| Kingdom | Plantae |
|---|---|
| Division (Phylum) | Pinophyta (Coniferophyta) |
| Class | Pinopsida |
| Order | Pinales |
| Family | Pinaceae |
| Genus | Pinus |
What is Pinus?
- Pinus, commonly known as pine trees, belongs to the family Pinaceae and encompasses a diverse group of evergreen conifers. These trees exhibit a wide range of heights, typically varying from 3 to 80 meters, with many species commonly reaching between 15 to 45 meters. Among the smallest members of this genus are the Siberian dwarf pine and Potosi pinyon, while the tallest pine recorded stands at an impressive height of 81.8 meters, located in southern Oregon’s Rogue River-Siskiyou National Forest.
- Pine trees are renowned for their longevity, with many species living between 100 to 1,000 years. Notably, the Great Basin bristlecone pine is among the oldest known organisms on Earth, with an estimated age of around 4,800 years. This remarkable lifespan is often complemented by their unique spiral growth patterns, which frequently align with Fibonacci number ratios. Such patterns reflect the tree’s adaptability and optimization for light exposure.
- Globally, the genus Pinus is represented by approximately 187 recognized species, according to the World Flora Online. In the United States, the American Conifer Society and the Royal Horticultural Society identify around 121 species. While pines predominantly thrive in the Northern Hemisphere, their ecological significance extends to various forest ecosystems where they provide essential habitat and sustenance for numerous wildlife species.
- From an economic perspective, pine trees hold significant value. They are primary sources of timber, utilized extensively in construction and manufacturing of paper products. Additionally, pines yield resin, which is processed into turpentine and rosin, and their seeds, known as pine nuts, are edible and nutritious. Moreover, various pine species are cultivated for ornamental purposes, reforestation initiatives, and as windbreaks, underscoring their versatility in land management.
- In terms of medicinal applications, pine-leaf oil is extracted for therapeutic uses, and various by-products, such as charcoal and fuel gases, further demonstrate the utility of pines in industrial contexts. The extensive variety of pines, with 818 named cultivars recognized by the American Conifer Society, reflects not only their aesthetic appeal but also their ecological and economic importance.
Pinus Habitat and Distribution
Pines, belonging to the genus Pinus, are among the most recognizable evergreen trees globally, found naturally across both hemispheres. Their adaptability to a wide range of climates and elevations makes them prominent in diverse ecosystems. Below is an organized exploration of their habitat and distribution based on existing knowledge.
- General Habitat Distribution:
- Pine trees are primarily located in the Northern Hemisphere but also inhabit regions in the Southern Hemisphere, especially in areas spanning from tropical to temperate climates.
- In the Northern Hemisphere, native pine species are widespread, extending from as far north as 66°N to as far south as 12°N.
- A notable species, the Sumatran pine (Pinus merkusii), is unique for growing south of the equator, specifically in Sumatra, Indonesia, at 2°S.
- Environmental Adaptability:
- Pine trees can thrive in diverse environments, including:
- Semi-arid deserts
- Rainforests
- High-altitude areas, with some species found at elevations up to 5,200 meters (17,100 feet) above sea level.
- These trees are capable of surviving in both the coldest and hottest environments on Earth.
- They are commonly found in mountainous regions where the soil is suitable and there is some access to water.
- Pine trees can thrive in diverse environments, including:
- Introduction and Cultivation in New Regions:
- Certain pine species have been introduced to temperate and subtropical regions in both hemispheres. These introductions are primarily for timber production or as ornamental plants in parks and gardens.
- Some introduced species have naturalized and, in certain instances, have become invasive, posing a threat to native ecosystems.
- Specific Distribution of Pinus:
- Pines are distributed across northern Europe, North and Central America, the subtropics of North Africa, and extend through Asia into countries like Afghanistan, Pakistan, India, Myanmar, and the Philippines. These regions are predominantly in the Northern Hemisphere.
- Some pine species spread beyond the equator, reaching Indonesia, with Pinus merkusii being the only species that crosses the equator.
- Indian Subcontinent and Neighboring Regions:
- In India, seven species of Pinus are found, four of which are restricted to the Himalayan region.
- Pinus armandii: Found in Arunachal Pradesh, along with central and western China and Taiwan.
- Pinus bhutanica: Found in Arunachal Pradesh and Bhutan.
- Pinus gerardiana: Occurs in the northwestern Himalayas, Afghanistan, Kashmir, and Himachal Pradesh.
- Pinus kesiya: Present in the Khasi and Naga hills, Manipur, Myanmar, and the Philippines.
- Pinus roxburghii: Distributed from Pakistan to Arunachal Pradesh at altitudes between 450 and 2,300 meters. This species is absent in Kashmir, where the full force of the monsoon is not felt.
- Pinus merkusii: Grows in Myanmar, Thailand, China, Indonesia, and the Philippines. It has recently been reported from Arunachal Pradesh as well.
- In India, seven species of Pinus are found, four of which are restricted to the Himalayan region.
- Forest Formation:
- Pines can form extensive pure forests, or they may grow in mixed forests in association with broad-leaved trees. These forests are characteristic of many temperate and subtropical mountain areas.
- The trees typically have a tall and pyramidal appearance in their early stages, with horizontal branches arranged in whorls. However, as they age, pines tend to lose their symmetry.
Characteristics Of Pinus
The key characteristics of Pinus are as follows:
- Evergreen Nature: Pines retain their green foliage year-round, even in harsh climates. Their needle-like leaves are coated with a waxy layer that helps conserve moisture and prevents freezing, enabling them to endure cold temperatures.
- Reproductive Cones: Reproduction in pines occurs via cones. There are two types of cones present on the tree:
- Male Cones: These are small, yellow, and responsible for producing pollen.
- Female Cones: Larger and green, they contain the seeds. The scales of the female cones hold the seeds until they are mature and ready for dispersal.
- Needle-like Leaves: The leaves of pine trees are long, slender, and pointed, closely resembling needles. These needles are grouped in clusters of two to five, depending on the species, and play a crucial role in water conservation.
