What is positive staining of Virus?
Positive staining of viruses is the process in which the viral particle is made dark on a light background. It is the opposite of negative staining where the virus image is light and the background becomes dark. It is used widely to study the diverse morphology of viruses especially from aquatic environments because the technique is simple and the stain directly covers the particle. It is the process where heavy metal salts like uranyl acetate or lead citrate interact with the virus so that the internal structures appear dense. The stain penetrates the particle and reacts with the viral components, and this is referred to as giving electron-dense contrast under TEM. It is mainly used when the ultrastructural features of viruses are observed in thin sections.
It is also used in molecular diagnostic studies where positive staining means the appearance of a visible signal after interaction between viral antigen and specific antibody. In this step antibody binds with viral antigen forming a colored precipitate in IHC or fluorescence in IF. These are used to detect and locate the virus inside tissues. The stain is considered positive when the viral antigen is recognized and the signal becomes visible.
Principle for positive staining of viruses
The principle of positive staining of viruses is based on the direct interaction between heavy metallic salts and the viral structures so that the particle itself becomes electron dense. It is similar to negative staining in preparation, but the stain penetrates the virus instead of only surrounding it. Ultra-thin sections are placed on a grid and then incubated with uranyl acetate or lead citrate, and these salts react with internal components of the virus. It is the process where these heavy metals absorb and scatter electrons during TEM observation, producing a dark viral image on a light background. Washing is important because it removes excess stain that remains outside the particle, and the grid is then air-dried before viewing. This is referred to as creating contrast by direct uptake of stain into the particle which allows clear visualization of morphology and internal structures.
Types of Positive staining of Viruses
The types of positive staining of viruses are two major groups. These are–
- Ultrastructural positive staining
- Immunological positive staining
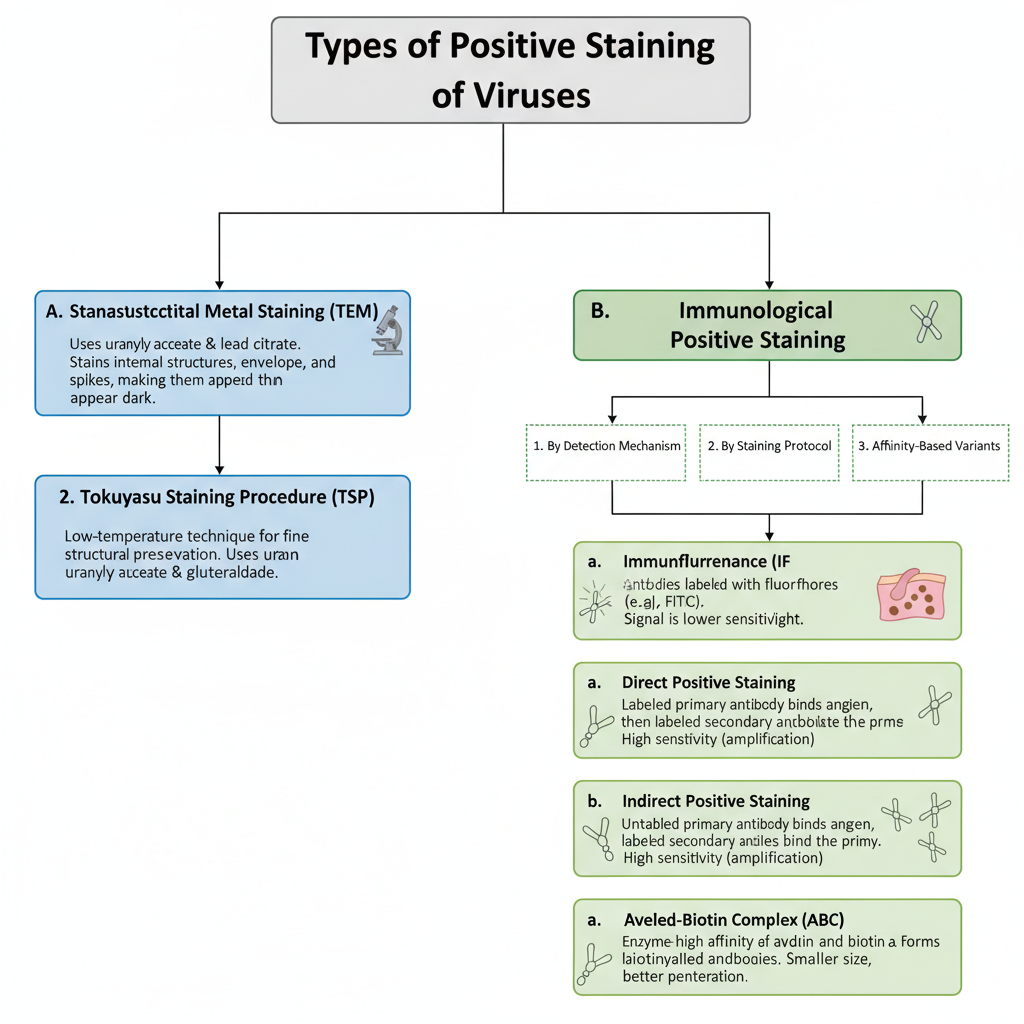
A. Ultrastructural Positive Staining (TEM)
It is the method used in Transmission Electron Microscopy (TEM) where heavy metal salts interact with the virus. In this process the stain binds with viral internal structures, envelope and spikes and makes the virus appear dark. It is mainly used to observe viral morphology in thin sections.
1. Standard Heavy Metal Staining
It is the process where sections are treated with heavy metal solutions such as uranyl acetate and lead citrate. These salts penetrate into the virus and into the cellular organelles. The reaction is as follows– the electron dense metals form contrast and so internal structures is dark and clearly visible.
