- The Pyrrolidonyl Arylamidase (PYR) test is a fast test used to identify group A beta-hemolytic streptococci and enterococci.
- Escherichia coli (-ve) is also identified using the PYR test, which distinguishes it from other indole-positive, lactose-positive, Gram-negative rods.
- It is also known as PYR (L-pyrrolidonyl—naphthylamide) and serves as a substrate for pyrrolidonyl peptidase detection.
- L-Pyrrolidonyl- -naphthylamide (PYR) is the substrate for the PYR test; it is hydrolyzed by a specific bacterial aminopeptidase enzyme (pyrrolidonyl peptidase).
- This enzyme’s hydrolysis of the substrate liberates -naphthylamide, which is identified by adding N, N-dimethylaminocinnamaldehyde. This detection reagent forms a crimson Schiff base with the naphthylamide.
- 98% of group A streptococci and 96% of group D enterococci hydrolyze PYR, according to Facklam, Thacker, Fox, and Eriquez. Although Aerococcus species are infrequently isolated in clinical laboratories, it is believed that these microbes can hydrolyze PYR.
- Facklam et al. also showed that 98% of group B streptococci, 100% of non-group A, B, and D streptococci, 100% of group D non-enterococci, and 82% of viridans streptococci are PYR-negative.
Principle of PYR Test
- The PYR hydrolysis test is indicative of both group A and group D enterococcal streptococci.
- The PYR test measures the activity of L-pyrrolidonyl arylamidase (PYR), an enzyme generated by Streptococcus pyogenes but not other bhaemolytic streptococci.
- The free b-napthylamide is then identified by adding the N,N-dimethylaminocinnamaldehyde diazo dye complex. The appearance of a crimson hue indicates PYR hydrolysis.
- The PYR test, which substitutes the bacitracin and salt tolerance (growth in 6.5% NaCl) assays, is a highly sensitive test.
- The Todd Hewitt Broth Base serves as the medium to which PYR substrate is introduced.
- Animal tissue digest and beef heart infusion give nitrogenous nutrition. Dextrose is the carbohydrate that provides energy.
- Disodium phosphate acts as a buffer, while sodium chloride maintains osmotic equilibrium.
- Chromogenic mixture offers substrate for PYR enzyme.
- After 18-24 hours of incubation at 35-37°C, add 1 drop of PYR reagent directly to the suspected surface growth on the plate. Observe for a change in colour after two minutes.
- S. pyogenes hydrolyzes the chromogenic mixture into L-pyrrolidone and b-naphthylamine.
- A favourable reaction is indicated when the PYR reagent combines with b-naphthylamine to create a red Schiffs Base.
Purpose of PYR Test
- Used for Streptococcus pyogenes isolation and identification.
- Determine the organism’s ability to synthesise L-pyrrolidonyl arylamidase.
Procedure of PYR Test
The PYR examination is accessible in many versions. The original PYR test took 16 to 20 hours, but a 4-hour broth assay is now available, as reported in this post. Other forms include quick (10-15 minute) assays in which organisms to be evaluated are inoculated onto filter paper discs or strips soaked with PYR reagent.
Requirements for PYR Test
PYR Reagent
The PYR reagent is utilised to detect the activity of the Pyrrolidonyl arylamidase enzyme in ß-hemolytic streptococci. PYR enzyme uses the chromogenic combination of PYR Agar (M1489) as a substrate. The formation of a crimson Schiff’s base by the PYR reagent and ß-naphthylamine indicates a favourable reaction.
Composition of PYR Reagent
| Ingredients | |
| N,N -Dimethylaminocinnamaldehyde | 1.0gm |
| Hydrochloric acid (concentrated) | 1.0ml |
| Distilled water | 99.0ml |
Preparation of PYR Reagent
- Inoculate PYR Agar (M1489) with test culture and incubate at 35-37°C for 18-24 hours.
- Directly apply 1 drop of PYR Reagent (R043) to the suspected colony.
- after 2 minutes, observe for colour change
- Red coloration denotes a PYR-positive organism.