- Bark Characteristics: The bark of pine trees is generally thick, rough, and scaly, providing insulation and protection from environmental elements. Its color can vary across species but is commonly brown, gray, or black.
- Monoecious Nature: Pines are monoecious, meaning that both male and female cones are present on the same tree. This allows for efficient reproduction since both reproductive organs coexist on the same plant.
- Wind Pollination: Pollination in pine trees is achieved through wind. Male cones release pollen grains into the air, which are then carried to the female cones, facilitating the fertilization process.
- Gymnosperms Classification: Pines fall under the classification of gymnosperms. Unlike angiosperms, gymnosperms have seeds that are not enclosed in an ovary. Instead, their seeds are exposed and located on the surface of the cones, relying on wind for dispersal.
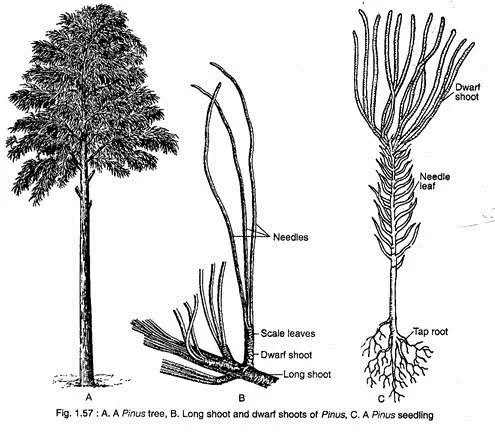
Vegetative structures of pinus
The vegetative structures of Pinus, or pines, exhibit a range of specialized adaptations that facilitate growth, nutrient acquisition, and environmental resilience. This overview elucidates the fundamental components of the root, stem, and leaf structures of Pinus, emphasizing their functions and interrelationships.
- Root Structures:
- Pinus displays two primary types of root systems:
- Long Roots: These roots possess the potential for indefinite growth and form the main root system.
- Dwarf Roots: Characterized by restricted growth and shorter lifespans, these roots contribute to the overall root structure.
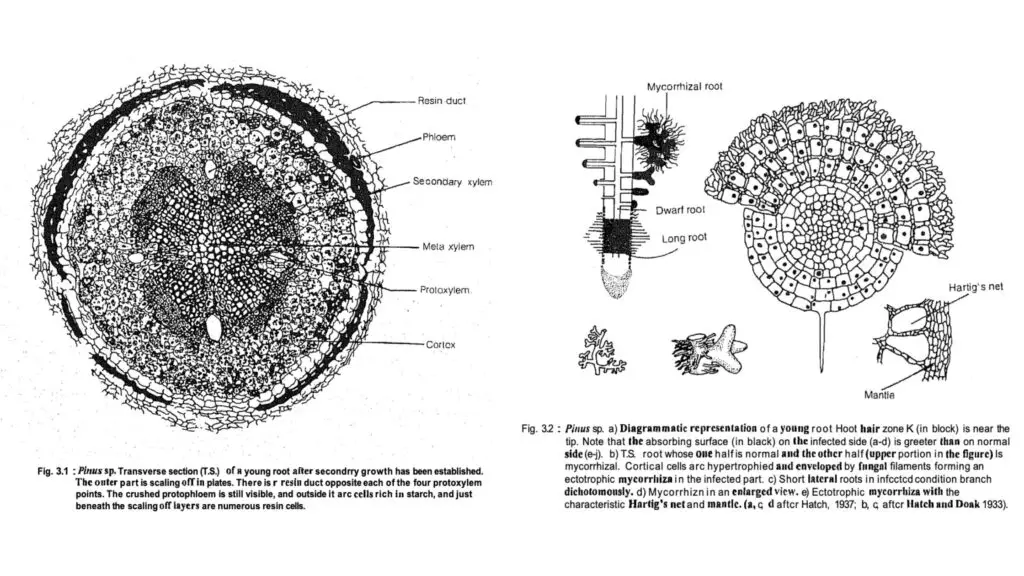
- The anatomical features of the roots include:
- Diarch or Tetrarch Arrangement: Both root types exhibit a diarch or tetrarch configuration, indicating the presence of two or four protoxylem points.
- Outer Structure: The root’s outer layer consists of an epidermis followed by a starch-filled cortex, which has an outer zone of small parenchymatous cells and an inner zone of larger cells. The endodermis is single-layered with Casparian strips, leading to a pericycle composed of 6 to 7 layers.
- Xylem and Phloem Arrangement: The xylem and phloem are radially arranged, with each protoxylem point associated with a resin duct. The metaxylem is composed of pitted tracheids, while the phloem consists of sieve cells and parenchyma.
- Secondary Growth: This process begins early, leading to structural similarities between the root and stem as they mature.
- Dwarf Roots:
- Anatomically similar to long roots, dwarf roots lack a root cap, resin ducts, starch in cortical cells, and secondary growth, resulting in a smaller cortex compared to long roots.
- Mycorrhizal Associations:
- Pinus establishes ectotrophic mycorrhizal relationships with over 50 different fungal species from the families Boletaceae and Agaricaceae. This association enhances the root system’s nutrient absorption capabilities, benefiting the pine tree through increased root surface area.