Some of the main features are–
- Useful for viewing viral envelope.
- It shows spikes and capsid arrangement.
- It is used in routine ultrastructural observation.
2. Tokuyasu Staining Procedure (TSP)
This is referred to as a specialised positive staining technique. It is used to visualize new morphological details of enveloped and non-enveloped viruses. It uses uranyl acetate, glutaraldehyde and polyvinyl alcohol. It is mainly used for viruses like Rotavirus, Rubella and HIV-1.
The process occurs when the section is treated at low temperature allowing fine structural preservation.
B. Immunological Positive Staining
It is the method where antigen–antibody reaction produces a visible coloured or fluorescent signal. This type is used in diagnostics for identifying viral antigens. Some of the important detection approaches are fluorescence based and enzyme based.
1. By Detection Mechanism
a. Immunofluorescence (IF)– It is the process where antibodies are attached with fluorophores like FITC or Rhodamine. The reaction is as follows– the fluorescent label emits light under fluorescence microscope showing the viral antigen site. It is used to observe viral protein accumulation inside nucleus or cytoplasm.
b. Immunohistochemistry (IHC)– It is the staining where enzyme labelled antibodies are used. The enzymes like HRP or Alkaline Phosphatase reacts with chromogen substrates such as DAB or AEC and forms coloured precipitate. These are important for tissue pathology as the staining preserves the tissue structure.
2. By Staining Protocol
a. Direct Positive Staining– It is the single step procedure where the primary antibody is directly conjugated with fluorophore or enzyme. These are–
- Rapid method.
- Low cross-reactivity.
- Lower sensitivity because no amplification occurs.
It is used in Direct Fluorescent Antibody test (DFA) for Rabies and HSV.
b. Indirect Positive Staining– It is the two step procedure. First an unlabeled primary antibody binds to the viral antigen and then a labeled secondary antibody binds to the primary antibody. This process is referred to as amplification because several secondary antibodies bind to one primary antibody.
Some of the main features are–
- High sensitivity.
- More time consuming.
- Suitable for detecting low abundance viral proteins.
3. Affinity-Based Variants
a. Avidin–Biotin Complex (ABC)– It is the method using the high affinity between avidin and biotinylated antibodies. This forms lattice like complex and results in strong signal.
b. Labeled Streptavidin Binding (LSB)– It is the method where enzyme labelled streptavidin binds with biotinylated primary antibodies. It is smaller in size and so the staining has better penetration in tissues.
Materials and reagents
1. Materials for Ultrastructural Positive Staining (TEM)
In this method, the staining solution is allowed to react with the viral particle so that electron-dense areas are formed inside the specimen. It is the process where heavy metal salts penetrate thin sections and produce a dark image under TEM. The main reagents and materials used are listed below.
