What is Shigella?
Shigella is a genus of Gram-negative, facultatively anaerobic, rod-shaped bacteria that is non-motile and non-spore-forming. It belongs to the Escherichia genus and was discovered by Kiyoshi Shiga in 1897. Shigella is a major cause of dysentery and primarily infects humans and certain primates, such as gorillas. This pathogen is not known to infect other mammals.
Shigella is responsible for a global health burden, with an estimated 80 to 165 million cases of shigellosis each year, leading to between 74,000 and 600,000 deaths, particularly in children in regions like sub-Saharan Africa and South Asia. It is considered one of the top four causes of moderate-to-severe diarrhea in these areas. The infection manifests as dysentery, characterized by severe diarrhea, often accompanied by blood and mucus in the stool, fever, and abdominal pain.
The Shigella genus is classified into four species, based on biochemical and serological properties: S. dysenteriae, S. flexneri, S. boydii, and S. sonnei. Each species consists of various serotypes, determined by distinct O antigens. These antigens help to identify the bacteria and classify them further.
- Shigella dysenteriae (Group A): This species is subdivided into 12 serotypes, each with unique antigens. S. dysenteriae serotype 1 is the strain originally described by Shiga and is thus known as Shiga’s bacillus. Other serotypes, 3 to 7, were identified in India and are referred to as the Large–Sachs group.
- Shigella flexneri (Group B): This species was first described by Flexner in 1900. It is characterized by mannitol fermentation and includes six serotypes (1 to 6), which are further subdivided into subtypes. This species is most commonly associated with infections in developing countries.
- Shigella boydii (Group C): Identified by Boyd in 1931, S. boydii is biochemically similar to S. flexneri but differs antigenically. This species contains 19 recognized serotypes and is found primarily in parts of Asia.
- Shigella sonnei (Group D): First described by Sonne in 1915, S. sonnei is the most common Shigella species in industrialized nations. It has 26 colicin types, classified using indicator strains, and is typically associated with milder forms of dysentery.
Shigella infections are transmitted primarily through the fecal-oral route, often via contaminated water or food. The bacteria invade the epithelial cells of the large intestine, leading to an inflammatory response. The pathogenesis of Shigella involves the production of toxins, including the Shiga toxin, which can cause cell damage and contribute to the severity of the disease.
Preventing Shigella infection involves improved sanitation, proper hand hygiene, and access to clean drinking water. Treatment typically includes rehydration therapy, and in severe cases, antibiotics may be required to control the infection. However, antibiotic resistance has been increasingly reported, complicating treatment strategies.
Geographical Distribution and Habitat of Shigella Infection
Shigella infection, known as shigellosis, is a global issue, but its prevalence and impact vary greatly depending on the region.
- Geographical Distribution
- Shigellosis occurs worldwide but is much more common in developing countries with poor sanitation.
- In industrialized nations, the incidence is lower but still exists.
- Endemic Infections:
- In developing regions, shigellosis is common across all age groups.
- All four species of Shigella contribute to the infection in these areas.
- India’s Situation:
- The most common species is S. flexneri, responsible for 50–85% of all Shigella cases.
- Other species found include S. dysenteriae (8–25%), S. sonnei (2–24%), and S. boydii (0–8%).
- Habitat
- Shigella is a strict human pathogen.
- These bacteria reside only in the large intestine of infected humans.
- Shigella species do not infect animals, which limits their spread to human hosts.
- The bacteria do not survive in environmental reservoirs and are transmitted directly between people.
Morphology of Shigella
Shigella is a type of bacteria that exhibits certain distinct morphological characteristics. These features help in its identification and classification. Here’s an overview of Shigella’s morphology:
- Shape and Size: Shigella bacteria are short, rod-shaped organisms. They typically measure about 0.5 to 1–3 micrometers in length.
- Gram-Negative: These bacteria are Gram-negative, which means they do not retain the crystal violet stain during the Gram staining process, appearing pink under the microscope.