- Pinus displays two primary types of root systems:
- Stem Structures:
- The stem of Pinus is characterized as erect and woody, covered in rugged, scaly bark that peels off. The branches can be categorized into two types:
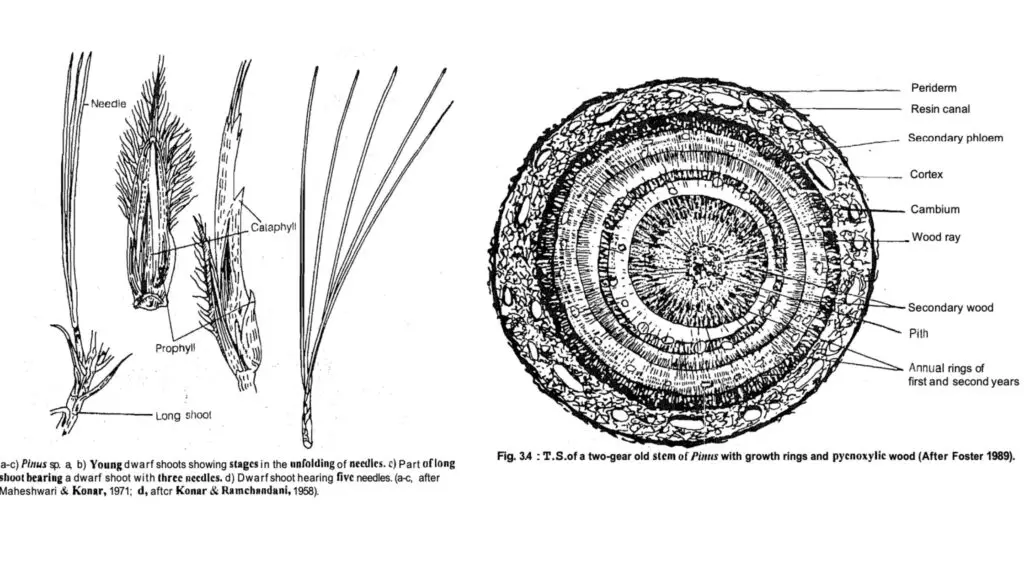
- Long Shoots: These branches exhibit unlimited growth and contain an apical bud encased in a protective bud scale. Long shoots arise as lateral buds in the axils of scale leaves, exhibiting nodal growth.
- Dwarf Shoots: Also referred to as brachyblasts or foliar spurs, these branches display limited growth and arise in the axils of scale leaves, each bearing two opposite scaly leaves (prophylls) and followed by 5-13 spirally arranged scaly cataphylls.
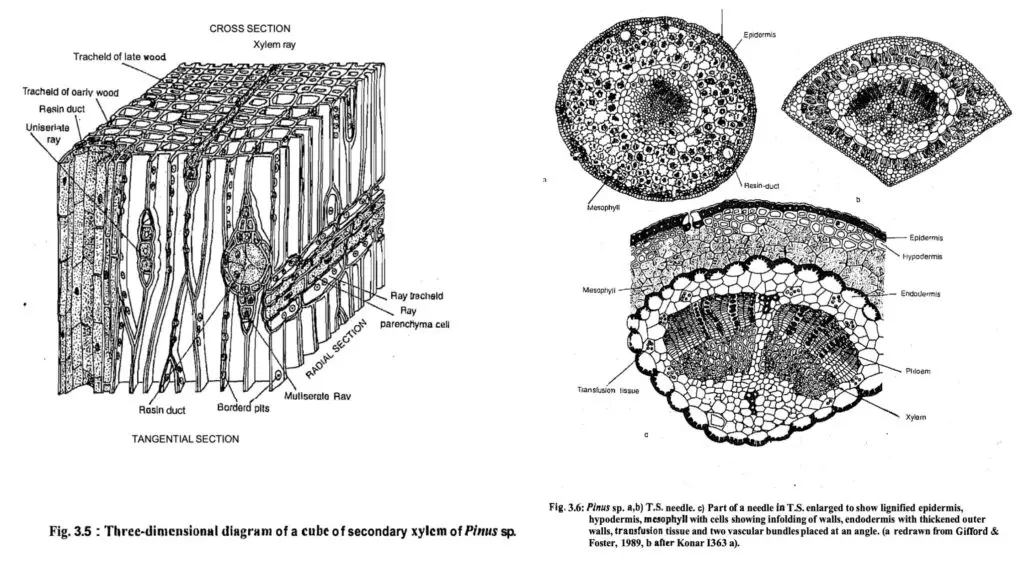
- The internal anatomy of the stem includes:
- Epidermis and Cortex: The epidermis is succeeded by a broad parenchymatous cortex, which provides structural support.
- Vascular Bundles: These consist of discrete collateral and open vascular bundles separated by broad medullary rays. Resin ducts lined with thin-walled epithelial cells are present, and their numbers increase following injury.
- Cambium and Growth: The cambium forms a continuous ring, producing secondary xylem towards the interior and secondary phloem outward. The xylem contains tracheids with bordered pits, while the phloem features sieve elements characterized by sieve plates.
- Ray Structures: The rays in the secondary xylem can be classified into uniseriate and multiseriate rays, which vary in height and structure.
- The stem of Pinus is characterized as erect and woody, covered in rugged, scaly bark that peels off. The branches can be categorized into two types:
- Leaf Structures:
- Leaves on dwarf shoots exhibit two primary types: long, needle-like foliage leaves and scale leaves, which serve protective functions. The number of needle leaves is species-specific and serves as a taxonomic characteristic.
- Primary and Secondary Leaves:
- The primary (deciduous) leaves appear shortly after germination and function as foliage. The secondary (permanent) leaves, or needles, emerge in bundles from short shoots or brachyblasts in the axils of scale leaves.
- Internal Structure of Needles:
- The needle structure consists of a single layer of epidermis, with a hypodermis beneath it. The mesophyll contains chlorenchyma with varying degrees of wall infolding and the presence of resin ducts.
- Vascular Bundle Composition: The vascular bundles comprise protoxylem and metaxylem elements, while the phloem consists of sieve cells and parenchyma. The endodermis consists of a single layer of barrel-shaped cells, surrounded by transfusion tracheids that facilitate lateral conduction of water and nutrients.
- Stomatal Arrangement: Stomata are found in longitudinal rows and are sunken, providing an adaptation for water conservation. The walls of the subsidiary and guard cells are partially lignified, enhancing their structural integrity.
Pinus Species
There are approximately 120 species of pine trees, primarily distributed across the Northern Hemisphere, and they are recognized for their needle-like leaves, woody cones, and scaly bark. These species demonstrate a variety of adaptations, enabling them to thrive in diverse environments. Below is an overview of key Pinus species:
- Scots Pine (Pinus sylvestris): A common species found across Europe and Asia, Scots pine is well-known for its resilience in poor soil conditions. This species is widely used for timber and other products due to its adaptability to various environmental factors.
- Eastern White Pine (Pinus strobus): Native to eastern North America, this species is valued for its tall, straight growth, making it highly desirable for timber. However, it is vulnerable to diseases like white pine blister rust, which can impact its health and survival in certain areas.