Reagents
- Uranyl acetate (usually prepared in purified water) which binds with proteins and nucleic acids.
- Lead citrate which is applied after uranyl acetate for increasing contrast.
- Purified water required for preparation and washing because excess stain must be removed.
- Sodium hydroxide pellets placed in the staining chamber to prevent formation of precipitate when lead citrate is used.
Equipment and materials
- Copper grids often coated with formvar used to hold ultra-thin sections.
- Syringe filter (0.02 µm) and syringe for filtering staining solutions before use.
- Tweezers suitable for handling EM grids.
- Plastic tubes or microcentrifuge tubes for preparing the stain.
- Filter paper wedges used to remove extra stain from the edge of the grid.
2. Materials for Immunological Positive Staining (IHC and IF)
In this type the stain is produced when antibody binds with viral antigen and a detectable signal is formed. It is the process used for identification of viruses in tissues or cells.
Reagents for tissue preparation
- Fixatives such as paraformaldehyde, formalin, chilled acetone or methanol which preserve the sample.
- Permeabilization agents like Triton X-100 or Tween-20 that allow antibody entry.
- Blocking reagents like BSA, non-fat milk or normal serum that reduce non-specific binding.
- Hydrogen peroxide when endogenous peroxidase needs to be blocked in IHC.
Detection reagents
- Primary antibody specific for the viral antigen.
- Secondary antibody conjugated with fluorophores (FITC, rhodamine, Alexa dyes) or enzymes (HRP, AP).
- Chromogens like DAB or AEC in IHC which produce colored precipitates when enzyme acts on them.
Counterstains and mounting media
- DAPI or Hoechst for nuclear staining in IF.
- Evans blue which gives background contrast in rabies testing.
- Hematoxylin or methyl green for chromogenic sections.
- Glycerol-based antifade media for fluorescence, or resin-based media for permanent mounting.
Procedure of Positive Staining of Viruses
There are present two methods of Positive Staining of Viruses;
- Ultrastructural Positive Staining (TEM method)
- Immunological Positive Staining (IHC and IF methods)
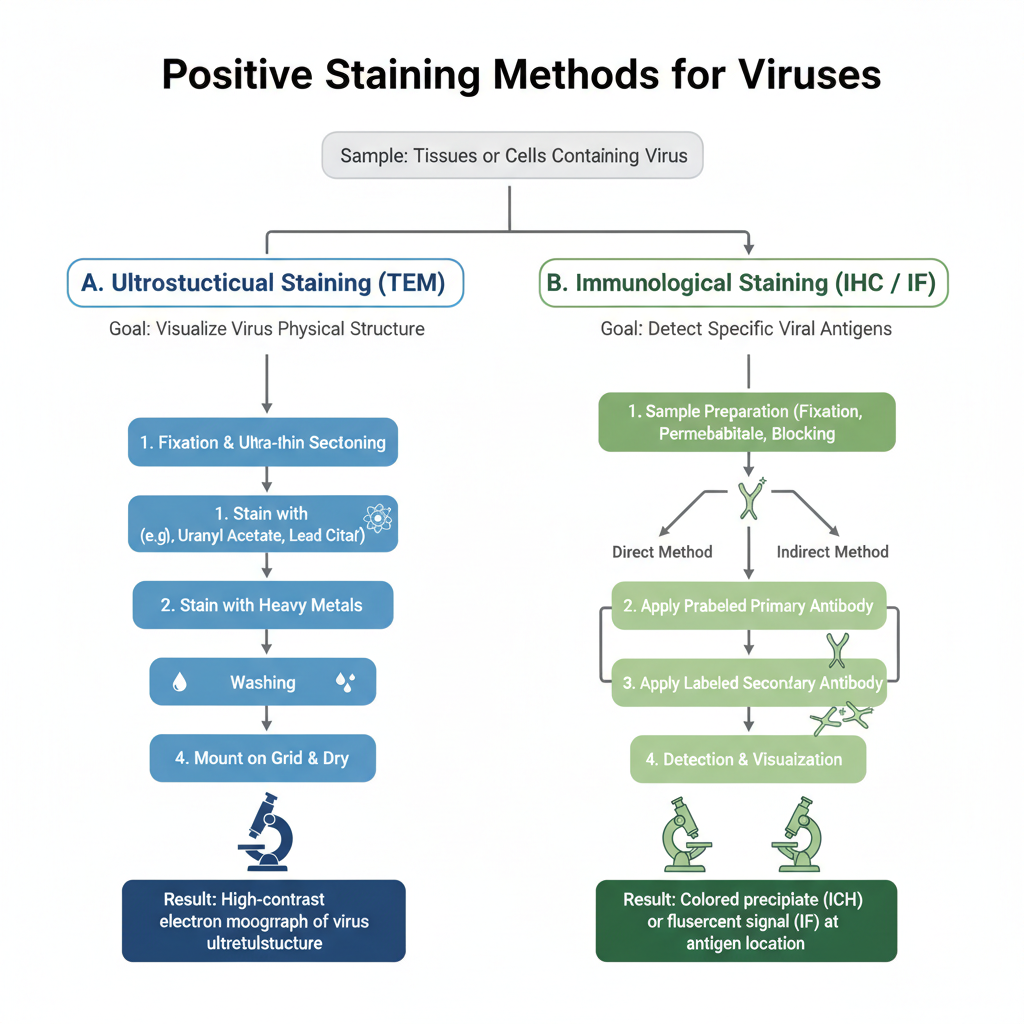
1. Ultrastructural Positive Staining Procedure (TEM)
This process is used when the virus ultrastructure is to be examined in very thin sections. The heavy metals is used to form high contrast.
Reagent Preparation
A 2% uranyl acetate solution is prepared in purified water. It is stirred for about 30 minutes to 1 hour. It is filtered through 0.02 µm filter so that undissolved particles is removed.