- Nonmotile: Unlike some other bacteria, Shigella are nonmotile. They lack flagella, which are the appendages used for movement.
- Nonsporing: Shigella does not form spores, distinguishing it from some other bacteria that can produce spores as a survival mechanism.
- Noncapsulated: Shigella species do not have a protective capsule around them, unlike some bacteria that have a capsule to shield them from the immune system.
- Fimbriae: Most Shigella species, with some exceptions (such as S. flexneri serotype 6 and certain strains of other serotypes), possess fimbriae. These are hair-like structures on the bacterial surface that aid in attachment to host cells.
Cultural Characteristics and Biochemical Reactions of Shigella
Shigella exhibits a range of cultural and biochemical features that aid in its identification. These traits are important for microbiological testing and clinical diagnosis.
Cultural Characteristics
- Aerobic and Facultative Anaerobic Growth: Shigella can grow both in the presence of oxygen and in its absence, making it a facultative anaerobe.
- Temperature and pH Range: These bacteria grow best at 37°C (body temperature), with a growth range from 10°C to 40°C. The optimal pH for growth is around 7.4.
- Growth on Nutrient Agar: On nutrient agar, Shigella forms small, circular, convex, smooth, and translucent colonies after overnight incubation. Some colonies may also appear rough, especially in subcultures.
- Growth on MacConkey Agar: On MacConkey agar, Shigella colonies appear colorless as they do not ferment lactose. However, S. sonnei will form pale pink colonies after extended incubation due to its delayed lactose fermentation.
- Selective Media:
- Deoxycholate Citrate Agar (DCA): This medium is effective for isolating Shigella from feces. On DCA, Shigella colonies are small and may turn pink upon prolonged incubation due to lactose fermentation.
- Xylose Lysine Deoxycholate (XLD) Agar: On XLD, Shigella species typically form red colonies, with minimal inhibition to S. dysenteriae and S. flexneri.
- Salmonella–Shigella (SS) Agar: Shigella colonies on SS agar are colorless.
- Hektoen Enteric (HE) Agar: Shigella on HE agar forms green colonies.
- Liquid Media: For enrichment, media like Selenite F broth and Gram-negative (GN) broth are often used. Fecal specimens enriched in GN broth for 4–6 hours, followed by subculture on selective media like XLD or HE, help isolate Shigella from clinical samples.
Biochemical Reactions
- Mannitol Fermentation: Shigella ferments mannitol, producing acid but no gas. This test differentiates mannitol-fermenting species from non-fermenters:
- Mannitol-fermenting species: S. flexneri, S. boydii, S. sonnei.
- Mannitol-nonfermenting species: S. dysenteriae.
- Glucose Fermentation: Shigella ferments glucose, producing acid but no gas. Some exceptions exist, like certain biotypes of S. flexneri type 6 and some strains of S. boydii, which do not ferment glucose.
- Lactose, Sucrose, and Other Carbohydrates: Shigella species do not ferment lactose, sucrose, salicin, adonitol, or inositol. However, S. sonnei may ferment lactose and sucrose late.
- Nitrate Reduction: Shigella can reduce nitrates to nitrites, but it does not produce hydrogen sulfide (H2S).
- Biochemical Test Results:
- Methyl Red (MR) Test: Shigella is MR positive, indicating acid production.
- Citrate Test: Shigella is citrate negative, meaning it does not utilize citrate as its sole carbon source.
- Oxidase Test: Shigella is oxidase negative.
- Catalase Test: Shigella is catalase positive, except for S. dysenteriae type 1, which is catalase negative.
Cell Wall Components and Antigenic Structure of Shigella
Shigella’s cell wall structure and antigenic components play key roles in its pathogenicity and classification. These features help identify and differentiate Shigella species in clinical settings.
Cell Wall Components
- Lipopolysaccharide (LPS): The cell wall of Shigella contains lipopolysaccharide (LPS), a key feature of Gram-negative bacteria.
- Endotoxin: The LPS functions as an endotoxin, contributing to the virulence of Shigella. This endotoxin is released during cell lysis and, to a lesser extent, during bacterial culture.
