Rabies is a viral disease that causes inflammation of the brain in humans and other mammals. It is transmitted through the saliva of infected animals, usually through a bite. The virus attacks the central nervous system, leading to symptoms such as fever, muscle spasms, and hallucinations. If left untreated, rabies is almost always fatal. It is important to seek medical attention as soon as possible after being bitten by an animal, as treatment is most effective when given before symptoms appear.
Epidemiology of Rabies Virus
- Rabies is found in many parts of the world, but it is most common in countries with a high number of stray dogs. It is also found in wild animals such as bats, foxes, and raccoons.
- In developed countries, rabies is relatively rare due to effective vaccination programs for domestic animals and public education about the importance of seeking medical attention after an animal bite.
- The World Health Organization (WHO) estimates that tens of thousands of people die from rabies each year, with the majority of cases occurring in Africa and Asia. However, it is difficult to accurately estimate the number of rabies cases, as many cases go unreported, especially in areas with limited access to healthcare.
- Rabies is a preventable disease, and efforts to control the disease include vaccination programs for animals, pre-exposure prophylaxis for high-risk individuals, and post-exposure treatment for people who have been bitten by an infected animal.
Rabies Virus Structure
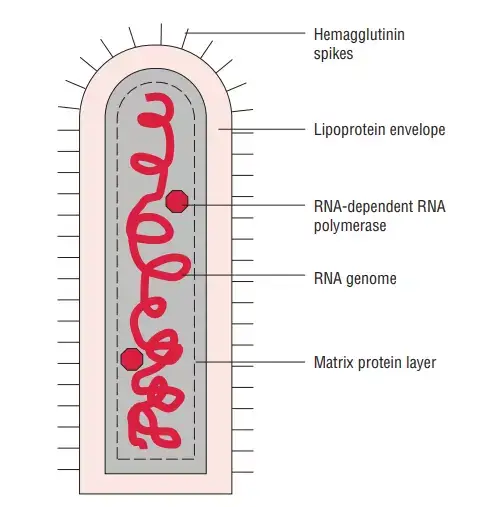
- Rhabdoviruses are roughly 180 nm in length and 75 nm in width.
- There are five proteins encoded by the rabies genome: nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and polymerase (P) (L).
- All rhabdoviruses contain two basic structural components: a ribonucleoprotein (RNP) core and an envelope. The nucleoprotein securely encases genomic RNA within the RNP.
- The RNP is coupled with two additional viral proteins, the phospoprotein and the large protein (L-protein or polymerase).
- On the surface of the virus, the glycoprotein forms around 400 trimeric spikes that are densely packed. The M protein is connected with both the envelope and the RNP and may be the key protein in the assembly of rhabdoviruses. The structure and composition of the rabies virus are represented in the diagram below.
- Rabies is an RNA virus. There are five proteins encoded by the genome, named N, P, M, G, and L. The order and relative size of the genome’s genes are depicted in the following diagram.
- Rabies virus structure is determined by the configuration of these proteins and the RNA genome.
Rabies Virus Shape
The rabies virus is a bullet-shaped virus with a diameter of about 75 nanometers and a length of about 180 nanometers. It is a member of the Rhabdovirus family and is classified as a negative-sense single-stranded RNA virus. The virus is enveloped, meaning that it has a protective outer layer made up of lipids that surrounds the viral genome. The virus is covered in spikes made up of a protein called glycoprotein, which helps it to bind to and infect host cells. The rabies virus is highly infectious and is transmitted through the saliva of infected animals, most commonly through bites or scratches. It can infect all warm-blooded animals, including humans, and is usually fatal once symptoms appear.
Genome organization of Rabies Virus
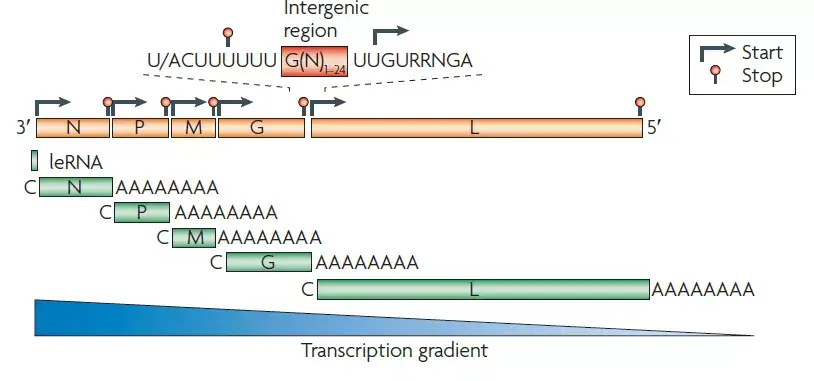
- The rabies virus genome is roughly 12 kb of single-stranded, antisense, nonsegmented RNA. There is an approximately 50 nucleotide leader sequence (LDR) followed by N, P, M, G, and L genes.
- The virion of a rhabdovirus is an enveloped, rod- or bullet-shaped structure that contains five protein species.
- The nucleoprotein (N) coats the RNA at a rate of one protein monomer every nine nucleotides, forming a helical nucleocapsid.