Steps
- Uranyl Acetate Incubation– The copper grid that hold the ultra–thin section is immersed into the uranyl acetate. Incubation time vary, sometimes 30 seconds and sometimes up to 15 minutes depending on how much penetration is needed.
- Washing– The grid is washed several times in purified water. This is necessary as it remove extra stain which otherwise form precipitates.
- Lead Citrate Incubation– The grid is now treated with lead citrate for about 4–5 minutes. This step is sensitive to CO₂ and so the staining chamber contain NaOH pellets to keep it free of CO₂.
- Drying and Viewing– Extra stain is removed by filter paper. The grid is allowed to dry in air and then observed under the TEM.
2. Immunological Positive Staining Procedures (IHC and IF)
These are used to detect viral antigens by means of antibodies. It is the process in which specific antibody bind to the antigen and produce either a fluorescent signal or a coloured precipitate.
Pre-Analytical Sample Preparation
Some of the important steps are–
- Fixation– Tissues or cells are fixed with agents like 4% PFA or chilled acetone (–20°C). It preserve morphology and help in retaining antigens.
- Antigen Retrieval (IHC only)– In fixed paraffin tissues the methylene bridges is broken by heat-induced retrieval or enzymes. This unmask the antigen.
- Permeabilization– For intracellular antigen Triton X-100 (0.1–0.5%) is used so that antibody can enter the cells.
- Blocking– Blocking is performed with normal serum or BSA. Endogenous peroxidase is blocked by hydrogen peroxide in IHC.
Direct Staining Protocol (Example: Rabies DFA)
- Application– The labelled antibody (like FITC anti-rabies antibody) is added to cover the impression smear.
- Incubation– The slide is kept in a humid chamber at 37°C for 30–45 minutes.
- Washing– Excess reagent is washed away with PBS stream for 1–2 minutes.
- Mounting– The slide is dried and mounted using a glycerol-based medium.
- Visualization– Under fluorescence microscope the apple-green bodies is seen where antigen is present.
Indirect Staining Protocol (IFA and IHC)
- Primary Antibody– The sample is incubated with the primary antibody for 1 hour at 37°C or overnight at 4°C.
- Washing– PBS is used to remove unbound antibody.
- Secondary Antibody– In IF, a fluorophore-conjugated secondary antibody is used and the step is done in dark. In IHC, an enzyme-linked secondary antibody (HRP or AP) is used.