PYR Broth
Composition of PYR Broth
| IngredientsGms / LitreBeef heart infusion from500.000Peptic digest of animal tissue20.000Dextrose2.000Sodium chloride2.000Disodium phosphate0.400Sodium carbonate2.500Chromogenic mixture0.100Final pH ( at 25°C)7.8±0.2 |
Preparation of PYR Broth
- Suspend 37 grammes in 1000 ml distilled water.
- If necessary, apply heat to dissolve the medium entirely.
- Mix thoroughly and distribute as desired.
- Autoclave at 15 pounds of pressure (121 degrees Celsius) for 15 minutes.
Method 1 – PYR Broth test
Preparation of Inoculum
- Isolate the to-be-identified organism on Brain Heart Infusion Agar (BHI).
- Collect a single well-isolated colony, streak it onto a BHI agar slant for enrichment, and incubate it at 37 degrees Celsius for 18 to 24 hours.
- Check for healthy growth.
- Wash the growth with two to three millilitres of sterile saline.
- Conform this suspension’s turbidity to McFarland Standard Number 0.5.
Inoculation of Vials
- Bring the liquid’s temperature to room temperature.
- Inoculate the vial with 100 L of the inoculum described above.
- 4-5 hours of incubation at 35-37°C.
- Observe for expansion.
- Add two to three drops of PYR Reagent.
Method 2 – PYR Disc Test
- Place a PYR Disk in an appropriate sterile container and allow it to reach room temperature.
- Rehydrate the disc with a little drop of phosphate buffer or sterile filtered water (pH 7.5).
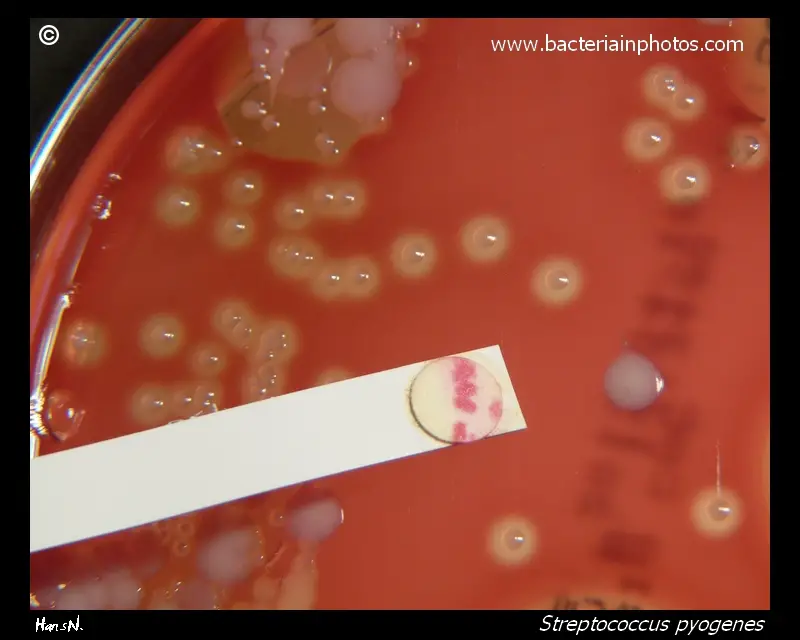
- Inoculate the disc surface with numerous pure colonies of the unidentified test organism grown on a blood agar plate for 18 to 24 hours.
- Incubate at room temperature for 10 minutes.
- Add one drop of PYR/LAP Reagent to the disc that has been infected.
- Interpret results within sixty seconds.
Interpretation of Results
- Positive: One minute after the addition of the reagent, the solution turns a deep cherry-red colour.