- P (phosphoprotein) and L (large protein) duplicates are associated with the nucleocapsid. The L protein is well called, as its gene occupies over half of the genome. Its vastness is justified by the fact that it is a protein with multiple functions.
- M (matrix) protein forms a layer between the nucleocapsid and envelope, while G (glycoprotein) trimers create protruding spikes from the envelope.
- All rhabdovirus genomes encode these five proteins, and in the case of Rabies Lyssavirus, all five are encoded.
Transmission of Rabies Virus
- People are typically infected via a deep bite or scratch from a rabid animal, and rabid dogs responsible for up to 99 percent of human infections.
- Bats are currently the leading cause of human rabies mortality in the Americas, as dog-mediated transmission has been mostly eliminated in this region.
- In addition to Australia and Western Europe, bat rabies is a rising public health problem in Australia. Human mortality caused by exposure to foxes, raccoons, skunks, jackals, mongooses, and other wild carnivore host species are extremely uncommon, and it is unknown if rodent bites transmit rabies.
- Transmission is also possible if infected animal saliva comes into close contact with human mucosa or fresh skin wounds. Rabies transmission through inhalation of virus-containing aerosols or organ transplantation is described, but highly uncommon.
- Theoretically, human-to-human transmission by bites or saliva is possible, but has never been confirmed. The same holds true for transmission to humans through eating of raw animal meat or milk.
Replication of Rabies Virus
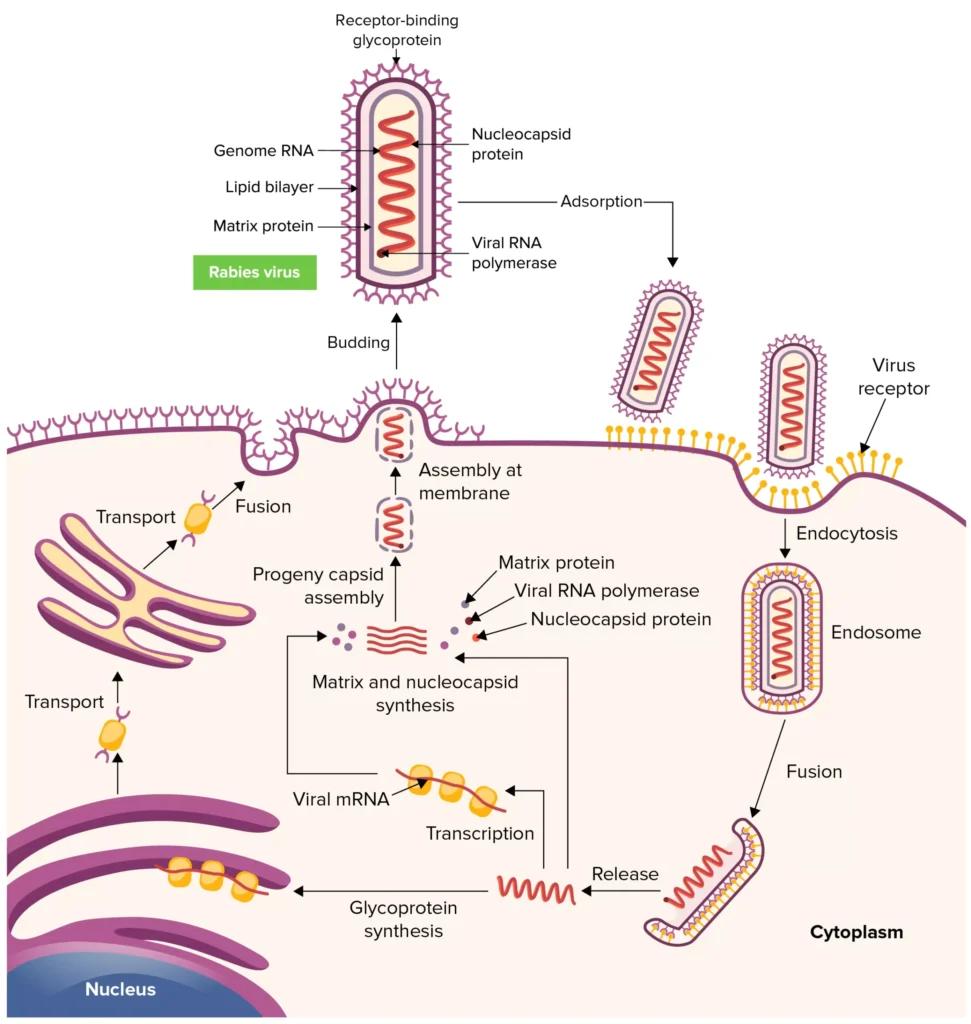
- The infection process is initiated by the fusion of the rabies virus envelope with the host cell membrane (adsorption). Possibly implicated is the interaction between the G protein and certain cell surface receptors.
- After adsorption, the virus enters the cytoplasm and penetrates the host cell. The virus particles congregate in the endosomes (cytoplasmic vesicles). The fusion of viral membranes with endosomal membranes releases viral RNP into the cytoplasm (uncoating). Because lyssaviruses have a linear, single-negative-stranded ribonucleic acid (RNA) genome, viral replication requires the transcription of messenger RNAs (mRNAs).