- Detection– In IF, counterstain (like DAPI) is done and antifade medium is used. In IHC, chromogen such as DAB or AEC is added. The coloured precipitate show the antigen site.
- Visualization– IF is viewed under fluorescence microscope. IHC is viewed with a brightfield microscope.
Results and interpretation of Positive Staining of Viruses
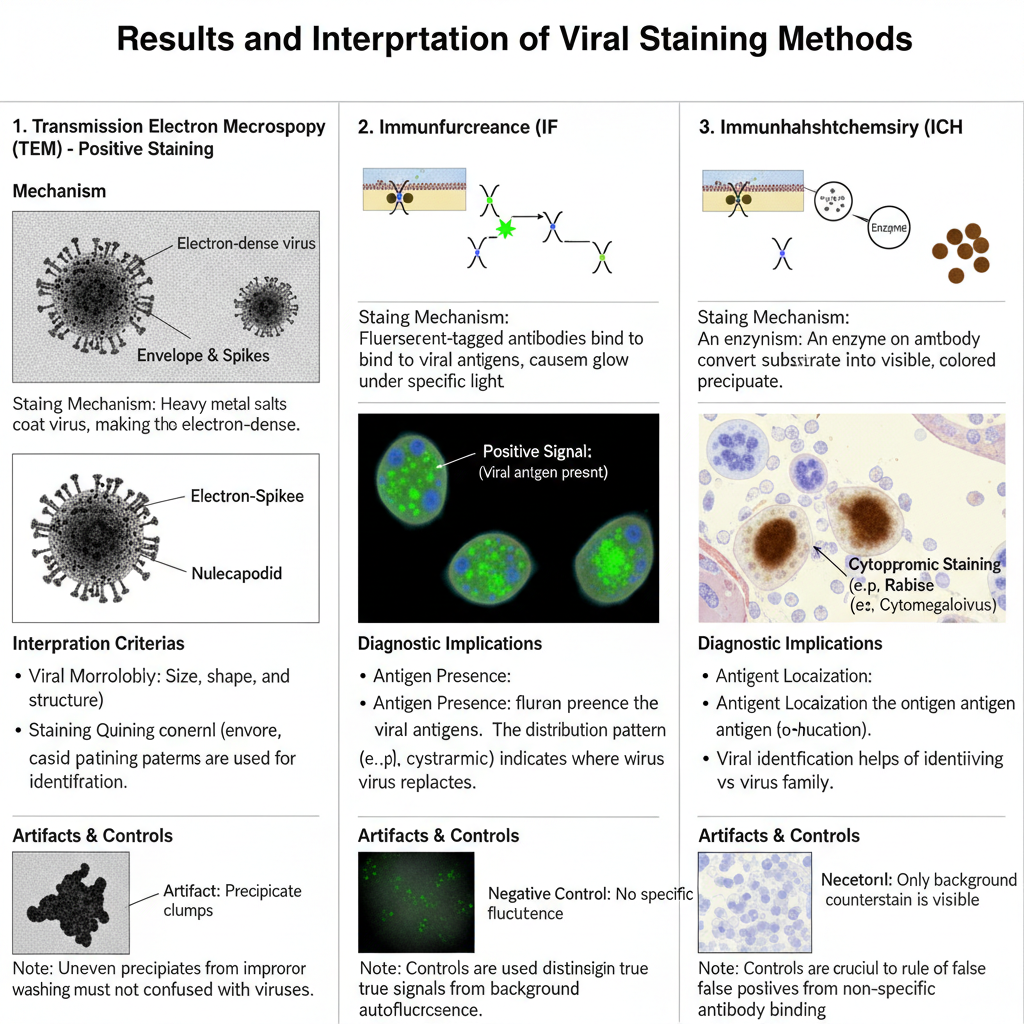
Results in Transmission Electron Microscopy (TEM)
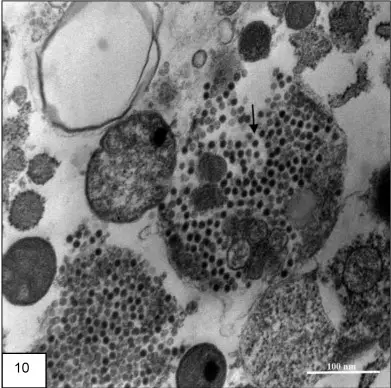
In this method the virus appear as a dark electron–dense particle. It is the process where heavy metal salts coat the viral body and absorb electrons strongly. So the background remain lighter and the viral outline is clearly visible.