- Loblolly Pine (Pinus taeda): As the most widespread pine species in the southeastern United States, loblolly pine is prized for its rapid growth and use in various forestry products. Its fast-growing nature makes it a preferred choice for commercial timber production.
- Monterey Pine (Pinus radiata): Native to coastal California, the Monterey pine is a fast-growing species that has been extensively planted in regions like New Zealand, Chile, and South Africa due to its adaptability and usefulness in forestry. Its rapid growth rate makes it a significant species for plantation forestry.
- Longleaf Pine (Pinus palustris): Indigenous to the southeastern United States, the longleaf pine is a slow-growing species that historically formed vast forests across the region. However, these forests have significantly diminished due to human activities such as logging and land conversion. Longleaf pine is also recognized for its ability to endure fire-prone environments.
- Lodgepole Pine (Pinus contorta): This species is found across western North America and is renowned for its resilience in harsh conditions, including high altitudes and poor soil. The lodgepole pine is adapted to environments that other trees may struggle to inhabit, making it a key species in such regions.
- Ponderosa Pine (Pinus ponderosa): A large and long-lived species, the ponderosa pine is native to western North America. It is highly valued for its timber, although it is vulnerable to wildfires, which pose a threat to its extensive growth in some areas.
- Jack Pine (Pinus banksiana): Native to northern North America, the jack pine is a small but rugged species. It plays a crucial role in maintaining the ecological balance of the boreal forest. Additionally, this species is adapted to survive and regenerate after fires, a common occurrence in its native habitat.
- Mexican Pinyon (Pinus cembroides): Found in the mountainous regions of Mexico and the southwestern United States, the Mexican pinyon is a small pine tree that produces edible seeds. These seeds, known as pine nuts, are an important food source for both wildlife and humans in the region.
Sporophyte of Pinus
The sporophyte of Pinus is the dominant, long-lived phase in its life cycle. It manifests as a tall, evergreen tree with a well-developed system of branching and root structures. The sporophyte stage of Pinus is characterized by its large size, pyramidal shape, and complex reproductive adaptations. Below are key details about the structure and function of the sporophyte in Pinus:
- Tree Structure: Pinus is a tall, evergreen tree with a main cylindrical stem covered in scaly bark. The stem grows vertically, while a series of widespread horizontal branches extend outwards each year, forming whorls in the axils of scale leaves. This branching is mostly restricted to the upper portion of the stem, resulting in a pyramid-like appearance.
- Branching Pattern: The branches of Pinus are dimorphic, meaning they consist of two distinct types:
- Long Shoots: These are responsible for the primary elongation of the tree and contribute to the overall height and shape.
- Dwarf Shoots (Brachyblasts or Spurs): These shorter shoots bear the needles and are responsible for photosynthesis. They contribute to the tree’s foliage.
- Leaf Types: Pinus exhibits two different types of leaves:
- Scale Leaves: Small and brownish, these are protective in nature and found at the base of long shoots.
- Needles (Acicular Foliage Leaves): These are the long, slender, green leaves that cluster together in groups and are found on dwarf shoots. The needles perform the essential function of photosynthesis and are adapted to conserve water through their waxy coating.
- Root System: The sporophyte of Pinus possesses a strong tap root system that becomes elongated as the tree matures. The taproot penetrates deep into the soil, providing anchorage and access to deep water sources. Additionally, the plant develops robust lateral roots that spread out horizontally, increasing the surface area for water and nutrient absorption.
- Monoecious Nature: Pinus is a monoecious plant, meaning that both male and female reproductive organs (cones) are present on the same tree, but on different branches:
- Male Cones: These are smaller, usually found on the lower branches of the tree, and produce pollen.
- Female Cones: Larger and located on the upper branches, these cones house the ovules, which, after fertilization, develop into seeds.
Origin and Relationship of Pinus
The origin and relationship of Pinus within the larger context of conifers are rooted in its evolutionary history and anatomical characteristics. As a prominent genus of the Pinaceae family, Pinus has evolved alongside other groups of gymnosperms, sharing notable similarities and distinctions. Below is a synthesis of the origin and relationships of Pinus with other gymnospermic groups:
- Evolutionary Background: Conifers represent a large and diverse group of extant gymnosperms, with the Pinaceae family being the most significant among modern conifers. The evolution of conifers can be traced back to the Permo-Carboniferous period, particularly to the members of Voltziales, commonly referred to as “transition conifers.” During the mid-Mesozoic era, these conifers reached their climax, resulting in extensive forest formations in Northern Europe.
- Primitive and Modern Families: Within the living families of conifers, the Pinaceae and Araucariaceae families are considered more primitive, likely evolving during the Triassic period. In contrast, the Cupressaceae and Cephalotaxaceae families are relatively younger, having evolved during the Upper Jurassic to Lower Cretaceous periods.
- Relationship with Cycadales:
- Pinus shares several anatomical and developmental similarities with the order Cycadales, including:
- Stem Anatomy: Both exhibit a broad pith, a large cortex, and centripetal wood, which is indicative of their growth patterns.
- Leaf Structure: The presence of haplocheilic, sunken stomata in leaves is a characteristic feature in both groups.
- Leaf Sclerenchyma: The presence of sclerenchyma tissue in leaves provides additional support.
- Seed Structure: The seeds of both Pinus and Cycadales possess a three-layered integument, offering protection during development.
- Gamete Development: Free-nuclear divisions occur during the development of the female gametophyte, a trait shared between these groups.
- Pinus shares several anatomical and developmental similarities with the order Cycadales, including:
- Relationship with Ginkgoales:
- Similarities between Pinus and Ginkgoales include:
- Branching Habit: Both exhibit profuse branching, typically showing an excurrent habit, which enhances their growth forms.
- Shoot Dimorphism: The presence of dimorphic shoots is evident, where both long and dwarf shoots are produced.
- Wood Type: Both groups have pycnoxylic wood, characterized by its dense and compact nature.
- Leaf Structure: Leaves in both groups feature haplocheilic sunken stomata, aiding in moisture retention.