- The genomic strand of rabies RNA is transcribed by a viral polymerase (L gene) into leader RNA and five capped and polyadenylated mRNAs, which are then translated into proteins. Translation, which involves the production of the N, P, M, G, and L proteins, happens on cytoplasmic ribosomes.
- Although G protein synthesis is initiated on free ribosomes, the endoplasmic reticulum (ER) and Golgi apparatus are responsible for its completion and glycosylation (processing of the glycoprotein). The ratio of intracellular leader RNA to N protein controls the transition from transcription to replication. When this switch is triggered, viral genome replication commences.
- The initial step in viral replication is the production of complete copies of the viral genome (positive strands). During the transition to replication, RNA transcription becomes “non-stop” and stop codons are disregarded.
- The viral polymerase enters a single spot on the 3′ end of the genome and then synthesises complete copies of the genome. These positive rabies RNA strands act as templates for the creation of complete negative strands of the viral genome.
- During the assembly process, the N-P-L complex envelopes negative-stranded genomic RNA to create the RNP core, while the M protein forms a matrix or capsule surrounding the RNP.
- The RNP-M complex migrates to a region of the plasma membrane with glycoprotein inserts, where the M-protein commences coiling. The M-RNP complex binds to the glycoprotein, causing the virus to emerge from the plasma membrane.
- Within the central nervous system (CNS), plasma membranes are favoured for viral budding. Viruses in the salivary glands, on the other hand, bud largely from the cell membrane into the acinar lumen.
- Viral budding in the salivary gland and virus-induced aggressive biting in the host animal increase the likelihood of viral transmission to a new host.
Pathogenesis of Rabies Virus
- The saliva of a rabid animal inoculates muscle and subcutaneous tissues with the rabies virus. During the incubation period, which lasts weeks to months, there is a delay in the movement of the virus at the inoculation site.
- Rabies virus attaches to nicotinic acetylcholine receptors at the neuromuscular junction and travels at a rate of 50–100 mm per day within the axons of peripheral nerves toward the spinal cord via retrograde rapid axonal transport.
- The virus disseminates along neuroanatomical channels within CNS axons. Rabies virus replicates in neurons and produces neuronal dysfunction via unidentified mechanisms, which presumably accounts for the disease’s clinical manifestations and deadly result.
- Changes in behaviour are characteristic of rabies, which is typically transmitted by biting infected animals. The virus then spreads centrifugally (away from the CNS) along nerves to many organs, including salivary glands, in animals that transmit the virus.
- The rabies virus is secreted in vectors’ saliva and transmitted to new hosts via biting. In North America, bats, raccoons, skunks, and foxes are significant rabies vectors, while canines are the most significant vector worldwide. There may be no recognition of bat bites, and there may be no known encounter with bats.
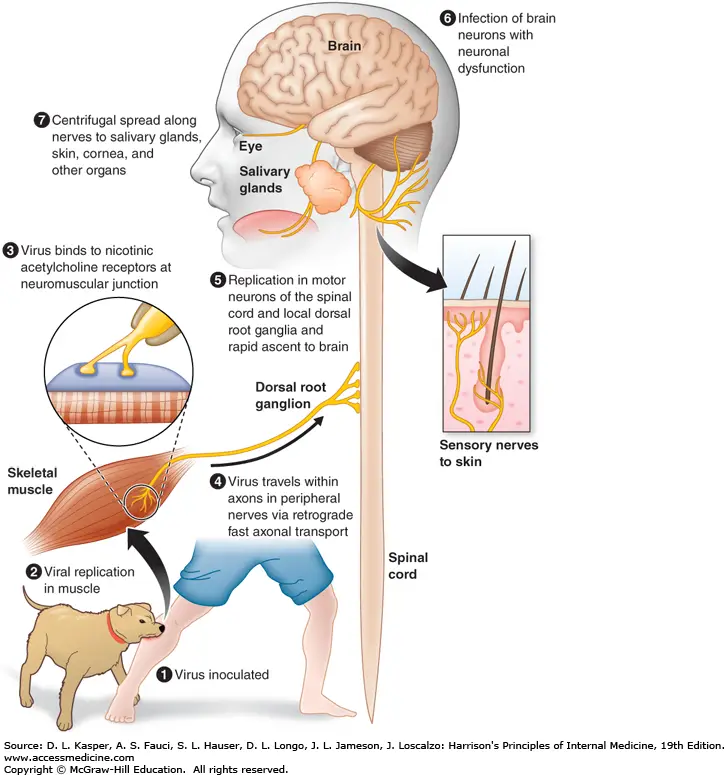
Clinical manifestations of Rabies Virus
- In humans, there are five distinct stages of rabies: incubation, prodrome, acute neurologic period, coma, and death (or, very rarely, recovery).
- Once clinical symptoms of rabies occur, no specific antirabies medicines are effective. The incubation period for rabies is more variable than that of any other acute illness, ranging from as few as 5 days to more than 2 years following initial exposure.
- Children and those bitten close to the central nervous system may experience somewhat shorter incubation times (e.g., the head). The prodromal stage, which typically lasts between 2 and 10 days, is when clinical symptoms initially manifest.