Antigenic Structure
- Somatic O Antigens: Shigella species possess somatic O antigens, which are used for serological identification and differentiation.
- K Antigens: Some strains of Shigella also have K antigens. These antigens can interfere with the agglutination reaction when testing with O antisera.
- Fimbrial Antigens: Shigella strains may carry fimbrial antigens, which are often present in S. flexneri. These antigens are important in the attachment and colonization of host cells.
- S. flexneri’s Complex Antigenic Structure:
- S. flexneri is the most antigenically diverse Shigella species.
- This species is classified into serotypes (I-VI) and subtypes (e.g., 1a, 1b; 2a, 2b) based on type-specific and group-specific antigens.
- It has additional X and Y variants, which lack type-specific antigens.
- S. flexneri serotype 6 is indole-negative and is further divided into three biotypes: Boyd 88, Manchester, and Newcastle.
- S. sonnei’s Homogeneous Antigenic Profile:
- Unlike S. flexneri, S. sonnei exhibits a homogeneous antigenic structure.
- It can exist in two phases: Phase I and Phase II.
- Phase I strains produce smooth colonies.
- Phase II strains form larger, flatter, and more irregular colonies.
- Phase II strains are more commonly found in convalescent cases and carriers than in active infections.
Virulence Factors of Shigella
Shigella is a notorious pathogen known for its ability to cause severe intestinal infections. Its virulence hinges on multiple factors, which allow it to invade host tissues, evade immune defenses, and cause damage. These factors are encoded by both chromosomal and plasmid genes.
- Endotoxins (LPS)
- The lipopolysaccharide (LPS) component of Shigella acts as an endotoxin.
- This endotoxin plays a key role in Shigella’s ability to invade tissues, replicate, and survive within the host.
- It helps the bacteria resist phagocytosis by tissue macrophages, a critical immune defense.
- Endotoxins enhance the cytotoxic effect of Shiga toxin, especially on human vascular endothelial cells.
- The genes responsible for producing this endotoxin are located on the chromosome of Shigella species.
- Intestinal Adherence Factor
- The intestinal adherence factor is a 97-kDa outer membrane protein, encoded by chromosomal genes.
- This protein is crucial for Shigella’s colonization in both human hosts and animal models.
- It ensures that Shigella can stick to and survive in the intestines during infection.
- Shiga Toxin
- Shiga toxin, produced by S. dysenteriae, is a potent exotoxin.
- It’s a heat-labile protein that functions both as an enterotoxin and a neurotoxin.
- The toxin is made up of two primary groups: Stx1 and Stx2, each with distinct immunological properties.
- These toxins are encoded by a bacteriophage integrated into the bacterial chromosome.Structure of Shiga Toxin:
- Composed of one A subunit and five B subunits.
- The B subunits bind to a surface receptor (Gb3) on host cell glycolipids, typically in the intestinal epithelium.
- Once bound, the A subunit enters the cell and disrupts protein synthesis by cleaving 28S rRNA in the 60S ribosomal subunit.Toxic Effects of Shiga Toxin:
- Neurotoxic Activity: Though termed “neurotoxin,” Shiga toxin primarily affects blood vessels, leading to vascular damage. Neurological symptoms are secondary effects.
- Enterotoxic Activity: In experimental settings, Shiga toxin causes fluid accumulation in rabbit intestines, mimicking the diarrhea and dysentery seen in human infections.
- Cytotoxic Activity: The toxin shows cytotoxic effects on various cells, including Vero and HeLa cells, and can damage human endothelial cells, such as renal vascular cells.
- Key Manifestations
- The primary clinical consequence of Shiga toxin is damage to the intestinal lining, leading to severe diarrhea and dysentery.
- In rare cases, the toxin can also damage the kidneys, triggering hemolytic uremic syndrome (HUS).