Some of the main results are–
- Viral particles appear dark and granular.
- The envelope, capsid and sometimes internal structures is visible.
- Inclusion bodies are also seen in the thin sections.
Interpretation– It is interpreted based on the morphology of the virus. The arrangement of spikes, the envelope thickness, and the nucleocapsid pattern is used to identify the virus. It is also interpreted whether the staining is uniform or irregular because uneven staining may indicate artifacts. Presence of electron-dense precipitates (lead carbonate crystals) is a common artifact when washing is improper or CO₂ react with the stain. So these dense clumps must not be confused with viral particles.
Results in Immunological Staining (IF and IHC)
These are the staining reactions where antibody bind to antigen giving a coloured or fluorescent signal. It show viral antigen distribution inside the cell.
Immunofluorescence (IF/DFA)
In IF the positive result appear as bright fluorescent bodies. When FITC is used the colour is apple-green. The fluorescence may be fine dust-like particles or large cytoplasmic inclusions.
Interpretation– It is interpreted that the antigen is present wherever the fluorescence is observed. The distribution show how the virus multiply inside the cell. A negative sample show no specific fluorescence. Autofluorescence must be distinguished from a true signal by using proper controls.
Immunohistochemistry (IHC)
In IHC the positive result appear as a coloured precipitate deposited at the antigen site. DAB produce brown colour while AEC produce red colour. These precipitates remain stable on the tissue section.
Interpretation– The colour tell the presence of viral antigen. The pattern show whether the virus is located in cytoplasm or nucleus. In some viruses the intranuclear inclusions appear as concentrated dark areas while in cytoplasmic viruses the deposits form clusters around the cell membrane or cytoplasm. A negative slide show only background staining with no localized precipitate.
Diagnostic Interpretation and Localization
It is the process in which the location and intensity of staining is used to conclude viral presence.
Some of the important points are–
- Control slides are necessary because false positive reaction can occur due to nonspecific binding or reagent aggregation.
- Absence of staining indicate that antigen is not present in the examined cell.
- The staining site show the viral replication compartment. Intranuclear staining indicate viruses like cytomegalovirus. Cytoplasmic staining is seen in rabies virus, alphaviruses and similar groups.
The interpretation thus tell both the identity of virus and the region of host cell where viral multiplication is occurring.

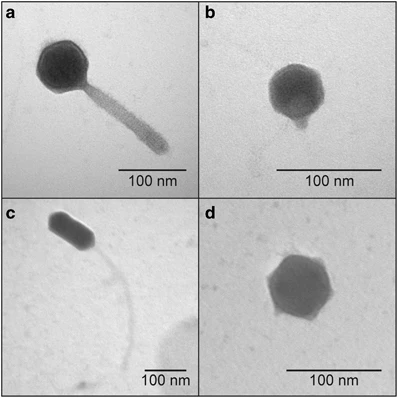
Applications of Positive Staining of Viruses
- It is used for observing internal structures of viruses present in ultra-thin sections.
- It is the process where heavy metal stains penetrate the virion so internal parts like nucleocapsid or viral core is clearly seen.
- It helps in locating viral replication sites inside host cell, and these are referred to as viral factories or inclusion bodies.
- It is used for identification of viruses based on morphology and presence of envelope or spikes.
- It is helpful for studying Orthomyxoviruses, Adenoviruses, Influenza viruses and other animal and human viruses.
- It is used in specialised staining procedures (Tokuyasu procedure) for both enveloped and non-enveloped viruses like Rotavirus, Rubella and HIV-1.
- It is used for detecting viral antigens in tissues by immunofluorescence or immunohistochemistry.
- It helps in the diagnosis of Rabies where apple-green fluorescence indicates presence of Rabies virus antigen.
- It is used for Cytomegalovirus detection in lung, gut or kidney showing intranuclear or cytoplasmic inclusions.
- It helps in identifying Herpes Simplex Virus antigens from collected lesion samples.