- These symptoms are frequently nonspecific (malaise, fever, and fatigue) or suggest involvement of the respiratory system (sore throat, cough, and dyspnea), the gastrointestinal system (anorexia, dysphagia, nausea, vomiting, abdominal pain, and diarrhoea), or the central nervous system (anorexia, dysphagia, nausea, vomiting, abdominal pain, and diarrhoea) (headache, vertigo, anxiety, apprehension, irritability, and nervousness).
- More notable abnormalities (agitation, photophobia, priapism, increased libido, insomnia, nightmares, and depression) may also occur, indicating the presence of encephalitis, psychiatric disorders, or brain diseases.
- The presence of pain or paresthesia at the site of viral injection, along with a recent history of animal bite, may prompt evaluation of rabies.
- The acute neurologic phase begins with objective symptoms of malfunction of the central nervous system. The disease may be categorised as furious rabies if hyperactivity (i.e., hydrophobia) predominates, or as dumb rabies if paralysis predominates.
- In both variants of the condition, fever, paresthesia, nuchal stiffness, muscle fasciculations, focal and widespread convulsions, hyperventilation, and hypersalivation are possible.
- At the conclusion of the acute neurologic phase, episodes of fast, erratic breathing may ensue, followed by paralysis and coma.
- Unless the patient is getting ventilatory assistance, which may prolong survival for days, weeks, or longer, death may occur as a result of additional complications.
- Although life support techniques can prolong the course of rabies, they rarely alter the disease’s outcome. However, the prospect of recovery must be acknowledged, and when resources allow, every effort must be made to assist the patient. At least seven known incidents of human “recovery” exist.
Diagnosis of Rabies Virus
Steps Summery
- absorbance (receptors and virion interation).
- Penetration (virus entry).
- De-coating (envelope removal).
- Transcription (synthesis of mRNAs).
- Interpretation (Synthesis of structural proteins).
- Procedure (G-protein gycosylation). Replication (synthesis of genomic RNA from the intermediate strand).
- construction
- Budding (complete virions).
A. Laboratory Diagnosis in Humans
Specimens
For the diagnosis of rabies, saliva, serum, cerebrospinal fluid (CSF), blood, urine, and skin and brain biopsies are often utilised specimens.
Microscopy
Demonstration of Negri bodies by microscopy is the typical histological feature of rabies. Impression smears of the human brain tissue taken at postmortem are dyed by Seller’s procedure for demonstration of Negri bodies. The stain contains methylene blue alcohol as fixative and basic fuchsin as staining reagent.
B. Direct Antigen Detection
For the diagnosis of rabies, viral antigens can be seen in the brain tissue after death or in antemortem saliva, skin biopsy taken from the face or neck, and corneal smear. A specialised technique to show rabies antigen in clinical specimens is the direct fluorescent antibody (DFA) test, which utilises certain monoclonal antibodies.
Isolation of the virus
By cultivating viruses in cell lines or on animals, viruses can be extracted from brain tissue, CSF, saliva, and urine.
- Culture: A delicate technique for isolating viruses is to do it in cell lines (such as WI-38, BHK-21, and CER). Since the virus has a negligible cytopathic effect, viral antigen is used to demonstrate virus presence in implanted cell cultures within 2-4 days. The DFA test is a regularly employed specific technique to demonstrate the antigen and uses monoclonal rabies antibodies labelled with fluorescein isothiocyanate.
- Animal inoculation: Mouse is the preferred animal for the animal vaccine. Injections of CSF, saliva, and urine are made intravenously. The mice who received the vaccine are watched for symptoms of clinical disease. The brain tissues are tested for the existence of Negri bodies by microscopy or for viral antigen by the DFA test after the mice have died or have been inoculated for 28 days.
Serodiagnosis
- Antibodies to rabies are present in the serum and CSF of human patients. The concentration of antibodies in the cerebrospinal fluid (CSF) is high.
- In fifty percent of instances, a quick fluorescence focus inhibition test titer demonstrates the presence of antibodies in the serum.
- After the first week of sickness, positive antibody titers (2–25% of serum titer) are detected in the cerebrospinal fluid (CSF).
Other tests
- Normal to increased white blood cell count with 6–8% atypical monocytes. It is possible to see albuminuria and sterile pyuria. In the prodromal and early acute neurologic episodes, hyperventilation causes respiratory alkalosis, which is followed by respiratory acidosis when respiratory depression advances.
- After one week of illness, 80% monocytosis is found in the cerebrospinal fluid (CSF). The findings of protein and glucose tests are normal.
Antemortem diagnosis of human rabies
- Until recently, the majority of rabies cases were lethal, and patients typically succumbed to the disease.
- With extensive patient support and improved patient management, however, few cases have survived.
- Consequently, laboratory procedures for antemortem diagnosis of rabies in people are proving to be more useful in such instances.
C. Laboratory Diagnosis in Animals
The laboratory diagnosis of rabies in dogs and other animals is essential for assessing infection risk and monitoring postexposure treatment in humans bitten by the animals.
Specimens
The brain of the deceased animal is the preferred specimen. The brain is carefully extracted from a deceased animal. For virus isolation, a portion of the specimen is collected in 50% glycerol saline (preservative). The hippocampus and cerebellum, which are plentiful in Negri bodies, are collected in Zenkers’ fixative for the demonstration of Negri bodies.
