement for additional tests for complete identification.
Can Simmons Citrate Agar be used for differentiating Salmonella strains?
Yes, Simmons Citrate Agar can be used to differentiate citrate-positive Salmonella strains (such as Salmonella enteritidis) from citrate-negative strains (such as Salmonella typhi, Salmonella paratyphi A, Salmonella pullorum, and Salmonella gallinarum).
What is the shelf life of Simmons Citrate Agar?
To ensure optimal performance, it is recommended to store the prepared tubes in a refrigerator and use them within 6 to 8 weeks.
Are there alternative media for citrate utilization testing?
Yes, other citrate utilization media include Koser’s citrate medium and Christensen’s citrate medium, each with its own advantages and specific applications.
What should be done if an organism exhibits weak or nonstandard test results?
In cases where an organism shows weak or nonstandard results on Simmons Citrate Agar, it is advisable to perform additional identification tests, such as biochemical, immunological, molecular, or mass spectrometry testing, for a comprehensive characterization of the organism.
References
- https://asm.org/ASM/media/Protocol-Images/Citrate-Test-Protocol.pdf?ext=.pdf
- https://assets.fishersci.com/TFS-Assets/LSG/manuals/IFU454651.pdf
- https://microbiologie-clinique.com/simmons-citrate-agar.html
- https://microbiologyinfo.com/simmons-citrate-agar-composition-principle-uses-preparation-and-result-interpretation/
- https://exodocientifica.com.br/_technical-data/M099.pdf
- https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/110/305/s3681dat.pdf
- https://www.bd.com/resource.aspx?IDX=22356
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.