- It is used for observing subcellular localization of viral proteins such as nuclear, cytoplasmic or ER-associated antigens.
- It helps in co-localization studies to understand interaction of viral proteins with cell organelles.
- It is used in quantifying infection levels during antiviral drug screening by high-throughput immunostaining.
Advantages of Positive Staining of Viruses
- It is helpful for clear visualization of internal viral structures because the stain penetrates the virion.
- It is the process which gives detailed morphology including envelopes, nucleocapsid and spikes.
- It allows locating viruses inside ultra-thin tissue sections, showing viral factories and inclusion bodies.
- It provides strong contrast since heavy metals absorb electrons and produce dark image of the virus.
- It is simple in procedure and the staining can be completed in a short time.
- It is used for highly specific detection of viral antigens because antibody–antigen recognition is involved.
- It shows viral proteins in their exact tissue position which helps in understanding pathogenesis.
- It allows subcellular localization of viral proteins when immunofluorescence is used.
- It is used for multiplex staining where different fluorophores help in observing more than one antigen.
- It gives high-resolution images and these can be quantified by digital analysis.
- It preserves tissue morphology well in immunohistochemistry so the infected cells and surrounding tissue are clearly seen.
- It provides stable chromogenic signal in IHC which does not fade and is suitable for long-term specimen storage.
- It is faster in direct staining methods because fewer steps are required and it reduces cross-reactivity.
- It gives higher sensitivity in indirect staining as signal amplification occurs by multiple secondary antibodies.
Limitations of Positive Staining of Viruses
- It is the process where heavy metal stain can obscure fine surface details of viruses, so spikes or projections is not clearly seen.
- It forms artifacts because stains may react with CO₂ and produce dense precipitates which interfere with interpretation.
- It is sensitive to light since silver or uranium salts get affected when exposed to light rays.
- It may alter the appearance or orientation of viral morphology due to external chemical reactions.
- It shows photobleaching in immunofluorescence as fluorophores degrade on exposure to light.
- It gives background noise because some tissues show natural autofluorescence.
- It has interference from endogenous enzymes in IHC which can produce false-positive signals.
- It may show cross-reactivity when antibodies bind to non-specific tissue components or microbes.
- It depends on the quality of specimen, and degraded tissues can give weak or false-negative staining.
- It has lower sensitivity in direct staining because there is no amplification of signal.
- It is less flexible in direct staining as every primary antibody must be labeled separately.
- It is time-consuming in indirect staining due to extra steps and washing procedures.
- It increases background staining in indirect methods since secondary antibodies bind non-specifically sometimes.
- It may show epitope masking because fixation can hide antigenic sites requiring retrieval steps.
- It needs high-cost instruments and trained personnel to interpret the staining correctly.
FAQ
What is positive staining of viruses?
Positive staining of viruses is a method of visually detecting and quantifying virus particles in a sample using a staining agent that specifically binds to the virus.
How does positive staining differ from negative staining?
In positive staining, the staining agent binds specifically to the virus particles, resulting in their visual detection, whereas in negative staining, the background is stained, and the virus particles remain unstained.
What are the advantages of positive staining of viruses?
Positive staining provides increased sensitivity, improved morphology, quantification, ease of use, and compatibility with other techniques.
What are the disadvantages of positive staining of viruses?
Some of the disadvantages of positive staining include selectivity, interference with virus function, artifacts, cost, and time-consuming procedures.
What are some applications of positive staining of viruses?
Positive staining is used in the diagnosis of viral infections, virology research, antiviral drug development, quality control, and virus quantification.
What precautions should be taken when performing positive staining of viruses?
Precautions when performing positive staining include the use of proper safety measures, proper storage of reagents, proper sample preparation, proper technique, and proper interpretation of results.
Is positive staining of viruses a reliable method for detecting viruses?
Yes, positive staining of viruses is a reliable method for detecting viruses, provided that the proper precautions and techniques are used.
Can positive staining be used to identify specific viruses?
Yes, positive staining can be used to identify specific viruses by using staining agents that are specific to a particular type of virus.
Is positive staining of viruses expensive?
Positive staining can be more expensive than other techniques, such as negative staining, due to the cost of the staining agents and specialized equipment.
How long does positive staining of viruses take?