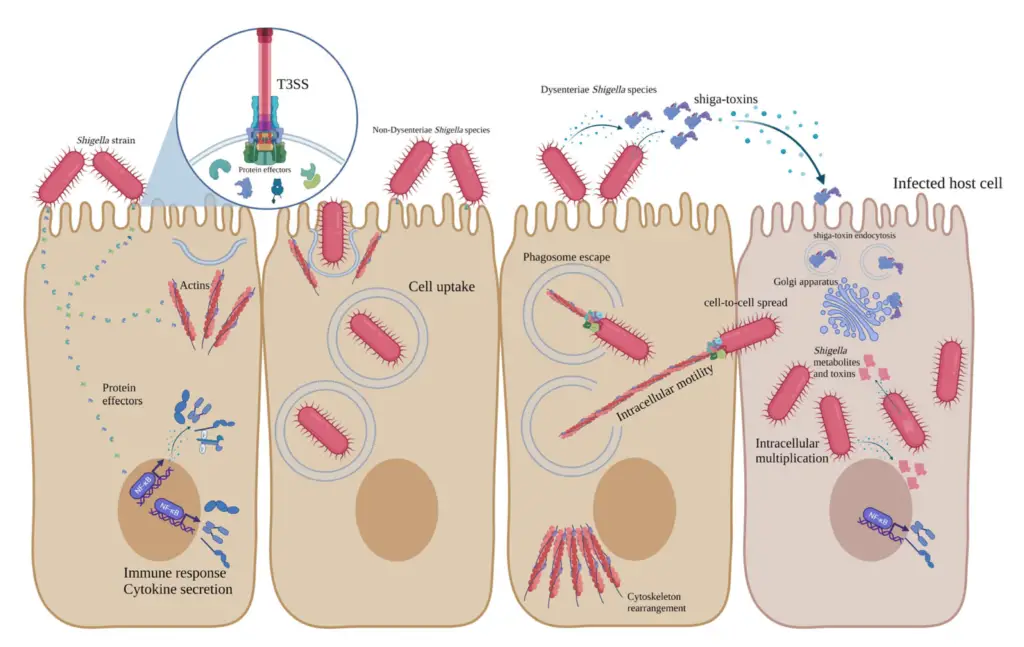
Pathogenesis of Shigella
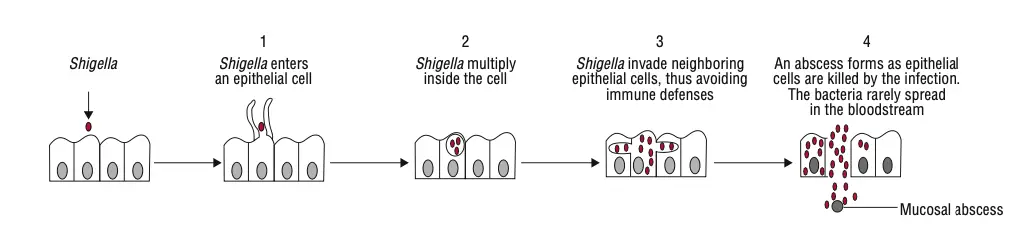
Shigella species are responsible for causing bacillary dysentery, a severe disease that primarily affects the colon. Infection occurs when the bacteria are ingested, and only a small number of bacteria are needed to initiate the disease.
- Infectivity Dose
- S. dysenteriae can cause disease with as few as 10 bacilli.
- S. sonnei and S. flexneri require about 100-200 bacilli to cause infection.
- Invasion and Colonization
- Once ingested, Shigella targets and invades the epithelial cells lining the large intestine.
- The bacteria use structural proteins, such as the intestinal adherence factor, endotoxins, and exotoxins to adhere to the intestinal cells.
- These factors enable Shigella to invade, replicate, and spread between cells.
- Shigella first infects the epithelial cells of the villi, where it multiplies.
- After initial infection, the bacteria spread laterally to adjacent cells, penetrating deeper into the tissue.
- Intracellular Replication and Spread
- Shigella can escape the phagocytic vacuole and replicate within the host cell cytoplasm.
- This ability to survive phagocytosis is vital for the bacteria’s persistence in the body.