Microscopy
Seller’s approach stains brain tissue impression smears to demonstrate the presence of Negri bodies. In laboratories lacking equipment for cell culture and antigen detection, this procedure is nonetheless useful.
Direct antigen detection
The demonstration of viral antigens in infected brain tissue and saliva by DFA is an effective way for diagnosing rabies in animals. Detection of antigen in the saliva indicates whether or not the animal excreted the virus in its saliva.
Isolation of the virus
As explained previously for the diagnosis of human rabies, viruses can be isolated from animal brain matter and saliva by cell line culture or in animals.
Treatment of Rabies Virus
There is no antirabies agent available. With extensive supportive care and management of sequelae, it has been proved that complete recovery from established rabies is possible. Previously, it was believed that rabies was always deadly.
Management, Prevention and Control of Rabies Virus
Pre-exposure prophylaxis and post-exposure prophylaxis can be used to describe rabies prophylaxis, depending on whether it was administered before or after exposure.
Pre-exposure prophylaxis
Pre-exposure prophylaxis is administered to high-risk individuals, including dog handlers, other animal handlers, and veterinarians. This is accomplished by employing safer cell culture vaccinations. Preexposure prophylaxis necessitates the administration of three doses of cell culture vaccinations on days 0, 7, 21, or 0, 28, and 56. A booster dose is indicated after one year, followed by an annual dose. In reducing the spread of rabies, animal control and immunisation techniques now play larger roles than postexposure prophylaxis.
Postexposure prophylaxis
Postexposure prophylaxis is initiated immediately after infection exposure. After exposure to a potentially infected dog or other rabid animals, urgent preventive measures consist of (a) local treatment, (b) confirmation that the animal is rabid, (c) delivery of hyperimmune serum, and (d) administration of an antirabies vaccination.
- Local treatment: Local treatment consists of a timely wound cleansing. The virus is transmitted through animal bites to the wounds. The wound should be cleaned quickly with soap and water, and then treated with quaternary ammonium compounds, such as Cetavlon, tincture, or aqueous solution of iodine or alcohol.
- Confirmation of the animal’s rabies status: This is possible through clinical observation of the questionable dog. If the dog is healthy 10 days after biting a human, rabies is extremely unlikely to have spread. In addition to the evidence of Negri bodies in brain tissue at autopsy or viral antigens in brain tissue or saliva, the diagnosis can also be made by seeing Negri bodies in brain tissue at autopsy. Treatment with hyperimmune serum: By injecting purified equine rabies immune globulin (ERIG) and human rabies immune globulin, passive immunisation is performed (HRIG). HRIG is rapidly administered to achieve passive immunisation against rabies. The suggested dose of HRIG is 20 IU per kilogramme of body weight. Fifty percent of the medication is administered through the incision and the remaining fifty percent is administered intramuscularly. It is often administered before to or simultaneously with the initial dose of rabies vaccine. It is not administered after rabies vaccination.
- Antirabies vaccine: Rabies is the only illness for which postexposure vaccination is widely and successfully employed. This is due to the disease’s lengthy incubation period. The likelihood of preventing rabies increases when persons are vaccinated as soon as possible following exposure. Since Pasteur’s successful use of a rabies vaccine derived from a dehydrated preparation of rabbit spinal cord in 1885, a wide variety of vaccines are used to protect humans against rabies, even after infection with the virus. Recent years have witnessed the gradual replacement of neural-tissue vaccinations with cell culture vaccines that are produced in human diploid cells and subsequently inactivated with propiolactone (refer the box Vaccines).
Rabies virus under microscope – Rabies virus image
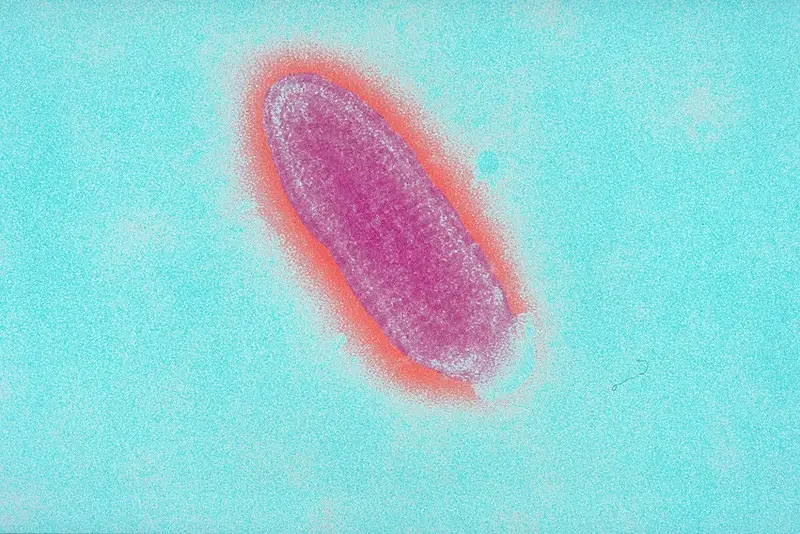
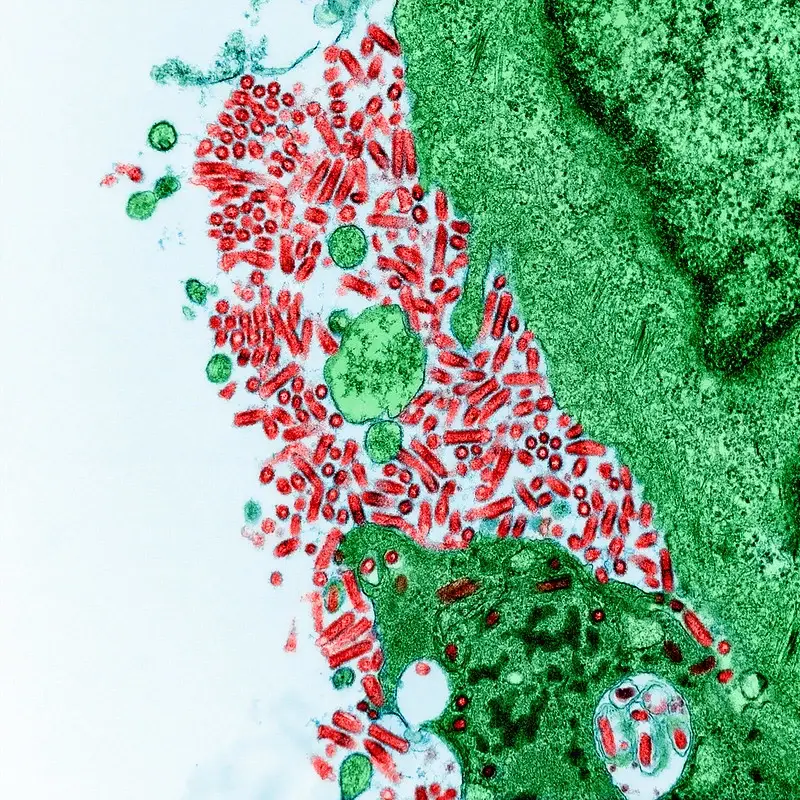
FAQ
Rabies Is Among Us