- Mature Wood Features: The mature wood shows pitting and Bars of Sanio, which are structural adaptations.
- Similarities between Pinus and Ginkgoales include:
- Relationship with Cordaitales:
- The evolutionary lineage of conifers can be traced back to the Voltziales, which are considered transitional forms between Cordaitales and modern conifers. Notable similarities between Pinus and Cordaitales include:
- Growth Habit: Both are characterized by tall, branched trees, which allows for significant vertical growth.
- Leaf Structure: The leaves are simple and exhibit parallel veins, indicating a similar structural design.
- Hypodermis Presence: The leaves contain a sclerenchymatous hypodermis, providing structural integrity.
- Wood Type: The presence of pycnoxylic wood is a trait shared between Pinus and Cordaitales.
- Pollen Characteristics: The pollen grains produced are winged, facilitating wind dispersal.
- Ovule Structure: The ovules are bilaterally symmetrical, a feature that aligns with characteristics seen in Cordaitales.
- The evolutionary lineage of conifers can be traced back to the Voltziales, which are considered transitional forms between Cordaitales and modern conifers. Notable similarities between Pinus and Cordaitales include:
Reproductive structures of pinus
Pinus, a genus of coniferous trees, exhibits distinct reproductive structures organized into male and female cones, essential for its reproductive cycle. Understanding these structures provides insights into the complex life cycle of this significant group of plants.
- Monoecious Nature: Pinus trees are monoecious, meaning they possess both male and female reproductive structures on the same individual, though these structures are located on separate branches. Male cones are typically found on lower branches, while female cones are situated on upper branches.
- Male Cone Structure:
- Male cones are formed from dwarf shoots and appear in clusters, with numbers varying by species (e.g., 15 cones in Pinus wallichiana to about 140 in Pinus roxburghii).
- Each male cone has a central axis where microsporophylls are spirally arranged. These microsporophylls bear two sporangia on their abaxial surfaces.
- The elongation of the cone axis exposes microsporangia, which dehisce longitudinally to release microspores.
- Development of Male Gametophyte:
- Development begins when one or more hypodermal cells differentiate into archesporial cells. These cells divide to form archesporial tissue, which further differentiates into a primary parietal layer and primary sporogenous cells.
- The primary sporogenous cells undergo divisions in multiple planes, leading to a mass of sporogenous tissue. Ultimately, the microspore mother cells emerge, leading to microsporogenesis.
- Meiosis occurs within microspore mother cells, producing a tetrad of haploid microspores. The microspores are released as uninucleate pollen grains, representing the first cell of the male gametophyte.
- The nucleus of the microspore divides, resulting in a small lens-shaped prothallial cell and a larger central cell, which further divides to form an antheridial initial and another prothallial cell. This division results in a mature four-celled pollen grain composed of two degenerating prothallial cells, an antheridial cell, and a tube cell.
- Female Cone Structure:
- Female cones replace long shoots and can vary in number, typically featuring 80 to 90 spirally arranged megasporophylls (ovuliferous scales).
- Each megasporophyll carries two inverted ovules on its adaxial surface. The ovules are positioned with the micropyle facing the cone’s central axis.
- In early stages, the ovuliferous scales are smaller than bracts, but post-pollination, they overgrow the bracts.
- Megasporangium and Megasporogenesis:
- Pinus ovules are characterized as unitegmic and crassinucellate, with the integument being mostly free from the nucellus except at the chalazal end. This forms a broad micropylar tube.
- A hypodermal archesporial cell, occasionally more than one, is formed at the micropylar end of the nucellus. This cell divides to produce a primary parietal cell and a primary sporogenous cell.
- The megaspore mother cell within the nucellus divides meiotically, resulting in a linear tetrad of megaspores, of which the lowest cell acts as the functional megaspore while the others degenerate.
- Development of Female Gametophyte:
- The functional megaspore enlarges, and its nucleus divides mitotically, leading to the formation of a free nuclear gametophyte. This structure maintains a constant number of nuclei specific to each species.
- As the gametophyte cellularizes, walls are laid down, forming elongated cells known as “alveoli.”
- Archegonial initials form at the micropylar end, where each initial divides into a large central cell and a small neck initial. The neck initial further divides to create a tier of cells forming the archegonial neck.
- The central cell undergoes rapid growth, resulting in a foam stage, leading to the formation of an ephemeral ventral canal cell and a large egg cell.
- The archegonium develops its own chamber through the growth of surrounding cells, which also form a jacket layer, ensuring connectivity with the surrounding gametophytic tissue.
The external morphology of Pinus species reveals a complex and specialized structure that supports its life as a large, perennial, evergreen plant. This discussion outlines the essential components of Pinus, including its branches, roots, leaves, and stem, and explores the anatomical features of its various parts, aiding students and educators in comprehending its biological significance.
- General Structure:
- Pinus is classified as a large, perennial, and evergreen plant, which means it retains its foliage year-round.
- The branches exhibit a spiral growth pattern, contributing to the plant’s characteristic conical or pyramidal appearance.
- The sporophytic body is differentiated into three primary parts: roots, stems, and acicular (needle-like) leaves.
- Roots:
- A prominent tap root is initially present, featuring few root hairs, but this root typically disappears over time.
- Subsequently, the plant develops numerous lateral roots that enhance absorption and anchorage in the soil.
- The ultimate branches of these lateral roots are covered with a layer of fungal hyphae known as ectotrophic mycorrhiza, which aids in nutrient uptake.
- Stem:
- The stem is cylindrical and erect, covered by a protective bark.
- It exhibits monopodial branching, where one main axis continues to grow while secondary branches arise.
- There are two primary types of branches: long shoots and dwarf shoots. Long shoots feature an apical bud and can grow indefinitely, while dwarf shoots lack an apical bud, limiting their growth. Dwarf shoots arise in the axils of scaly leaves on long shoots.
- Branch and Leaf Structure:
- Long shoots are characterized by numerous scaly leaves, while dwarf shoots possess two scaly leaves (called prophylls) followed by 5-13 cataphylls arranged in 2/5 phyllotaxy and 1-5 needles.