The time required for positive staining of viruses can vary, but it is typically a time-consuming process, especially when preparing large numbers of samples or when using techniques that require multiple steps.
- ARUP Laboratories. (n.d.). Herpes Simplex Virus DFA with Reflex to Herpes Simplex Virus Culture. ARUP Laboratories Test Directory. Retrieved from https://ltd.aruplab.com/Tests/Pub/0060280
- Boster Bio. (n.d.). IHC Principle: How Immunohistochemistry Staining Works. Retrieved from https://www.bosterbio.com/protocol-and-troubleshooting/immunohistochemistry-ihc-principle
- Centers for Disease Control and Prevention. (2025, August 4). Laboratory Methods for Rabies Testing. Retrieved from https://www.cdc.gov/rabies/php/labs-specimens/testing.html
- County of San Luis Obispo Public Health Laboratory. (n.d.). Rabies DFA. Retrieved from https://www.slocounty.ca.gov/departments/health-agency/public-health/forms-documents/laboratory/test-summaries/rabies-dfa
- Creative Diagnostics. (n.d.). Direct vs Indirect Immunofluorescence: Which is the Better Technique. Retrieved from https://www.creative-diagnostics.com/direct-vs-indirect-immunofluorescence-which-is-the-better-technique.htm
- Deshpande, G. (2025, February 17). 5 Controls for Immunofluorescence: An Easy Guide. Bitesize Bio. https://bitesizebio.com/44370/controls-for-immunofluorescence-a-beginners-guide/
- Dwivedi, B. (2022, May 29). Immunofluorescence- Definition, Principle, Types, Uses, Limitations. Microbe Notes. https://microbenotes.com/immunofluorescence/
- Expert Analysis of Positive Staining Methodologies for Viral Detection: From Ultrastructure to Molecular Diagnostics. (n.d.).
- ibidi GmbH. (2019). Immunofluorescence Assays [Application Guide]. https://ibidi.com/img/cms/resources/AG/FL_AG_039_Immunofluorescence_150dpi.pdf
- Mokobi, F. (2022, September 5). Positive staining of Viruses. Microbe Notes. https://microbenotes.com/positive-staining-of-viruses/
- NeoGenomics Laboratories. (2025, October 2). CMV (Cytomegalovirus) by IHC. Retrieved from https://www.neogenomics.com/providers/test/APH-CMIX-01AX/cmv-cytomegalovirus-by-ihc/
- Netherton, C., Moffat, K., Brooks, E., & Wileman, T. (2007). A Guide to Viral Inclusions, Membrane Rearrangements, Factories, and Viroplasm Produced During Virus Replication. Advances in Virus Research, 70, 101–182. https://doi.org/10.1016/S0065-3527(07)70004-0
- Sharada, R., & Kavitha, G. (2020). Direct Fluorescent Antibody (DFA) Test [Training presentation]. World Organisation for Animal Health (WOAH). https://rr-asia.woah.org/app/uploads/2020/11/day2_3-newdr-sharada_dfa_oie-vt_final_06112020.pdf
- Sino Biological. (n.d.). Immunofluorescence Protocol (IF Protocol). Retrieved from https://www.sinobiological.com/category/if-protocol
- Sino Biological. (n.d.). Which is the best method, IF or IHC? Retrieved from https://www.sinobiological.com/category/best-method-if-or-ihc
- Sino Biological. (n.d.). Which one is better, indirect IF or dierect IF? Retrieved from https://www.sinobiological.com/category/if-icc-faq-indirect-dierect-if
- The Native Antigen Company. (2019, January 28). Visualising viral infection with immunofluorescence microscopy. Retrieved from https://thenativeantigencompany.com/visualising-viruses-with-immunofluorescence-microscopy/
- Thermo Fisher Scientific. (n.d.). Immunofluorescence Method for IHC Detection. Retrieved from https://www.thermofisher.com/es/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/immunofluorescence-method-ihc-detection.html
- University of Florida. (2016). Laboratory No. 4: Electron Microscopy [Lecture notes]. https://plantpath.ifas.ufl.edu/classes/PLP4222-PLP6223/documents/LABORATORYNO.4LECTUREElectronMicroscopyandNegativeStaining2016_001.pdf
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.