Yes, rabies is a serious and often fatal disease that affects animals and humans. It is caused by a virus that is transmitted through the saliva of infected animals, most commonly through bites or scratches. The virus attacks the central nervous system and can cause symptoms such as fever, headache, muscle weakness, and abnormal behavior. If left untreated, rabies can be fatal. It is important to take precautions to prevent rabies, such as avoiding contact with wild animals and making sure that your pets are up to date on their rabies vaccinations. If you suspect that you or a loved one may have been exposed to rabies, it is important to seek medical attention as soon as possible, as early treatment can be life-saving.
Which mechanism allows the rabies virus to gain access to the central nervous system (cns)?
The rabies virus is able to gain access to the central nervous system (CNS) through a process called axonal transport. After being introduced into the body, the virus travels from the site of the bite or scratch to the brain and spinal cord through the peripheral nerves. This process, known as retrograde axonal transport, occurs when the virus infects a nerve cell and is transported back to the cell body, which is located in the CNS. Once the virus reaches the cell body, it can replicate and spread to other cells in the CNS, leading to the development of the characteristic symptoms of rabies.
In addition to axonal transport, the rabies virus can also gain access to the CNS through a process called anterograde axonal transport. This occurs when the virus infects a nerve cell in the CNS and is transported out to the periphery, where it can infect other cells. This can occur when the virus is transmitted from an infected animal to a human or other animal through bites or scratches.
What does the rabies virus look like?
The rabies virus is a bullet-shaped virus with a diameter of about 75 nanometers and a length of about 180 nanometers. It is a member of the Rhabdovirus family and is classified as a negative-sense single-stranded RNA virus. The virus is enveloped, meaning that it has a protective outer layer made up of lipids that surrounds the viral genome. The virus is covered in spikes made up of a protein called glycoprotein, which helps it to bind to and infect host cells.
At what temperature does the rabies virus die?
The rabies virus is sensitive to temperature changes and can be killed by high temperatures. The virus is most stable at low temperatures, but it can be killed by exposing it to temperatures above 60°C (140°F) for a period of time. For example, the virus can be killed by boiling for one minute, or by heating at 56°C (133°F) for 30 minutes. It is also sensitive to pH changes and can be inactivated by exposure to pH levels below 6 or above 11. The virus can also be killed by certain chemicals, such as alcohol and chlorine.
It is important to note that while the rabies virus can be killed by high temperatures and certain chemicals, it is important to use caution when handling potentially infected materials, as the virus can be transmitted through contact with saliva or brain tissue. Proper infection control measures, such as wearing gloves and using disinfectants, should be followed to prevent exposure to the virus.
How long does the rabies virus live on surfaces?
The rabies virus is not particularly hardy and can be easily killed by exposure to heat, sunlight, and certain chemicals. It does not survive long outside of the body, and is usually transmitted through the saliva of infected animals, most commonly through bites or scratches.
In general, the rabies virus does not live long on surfaces. It can survive for a short period of time in moist environments, such as in water or in the tissue of a recently deceased animal, but it quickly dies when exposed to air. The virus is sensitive to UV light and is quickly inactivated by sunlight, so it does not survive long outdoors.
It is important to note that while the rabies virus does not survive long on surfaces, it is important to use caution when handling potentially infected materials, as the virus can be transmitted through contact with saliva or brain tissue. Proper infection control measures, such as wearing gloves and using disinfectants, should be followed to prevent exposure to the virus.