- Shigella triggers programmed cell death (apoptosis) in the host cells, which helps it avoid immune defenses.
- Inflammatory Response
- Apoptosis of infected cells leads to the release of interleukin-1, a signaling molecule that attracts polymorphonuclear leukocytes (PMNs) to the site of infection.
- These immune cells amplify the inflammatory response, which contributes to tissue damage.
- The inflammation weakens the intestinal wall and allows the bacteria to invade deeper epithelial layers.
- Role of Shiga Toxin
- Shiga toxin produced by S. dysenteriae plays a critical role in disease progression.
- After the bacteria invade the colonic cells, Shiga toxin exacerbates mucosal damage.
- The toxin causes vascular damage in the colon, further contributing to tissue destruction.
- Pathological Features
- The infection leads to edema, erythema, and friability of the mucosa.
- Superficial ulceration and focal mucosal hemorrhages are common, particularly in the rectosigmoid junction.
- These pathological changes cause the severe symptoms of dysentery, including diarrhea and abdominal pain.
Mechanism of Toxicity of Shigella
Shigella’s toxicity is driven by multiple virulence factors that allow it to evade the host immune system and cause significant cellular damage.
- Virulence Factors and Invasion
- Shigella has several proteins, known as Ipa proteins, which are key for host cell adherence and invasion.
- These proteins are secreted through a type III secretion system made up of Mxi-Spa proteins, which allow the bacteria to enter host cells.
- Once inside the colonic epithelial cells, Shigella rapidly multiplies and spreads to adjacent cells, creating a path of tissue destruction.
- Effector Proteins and Immune Modulation
- Shigella secretes outer surface proteins (Osp) that are crucial for modulating the immune response.
- These proteins affect the release of cytokines and promote neutrophil migration, which contributes to inflammation and tissue damage.
- Shiga Toxin
- Shigella dysenteriae produces Shiga toxin, a potent exotoxin.
- The toxin is only released when the bacterial cell lysates and is then able to bind to host cells.
- Once bound, Shiga toxin enters the host cell through endocytosis, where it halts protein synthesis, leading to cell death.
- Shiga toxin has multiple toxic activities, acting as an enterotoxin, neurotoxin, and cytotoxin, depending on the tissue it affects.
- Lipopolysaccharides (LPS) as Endotoxins
- LPS, found in the outer membrane of Gram-negative bacteria like Shigella, acts as an endotoxin.
- LPS is not necessary for invasion, replication, or spread but contributes to tissue damage once the bacteria are destroyed.
Each of these mechanisms works together to allow Shigella to cause the intense symptoms and cellular damage seen in shigellosis.
Clinical Syndromes of Shigella Infection
Shigella infection leads to shigellosis, which includes a variety of clinical manifestations ranging from mild diarrhea to severe bacillary dysentery.
- Bacillary Dysentery
- This is the most severe form of shigellosis.
- Symptoms:
- Sudden onset of high-grade fever
- Abdominal cramps
- Tenesmus (painful urge to pass stool)
- Urgency and passage of small amounts of blood- and mucus-filled stools.
- The incubation period is typically short, lasting between 12 hours and 7 days, with the peak around 48 hours.
- The severity is inversely related to the number of bacteria ingested.
- The infection is self-limiting in most cases.
- Asymptomatic Colonization
- In some cases, patients may harbor Shigella in the colon without showing symptoms.
- These individuals act as persistent reservoirs, capable of transmitting the bacteria to others.
- Complications
- S. dysenteriae type 1 infections are more likely to result in complications, such as:
- Arthritis
- Toxic neuritis
- Conjunctivitis
- Intussusception in children.
- Hemolytic Uremic Syndrome (HUS) may develop due to vasculopathy caused by Shiga toxin, leading to kidney failure.
- Reiter Syndrome (a combination of arthritis, urethritis, and conjunctivitis) can occur, particularly in adults with the HLA-B27 histocompatibility antigen.