- Leaves are categorized into two types: foliage leaves and scaly leaves. Scaly leaves are thin, brown, and scale-like, while foliage leaves are large and needle-like, varying from 1 to 5 per shoot depending on the species.
- Spurs are classified based on the number of leaves present: unifoliar (one leaf), bifoliar (two leaves), trifoliar (three leaves), and so forth. Various species exhibit different spur types, such as:
- Pinus monophylla: unifoliar
- P. sylvestris: bifoliar
- P. gerardiana: trifoliar
- P. quadrifolia: quadrifoliar
- P. wallichiana: pentafoliar
- Anatomy of Different Parts:
- T.S. Young Root:
- The outer layer consists of thick-walled epiblema with numerous root hairs, followed by several layers of parenchymatous cortex.
- An endodermis layer is present, along with several layers of the pericycle.
- Vascular bundles are arranged radially, ranging from diarch to tetrarch, with exarch protoxylem. The protoxylem has a bifurcated (Y-shaped) structure, interspersed with resin canals, while the phloem alternates with the protoxylem.
- T.S. Old Root Showing Secondary Growth:
- The outer region shows several layers of cork produced by the meristematic activity of the cork cambium.
- The cork cambium also generates secondary cortex layers, containing many resin canals and stone cells.
- Below the phloem, cambium develops, producing secondary phloem outward and secondary xylem inward.
- Crushed primary phloem is located outside the secondary phloem, with uniseriate medullary rays present in the secondary xylem.
- T.S. Long Shoot (Young):
- The stem has multiple leaf bases, giving it a wavy appearance. The outer epidermis is single-layered, thick-walled, and heavily cuticularized, followed by multilayered cortex.
- The stele is identified as eustelic or polyfascicular endarch siphonostele, with vascular bundles arranged in a ring.
- The xylem consists solely of tracheids, while phloem on the ventral side comprises sieve cells, sieve plates, and albuminous cells.
- T.S. Long Shoot (Old):
- Similar to a dicotyledonous stem, the old stem exhibits secondary growth, with cork cambium producing cork outward and secondary cortex inward.
- Cambium develops between the secondary phloem and secondary xylem, with annual rings of thin-walled spring wood alternating with thick-walled autumn wood, termed pycnoxylic.
- The central region of the stem contains parenchymatous pith, while resin canals are dispersed throughout various parts.
- T.S. Dwarf Shoot (Young):
- The structure closely resembles that of the young long shoot but features a fixed number of resin canals (typically six) and vascular bundles.
- The pith is smaller compared to the long shoot.
- T.S. Dwarf Shoot (Old):
- Similar to the old long shoot, the epidermis is surrounded by scaly leaves and multilayered cortex, but lacks cork and cork cambium.
- The inner cortex consists of crushed primary phloem, secondary phloem, cambium, and secondary xylem.
- T.S. Needle (Foliage Leaf):
- The leaf shape varies by species; it can be circular, semicircular, or triangular.
- The outer epidermis features thick-walled cells and is covered with a robust cuticle, while sunken stomata are present on the epidermis.
- The leaf structure contains several layers of sclerenchymatous hypodermis, mesophyll tissue with chloroplast-filled cells, and variable resin canals.
- T.S. Young Root:
Reproduction of Pinus
Reproduction in Pinus is a well-organized process characterized by its sexual mode, involving distinct male and female reproductive structures. This process showcases the plant’s adaptations for survival and reproduction within its environment. Below is a detailed synthesis of the reproductive mechanisms of Pinus.
- Reproductive Strategy:
- Pinus reproduces sexually and is classified as monoecious, meaning that both male and female reproductive structures are present on the same individual. However, these structures are produced on separate branches, with male cones developing on the lower branches and female cones on the upper branches.
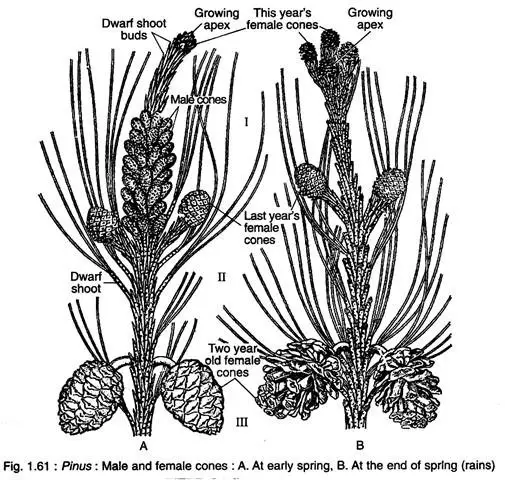
- Male Cones:
- Male cones are small, typically ranging from 2 to 4 cm in length and exhibit an oval shape. They arise in clusters from the base of the current year’s long shoot during early spring.
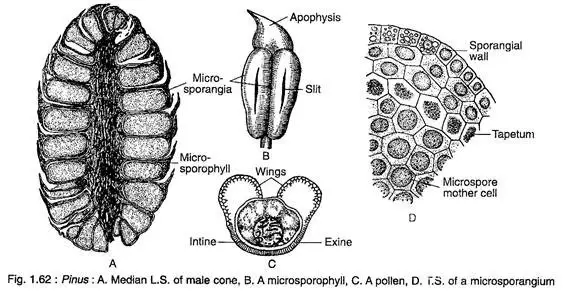
- Each male cone has a central axis with 60 to 150 spirally arranged microsporophylls. Each microsporophyll is a membranous structure with a distal part called the apophysis, which has a roughly triangular shape.
- On the abaxial surface of each microsporophyll, two sac-like microsporangia develop, originating from hypodermal cells. These microsporangia consist of a multilayered wall, a tapetum, and microspore mother cells.
- Through meiotic division, each microspore mother cell produces four microspores or pollen grains. Consequently, a mature microsporangium contains numerous pale yellow pollen grains that are boat-shaped with monosulcate apertures, enclosed by two wall layers: a thick outer exine and a thin inner intine.