How large is the rabies virus?
The rabies virus is a small virus with a diameter of about 75 nanometers and a length of about 180 nanometers. It is classified as a member of the Rhabdovirus family and is a negative-sense single-stranded RNA virus. The virus is enveloped, meaning that it has a protective outer layer made up of lipids that surrounds the viral genome. The virus is covered in spikes made up of a protein called glycoprotein, which helps it to bind to and infect host cells.
For comparison, here are some other objects and their sizes to give you an idea of the scale of the rabies virus:
A human red blood cell: 7-8 micrometers (μm) in diameter
A human hair: 50-100 μm in diameter
A grain of salt: 500-1000 μm in size
As you can see, the rabies virus is extremely small, and is only visible under a microscope. It is transmitted through the saliva of infected animals, most commonly through bites or scratches, and can infect all warm-blooded animals, including humans.
How long rabies virus survive outside?
The rabies virus is not particularly hardy and can be easily killed by exposure to heat, sunlight, and certain chemicals. It does not survive long outside of the body, and is usually transmitted through the saliva of infected animals, most commonly through bites or scratches.
In general, the rabies virus does not survive long on surfaces. It can survive for a short period of time in moist environments, such as in water or in the tissue of a recently deceased animal, but it quickly dies when exposed to air. The virus is sensitive to UV light and is quickly inactivated by sunlight, so it does not survive long outdoors.
It is important to note that while the rabies virus does not survive long on surfaces, it is important to use caution when handling potentially infected materials, as the virus can be transmitted through contact with saliva or brain tissue. Proper infection control measures, such as wearing gloves and using disinfectants, should be followed to prevent exposure to the virus.
The rabies virus is most commonly transferred through what?
The rabies virus is most commonly transmitted through the saliva of infected animals, most commonly through bites or scratches. The virus can infect all warm-blooded animals, including humans, and is usually fatal once symptoms appear.
Rabies is primarily a disease of animals, and most human cases of rabies are the result of exposure to infected animals. Dogs are the most common source of rabies infection in humans, followed by bats. Other animals that can transmit rabies include cats, cows, foxes, raccoons, and skunks.
It is important to take precautions to prevent rabies, such as avoiding contact with wild animals and making sure that your pets are up to date on their rabies vaccinations. If you suspect that you or a loved one may have been exposed to rabies, it is important to seek medical attention as soon as possible, as early treatment can be life-saving.
What is one difference between rabies virus and influenza virus?
Rabies virus and influenza virus are two different types of viruses that belong to different families of viruses. Here are some differences between the two viruses:
Shape: The rabies virus is a bullet-shaped virus with a diameter of about 75 nanometers and a length of about 180 nanometers, while the influenza virus is a spherical or rod-shaped virus with a diameter of about 80-100 nanometers.
Genome: The rabies virus is a negative-sense single-stranded RNA virus, while the influenza virus is a positive-sense single-stranded RNA virus.
Transmission: The rabies virus is transmitted through the saliva of infected animals, most commonly through bites or scratches, while the influenza virus is transmitted through the respiratory secretions of infected individuals, such as through coughing or sneezing.
Symptoms: The symptoms of rabies and influenza can be similar, but there are some key differences. Rabies typically causes symptoms such as fever, headache, muscle weakness, and abnormal behavior, while influenza typically causes symptoms such as fever, cough, sore throat, and muscle aches.
Treatment: There is no specific treatment for rabies once symptoms appear, and the disease is usually fatal. However, early treatment with a series of rabies vaccines can be life-saving. Influenza can be treated with antiviral medications, such as oseltamivir or zanamivir, which can help to reduce the severity and duration of symptoms.
References
- Rupprecht CE. Rhabdoviruses: Rabies Virus. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 61. Available from: https://www.ncbi.nlm.nih.gov/books/NBK8618/
- WUNNER, W. H. (2007). Rabies Virus. Rabies, 23–68. doi:10.1016/b978-012369366-2/50004-x
- Kuzmin, I. V., & Rupprecht, C. E. (2008). Rabies Virus. Encyclopedia of Virology, 367–373. doi:10.1016/b978-012374410-4.00482-9
- Jackson, A. C. (2014). Rabies Virus. Encyclopedia of the Neurological Sciences, 1027–1030. doi:10.1016/b978-0-12-385157-4.00386-9
- Wunner, W. H., & Conzelmann, K.-K. (2013). Rabies Virus. Rabies, 17–60. doi:10.1016/b978-0-12-396547-9.00002-x
- Jackson, A. C. (2006). Rabies Virus Infection. Current Therapy in Neurologic Disease, 142–144. doi:10.1016/b978-0-323-03432-6.50036-2
- Jackson, A. C. (2017). Rabies Virus☆. Reference Module in Neuroscience and Biobehavioral Psychology. doi:10.1016/b978-0-12-809324-5.03909-2
- https://www.brainkart.com/article/Rabies-Virus–Properties-of-the-Virus_18505/
- https://universe84a.com/rabies-virus-introduction-morphology/
- https://www.verywellhealth.com/rabies-overview-4156466
- https://microbeonline.com/virology-note-rabies-virus-structure-pathogenesis-and-clinical-findings/
- https://microbenotes.com/rabies-virus/
- https://www.cdc.gov/rabies/about.html
- https://www.who.int/news-room/fact-sheets/detail/rabies
سلام اگه با دست به زمینی که سگ وگربه اونجا زیاد تردد داره بزنیم و با دست که شسته نشده چیزی بخوریم یا داخل دهن بکنیم ایا هاری میگیریم نیاز به واکسن هست؟ اگه دست به لباسمون هم بزنیم به لباسمون میچسیه
رابیس (هاری) معمولاً از طریق تماس مستقیم با بزاق حیوان مبتلا، معمولاً از طریق گزش یا خراشیدگی منتقل میشود. ویروس رابیس در خارج از میزبان، مانند روی زمین یا روی اشیاء مانند کفشها، به طور معمول زنده نمیماند.