- Negative: no colour variation or yellow or orange hue
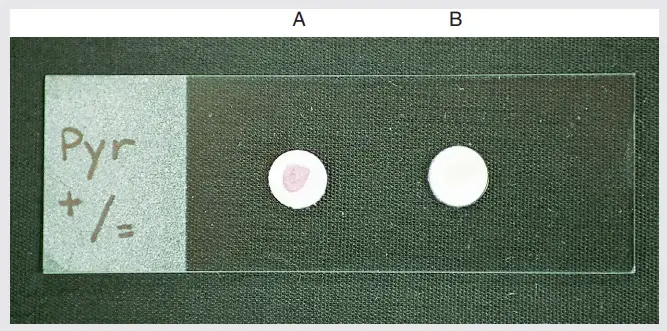

| Organism | Result |
| Streptococcus pyogenes | positive |
| Enterococcus faecalis | positive |
| Escherichia coli | negative |
| Streptococcus agalactiae | negative |
| S. pneumonuae | positive |
| Abiotrophia adiacens | positive |
| Abiotrophia defectiva | positive |
| Aerococcus viridans | positive |
| Aerococcus urinae | negative |
| Alliococcus otitis | positive |
| Gemella hemolysans | V |
| Gemella morbillorum | positive |
| Helcococcus kunzii | positive |
| Lactococcus spp. | positive |
| Leuconostoc spp. | negative |
| Pediococcus spp. | negative |
| Tetragenacoccus spp. | negative |
| Vagacoccus spp. | positive |
Quality control
- Positive control: Enterococcus faecalis, Streptococcus pyogenes
- Negative control: Streptococcus agalactiae
Limitations
- If the disc is excessively damp, a false-negative result may result.
- If selective media or biochemical tube agars are employed to provide inocula, false-negative test results may be obtained.
- Before performing the PYR test, ensure that the test organism is -hemolytic, catalase negative, and a gram-positive coccus.
- Streptococcus porcinus and S. iniae are -hemolytic bacteria linked with animals that contain the required enzyme for a positive response.
- Escherichia coli and indole-positive Proteus grown in media with a high level of tryptophan may develop a blue-green hue, which is regarded a negative result.
- PYR-positive Enterococci and group A streptococci should be distinguishable based on differences in colony size and appearance.
- Use an adequate inoculum or false-negative results may occur
- Rare gram-positive cocci will test positive for this disease, but they are not enterococci. Gram stain is quite useful. They are typically arranged in tetrads or clusters on the smear, consist of minute colonies, or are insignificant pathogens.
- The disc test for Staphylococcus aureus produces weak, pale results; positive results may need to be verified with additional tests or the tube PYR test, which is available in commercial quick identification kits.
Uses of PYR Test
PYR is the abbreviation for L-pyrrolidinyl—naphthylamide. This PYR test identifies an organism’s capacity to utilise substrate PYR by L-pyrroglutamyl amino-peptidase. This test’s applications are as follows:
- Streptococcus pyogenes (PYR positive) distinction from other beta-hemolytic Streptococci (Negative)
- Differentiation of PYR-positive Enterococcus species from PYR-negative group D Streptococci (Streptococcus bovis, Streptococcus equinus).
- It is utilised to distinguish Escherichia coli (PYR Negative) from other indoles-positive, lactose-positive, gram-negative rods.
- It is advantageous to distinguish between the coagulase-negative Staphylococci in order to screen for Staphylococcus lugdunensis (PYR positive) and identify additional staphylococci to the species level.
References
- https://www.labce.com/spg15087_pyrrolidonyl_arylamidase_pyr_differential.aspx
- https://microbenotes.com/l-pyrrolidonyl-arylamidase-pyr-test/
- https://microbeonline.com/pyrrolidonyl-arylamidase-pyr-test-principle-procedure-results/
- https://www.microxpress.in/uploads/product/rapid-pyr-test-kit_technicaldetails_4420200704.103652.pdf
- https://microbe-canvas.com/tests.php?p=2082
- https://himedialabs.com/TD/R043.pdf
- https://himedialabs.com/TD/M1789.pdf
- https://universe84a.com/pyr-test/
- https://www.dalynn.com/dyn/ck_assets/files/tech/DP95K.pdf
- https://microbiologyinfo.com/pyr-test-principle-uses-procedure-and-result-interpretation/
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.