- Shigella septicemia is rare, but can occur in malnourished children infected with S. dysenteriae.
- This may progress to disseminated intravascular coagulation, bronchopneumonia, and even multiple organ failure in lethal cases.
- S. dysenteriae type 1 infections are more likely to result in complications, such as:
- Chronic and Relapsing Infection
- In patients with HIV, Shigella infection often becomes prolonged and relapsing, even with antibiotic treatment.
- Such infections are more likely to be complicated by bacteremia.
- Variation Among Species
- S. sonnei tends to cause the mildest form of bacillary dysentery, often presenting as just mild diarrhea.
- S. flexneri and S. boydii typically lead to more severe symptoms than S. sonnei.
Reservoir, Source, and Transmission of Shigella Infection
Shigella infection, or shigellosis, spreads through various routes, with humans being the primary source of the bacteria.
- Reservoirs of Infection
- Infected individuals or, less commonly, carriers serve as reservoirs.
- Chronic carriers are rare, as the bacteria are typically excreted from the body within a few weeks.
- In some cases, malnourished children or individuals with AIDS may continue to harbor the bacteria longer.
- Transmission Routes
- Fecal-oral transmission:
- This is the most common route, where the infection spreads through hand-to-mouth contact with contaminated fingers.
- Only 10-200 bacilli are needed to cause disease, making it easy for the infection to spread in places with poor sanitation and hygiene practices.
- Contaminated food and water:
- Shigella spreads when food or water is contaminated with human feces carrying the bacteria.
- Fomites:
- Inanimate objects such as door handles, water taps, and lavatory seats can act as vehicles for transmission if they are contaminated with fecal matter.
- Flies:
- These insects can transfer Shigella as mechanical vectors, spreading the bacteria from feces to surfaces or food.
- Sexual transmission:
- Oro-anal contact is a known route of transmission, particularly among young male homosexuals.
- Fecal-oral transmission:
- Populations at Highest Risk
- Children are the most affected group, with nearly 70% of all infections occurring in those under 15 years old.
- Malnourished children are at an even higher risk, with the infection often worsening their nutritional status and leading to a cycle of recurrent infections and growth retardation.
- Other high-risk groups include young children in daycare centers, nurseries, and custodial institutions, as well as their families.
- Shigellosis is also common in household contacts of infected children and in certain adult populations, such as male homosexuals.
Laboratory Diagnosis of Shigella Infection
Diagnosing Shigella infection involves several steps, from collecting the right specimen to confirming the bacteria in the lab.
- Specimens
- Stool is the primary specimen for diagnosis.
- Fresh stool samples should be collected and inoculated immediately or transported in a suitable medium, such as Sachs’ buffered glycerol saline (pH 7.0–7.4).
- Rectal swabs may also be taken if the infection site is visible during sigmoidoscopy. However, swabs without a significant amount of stool or mucus are not reliable.
- Microscopy
- In routine microscopy, the stool may show clumps of polymorphonuclear leukocytes.
- Around 70% of cases show fecal blood or leukocytes in the stool, which aids in diagnosis.
- Culture
- A stool sample is mandatory in all suspected cases of shigellosis for culture.
- Typically, more than one stool or rectal swab is collected.
- Samples should be inoculated on at least two different culture media, such as MacConkey, XLD, DCA, or eosin-methylene blue agars.
- For enrichment, inoculate one tube each of selenite F and GN broth, then incubate at 37°C for 12–18 hours. After this, subculture onto selective media.
- After overnight incubation, Shigella will produce:
- Pale non-lactose-fermenting colonies on MacConkey and DCA media.
- Red colonies on XLD medium.
- Colorless colonies on SS agar.
- Identification of Bacteria
- Pale, non-lactose-fermenting colonies on MacConkey agar are identified through:
- A motility test.
- Biochemical tests.
- Slide agglutination tests using specific Shigella antisera (both polyvalent and monovalent sera).
- Pale, non-lactose-fermenting colonies on MacConkey agar are identified through:
- Serodiagnosis
- Serological tests are not useful for diagnosing Shigella infection.