سناریویی که شما توصیف کردهاید - وجود ویروس رابیس روی زمین، چسبیدن به کفشها، و سپس انتقال به دستها و احتمالاً بلعیده شدن - بسیار بعید است به دلایل زیر:
۱. بقای ویروس در خارج از میزبان: ویروس رابیس در خارج از میزبان پستاندار زنده نمیماند طولانی مدت. این ویروس به ویژه نسبت به نور خورشید و خشک شدن حساس است. بنابراین، حتی اگر بزاق حیوان آلودهای روی زمین باشد، ویروس احتمالاً برای مدت طولانی عفونی نخواهد ماند.
۲. انتقال ثانویه: انتقال ویروس از زمین به کفشها، سپس به دستها، و در نهایت به دهان شامل چندین مرحله است. با هر انتقال، مقدار ویروس (اگر هنوز قابلیت بقا داشته باشد) به طور قابل توجهی کاهش مییابد که احتمال عفونت را کمتر میکند.
۳. مسیر عفونت: ویروس رابیس معمولاً باید از طریق شکستگی در پوست وارد بدن شود، مانند گزش یا خراش. بلعیدن ویروس (به عنوان مثال، با دستهایی که کفشهای آلوده را لمس کردهاند) مسیری معمول برای انتقال نیست و برای عفونت بسیار کم خطر محسوب میشود.
اگرچه همیشه خوب است که بهداشت را با شستن دستها و عدم لمس صورت با دستهای نشسته حفظ کنیم (برای جلوگیری از انواع دیگر عفونتها)، نگرانی خاص در مورد انتقال ویروس رابیس به روشی که شما توصیف کردهاید بسیار کم است. با این حال، اگر نگرانی در مورد قرار گرفتن در معرض رابیس وجود دارد، مهم است که برای مشاوره و احتمال واکسیناسیون با متخصصان بهداشت مشورت کنید.
ببخشید ویروس هاری اگه روی زمین که سگ و گربه تردد دارن باشه بادر آوردن کفشی که از روی آن رد میشیم به دست ما هم میچسبه؟ آیا اگر دست را در دهن بکنیم ویروس منتقل میشه؟
رابیس (هاری) معمولاً از طریق تماس مستقیم با بزاق حیوان مبتلا، معمولاً از طریق گزش یا خراشیدگی منتقل میشود. ویروس رابیس در خارج از میزبان، مانند روی زمین یا روی اشیاء مانند کفشها، به طور معمول زنده نمیماند.
سناریویی که شما توصیف کردهاید - وجود ویروس رابیس روی زمین، چسبیدن به کفشها، و سپس انتقال به دستها و احتمالاً بلعیده شدن - بسیار بعید است به دلایل زیر:
۱. بقای ویروس در خارج از میزبان: ویروس رابیس در خارج از میزبان پستاندار زنده نمیماند طولانی مدت. این ویروس به ویژه نسبت به نور خورشید و خشک شدن حساس است. بنابراین، حتی اگر بزاق حیوان آلودهای روی زمین باشد، ویروس احتمالاً برای مدت طولانی عفونی نخواهد ماند.
۲. انتقال ثانویه: انتقال ویروس از زمین به کفشها، سپس به دستها، و در نهایت به دهان شامل چندین مرحله است. با هر انتقال، مقدار ویروس (اگر هنوز قابلیت بقا داشته باشد) به طور قابل توجهی کاهش مییابد که احتمال عفونت را کمتر میکند.
۳. مسیر عفونت: ویروس رابیس معمولاً باید از طریق شکستگی در پوست وارد بدن شود، مانند گزش یا خراش. بلعیدن ویروس (به عنوان مثال، با دستهایی که کفشهای آلوده را لمس کردهاند) مسیری معمول برای انتقال نیست و برای عفونت بسیار کم خطر محسوب میشود.
اگرچه همیشه خوب است که بهداشت را با شستن دستها و عدم لمس صورت با دستهای نشسته حفظ کنیم (برای جلوگیری از انواع دیگر عفونتها)، نگرانی خاص در مورد انتقال ویروس رابیس به روشی که شما توصیف کردهاید بسیار کم است. با این حال، اگر نگرانی در مورد قرار گرفتن در معرض رابیس وجود دارد، مهم است که برای مشاوره و احتمال واکسیناسیون با متخصصان بهداشت مشورت کنید.