- The exine on the sides of the pollen expands to form two wings, rendering the pollen bisaccate. This adaptation is significant for its anemophilous mode of pollination, as the pollen grains are released in large quantities, creating a phenomenon colloquially referred to as “sulphur showers” during the spring.
- Female Cones:
- Female cones emerge in pairs or clusters, developing very slowly over several years. The initial young cone is small (1-2 cm in length), soft, and red-green, while the second-year cone grows larger (5-8 cm), becoming woody and green. The fully matured third-year cone can reach lengths of 15-60 cm, characterized by a loose and brown structure.
- Structurally, the female cone is a compound shoot consisting of a central axis bearing 80-90 megasporophylls arranged spirally. Each megasporophyll features two types of scales: a large woody ovuliferous scale on the adaxial surface and a bract scale on the abaxial surface.
- Initially, the ovuliferous scale is smaller than the bract scale, but post-pollination, it enlarges significantly. The ovuliferous scale, a thick, woody, roughly triangular structure, develops an upper exposed part known as the apophysis, with its tip becoming the umbo in the mature cone.
- Each megasporangium (ovule) develops from superficial cells of the ovuliferous scale, a process classified as eusporangiate.
- Pollination and Fertilization:
- The pollination process in Pinus is facilitated by wind, with pollen grains dispersed and caught by the female cones. The distinct spatial arrangement of the male and female cones ensures effective pollen transfer, maximizing reproductive success.
Ovule and Megasporogenesis of Pinus
The ovule and megasporogenesis of Pinus exemplify intricate biological processes that contribute to the reproductive success of this genus. These processes involve several specialized structures and developmental stages, which are essential for understanding the lifecycle of Pinus. Below is a detailed synthesis of the ovule structure and the process of megasporogenesis.
- Ovule Structure:
- The ovules of Pinus are classified as anatropous, unitegmic, and crassinucellate.
- Each ovule features a single integument that remains detached from the nucellus except at the chalazal end.
- A notable characteristic of the ovule is the broad micropylar tube, which exhibits an inward curvature during pre-pollination stages and an outward curvature during pollination.
- The integument consists of three distinct layers: an outer fleshy layer, a middle stony layer, and an inner fleshy layer. These layers play significant roles in protecting the ovule and facilitating fertilization.
- Megasporogenesis:
- The process of megasporogenesis begins with a hypodermal cell within the nucellar tissue at the micropylar end, which differentiates into an archesporial cell.
- This archesporial cell undergoes a periclinal division, resulting in an upper parietal cell and a lower megaspore mother cell.
- The parietal cell further divides to form a tapetal layer that supports the developing megaspores.
- Subsequently, the megaspore mother cell undergoes meiotic division, producing a linear tetrad of four megaspores.
- Of these four megaspores, the outer three degenerate, leaving only the lowermost megaspore functional.
- Pollen Chamber and Pollination:
- The upper free opening of the integument forms the micropyle, while a concavity between the integument and the nucellus at the upper portion of the ovule constitutes the pollen chamber.
- After pollination occurs, pollen grains are retained in the pollen chamber, where further development takes place within the nucellar tissue, leading to the formation of the female gametophyte.
- Morphological Nature of the Ovuliferous Scale:
- There is considerable debate among scientists regarding the morphological nature of the ovuliferous scale, with several historical hypotheses proposed over time.
- Robert Brown (1827) suggested that the ovuliferous scale is an open foliar carpel bearing naked ovules situated in the axil of a bract scale.
- Schleiden (1839) posited that it represents an axillary placenta within the axil of an axillary leaf (bract scale).
- Alexander Brown (1842) theorized that the ovuliferous scale is akin to the first two leaves of a fused axillary shoot.
- Van Tieghem (1869) argued that it is a single leaf branch present in the axil of a bract.
- Delpino (1889) suggested a tripartite bract theory, where the ovuliferous and bract scales are parts of a fused bract.
- The ligular or excrescence theory of Sachs (1882) and Eichler (1889) characterized the female cone as a simple flower, with the cone axis representing the receptacle and the bract scale as free carpels.
- Braun’s brachyblast theory described the female cone as an inflorescence, with the ovuliferous scale representing a determinate axillary shoot.
- The modern hypothesis by Florin (1951) introduces the term “seed-cone complex,” proposing that the female cone of Pinus is an inflorescence with the cone axis as the peduncle, the bract scale as a true bract, and the ovuliferous scale as a rudimentary female flower. This hypothesis is substantiated by fossil evidence showing the evolutionary development of these structures.
- There is considerable debate among scientists regarding the morphological nature of the ovuliferous scale, with several historical hypotheses proposed over time.
Gametophyte of Pinus
Gametophytes of Pinus are essential components in the reproductive cycle of this conifer, representing distinct male and female structures that develop from spores. The male gametophyte is formed from microspores, while the female gametophyte originates from megaspores. This section outlines the intricate development processes of both male and female gametophytes in Pinus, providing insights into their structure, function, and role in fertilization.
- Male Gametophyte Development Before Pollination:
- The male gametophyte development in Pinus follows a pattern akin to that observed in Ginkgo. Pollen grains undergo endosporic development, which means that development occurs within the spore wall.
- Initially, the pollen nucleus divides mitotically to produce a small lens-shaped first prothallial cell towards the proximal end and a larger central cell at the distal end.
- The central cell subsequently produces a second prothallial cell and an antheridial initial, where the latter divides into a small antheridial cell and a large tube cell.
- The pollen grains are released from the microsporangium at the four-celled stage, which comprises two prothallial cells, one antheridial cell, and one tube cell.
- Male Gametophyte Development After Pollination:
- Upon pollination, the four-celled pollen grains reside in the pollen chamber for about eleven months before germinating.
- The tube cell extends through the pollen aperture to form a pollen tube that penetrates the nucellar tissue of the ovule.
- Within the pollen tube, the antheridial cell divides to yield a stalk cell and a spermatogenous (body) cell. The spermatogenous cell further divides, producing two male nuclei just prior to fertilization, which are non-motile and ephemeral.
- Female Gametophyte Development:
- The female gametophyte originates from the functional megaspore, which enlarges considerably following fertilization.