Treatment of Shigella Infection
The approach to treating Shigella infections depends on the severity of the case and the patient’s condition.
- Uncomplicated Shigellosis
- In cases where the infection is mild, no antibiotics are necessary.
- Self-limiting cases usually resolve on their own within a few days.
- Focus on managing dehydration, especially in infants and young children.
- Oral fluid and electrolyte replacement is crucial for maintaining hydration.
- Severe Shigellosis or At-Risk Groups
- Antibiotic treatment is recommended in the following scenarios:
- Severe or toxic cases.
- For vulnerable populations, including young children, debilitated individuals, and the elderly.
- Antibiotics help in:
- Reducing the duration of illness.
- Limiting person-to-person spread.
- Preventing the spread to household contacts.
- In developing countries, treating malnourished children reduces the risk of worsening malnutrition after shigellosis.
- Prophylactic antibiotics are not recommended for contacts.
- Antibiotic treatment is recommended in the following scenarios:
- Antibiotic Options
- Common antibiotics used include:
- Trimethoprim-sulfamethoxazole (effective and the drug of choice if antibiotic susceptibility is unknown).
- Ampicillin (still the drug of choice for susceptible Shigella strains).
- Tetracycline.
- Quinolones like nalidixic acid and ciprofloxacin.
- Trimethoprim-sulfamethoxazole works by blocking folic acid synthesis, targeting Shigella bacteria effectively.
- Common antibiotics used include:
- Antibiotic Resistance
- Antibiotic-resistant Shigella strains are a growing concern.
- Resistance to multiple antibiotics, such as streptomycin, chloramphenicol, and sulfonamides, was first observed in Japan in the 1950s.
- The indiscriminate use of antibiotics has contributed to the rise of resistant strains.
- It’s critical to base treatment on in vitro antibiotic susceptibility testing to ensure the effectiveness of the prescribed antibiotics.
Prevention and Control of Shigella Infection
Preventing and controlling Shigella infection is centered on breaking the transmission cycle and improving sanitation practices.
- Main Source of Transmission
- Person-to-person transmission is the most common in developed countries.
- In areas with poor sanitation, water contaminated with human feces is the leading cause of infection.
- Sanitation Practices
- Control efforts focus primarily on improving sanitation—both personal and environmental.
- Regular hand washing with soap is a key preventive measure.
- Ensuring safe drinking water and proper waste disposal is essential.
- Food safety measures, such as avoiding contamination during preparation, can limit transmission.
- Prophylaxis and Vaccines
- Antibiotics are not used for prevention in individuals who are not infected.
- Currently, there is no effective vaccine available for Shigella.
- Public Health Measures
- Improving hygiene in daycare centers, schools, and public spaces can help reduce outbreaks.
- Awareness campaigns focusing on proper sanitation and food safety are essential, particularly in areas with high infection rates.
- Mumy, K. L. (2014). Shigella. Encyclopedia of Toxicology, 254–255. doi:10.1016/b978-0-12-386454-3.00538-8
- Pakbin, B., Brück, W. M., & Brück, T. B. (2023). Molecular Mechanisms of Shigella Pathogenesis; Recent Advances. International Journal of Molecular Sciences, 24(3), 2448. https://doi.org/10.3390/ijms24032448
- https://www.mayoclinic.org/diseases-conditions/shigella/symptoms-causes/syc-20377529
- https://www.cdc.gov/shigella/about/index.html
- https://my.clevelandclinic.org/health/diseases/17826-shigellosis
- https://www.ncbi.nlm.nih.gov/books/NBK8038/
- https://www.webmd.com/food-recipes/food-poisoning/what-is-shigella
- https://en.wikipedia.org/wiki/Shigella
- https://www.cdc.gov/shigella/signs-symptoms/index.html
- https://medlineplus.gov/ency/article/000295.htm
- https://www.sciencedirect.com/topics/immunology-and-microbiology/shigella
- https://www.ncbi.nlm.nih.gov/books/NBK482337/