- The nucleus of the megaspore undergoes mitotic divisions, leading to a substantial increase in the number of free nuclei, which is constant for specific species. For example, P. gerardiana has about 2,000 nuclei, while P. roxburghii and P. wallichiana have approximately 2,500.
- As the megaspore enlarges, a central vacuole forms, pushing the cytoplasm and nuclei towards the periphery, resulting in the nuclei being enveloped by a thin film of cytoplasm.
- Cell wall formation begins from the periphery, creating multinucleate tube-like structures called alveoli. Cross-walls are then laid down within each alveolus, resulting in uninucleate cells that form the cellular tissue of the female gametophyte, known as the endosperm or female prothallus.
- Archegonium Development:
- The archegonia in Pinus develop similarly to those in Ginkgo. A few cells at the micropylar end of the female gametophyte enlarge, becoming archegonial initials.
- Each archegonial initial divides to form an outer primary neck initial and a large central cell. The primary neck initial divides further, producing a neck composed of four cells arranged in a single tier.
- The central cell grows rapidly and develops vacuoles, eventually leading to the formation of an ephemeral ventral canal cell and a large egg cell.
- A nutritive layer, called the archegonial jacket, surrounds the archegonium, while the surrounding nucellar tissue reorganizes to form an archegonial chamber.
- Pollination Process:
- Pinus is characterized as an anemophilous species, meaning it relies on wind for pollination. Pollen grains can remain suspended in the air for a time before settling.
- Concurrently, the nucellar beak in the ovule disorganizes, producing a viscous sugary liquid that emerges cyclically through the micropyle as a pollination drop. This drop captures pollen grains, which are then directed into the pollen chamber as the fluid evaporates.
- Fertilization Process:
- Fertilization occurs approximately one year after pollination. The pollen tube penetrates the archegonium’s tip, pushing through the nucellar cells.
- Enzymes secreted by the egg cell break down the pollen tube wall, releasing the two male nuclei. One of these nuclei fuses with the egg cell, resulting in the formation of a zygote.
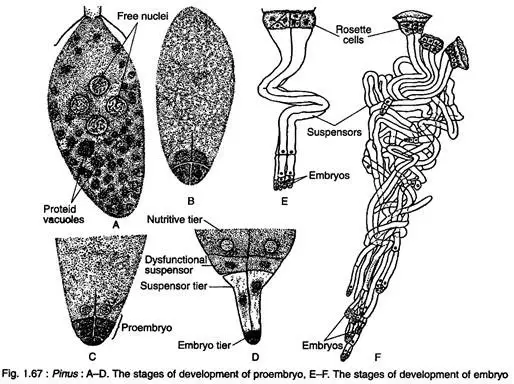
- Development of Proembryo:
- Following fertilization, the zygote nucleus undergoes two mitotic divisions, producing four nuclei that align at the base of the zygote.
- These nuclei then divide synchronously to form eight nuclei arranged in two tiers, leading to the proembryo comprising sixteen cells.
- The lowest tier is identified as the embryonal tier, which further divides to form the embryo, while other tiers fulfill various roles, including providing nutrition.
- Embryogeny:
- The developing embryonal cells are embedded within the gametophyte due to the elongation of the embryonal suspensor, which differentiates various suspensors labeled Es1, Es2, Es3, etc.
- The embryonal mass undergoes unequal elongation, leading to the formation of distinct embryonal tubes, culminating in polyembryony, where multiple embryos can arise from a single zygote. This is a specific type of cleavage polyembryony.
- Ultimately, the proembryo divides transversely to develop into an embryo, characterized by three to eighteen cotyledons, a defined epicotyl root axis, and a hypocotyl shoot axis.
- Reproductive Cycle:
- Pinus exhibits both two-year and three-year reproductive cycles. In the two-year cycle, pollination occurs in late spring of the first year, with fertilization following in the second year. In the three-year cycle, pollination occurs in the spring of the first year, while fertilization takes place in the spring of the third year, resulting in seeds being shed in autumn.
Seeds of Pinus
Seeds of Pinus are remarkable structures that play a crucial role in the plant’s reproductive cycle and dispersal strategies. Characterized by their unique winged morphology, these seeds exhibit features that enhance their survival and spread within various environments. This discussion provides an in-depth exploration of the structure, function, and germination of Pinus seeds.
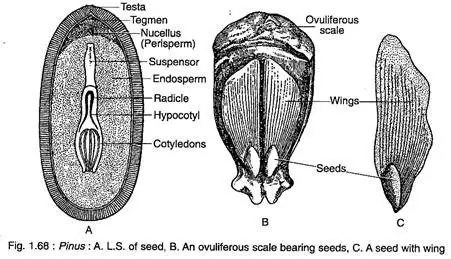
- Seed Structure:
- The seeds of Pinus are equipped with a well-developed wing that is thin, papery, and easily detachable upon reaching maturity. This wing aids in wind dispersal, allowing seeds to travel significant distances from the parent tree.
- The formation of the wing involves contributions from the outer fleshy layer of the integument and parts of the ovuliferous scale, resulting in a specialized structure that facilitates aerial dissemination.
- Seed Dispersal:
- Pinus seeds are primarily dispersed by wind. The lightweight nature of the seeds, combined with their wing structure, allows them to be carried away from the parent plant, which is essential for reducing competition and establishing new growth in suitable habitats.
- Viability and Longevity:
- One notable feature of Pinus seeds is their ability to remain viable for extended periods. This longevity increases the likelihood of successful germination when environmental conditions become favorable.
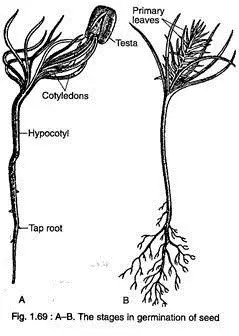
- Germination Process:
- The germination of Pinus seeds occurs through an epigeal process. In this type of germination, the cotyledons, which serve as the initial leaves of the plant, emerge above the soil surface. This adaptation allows the young plant to start photosynthesis quickly, promoting its early growth and establishment in its environment.
Pinus Uses
Pinus trees, commonly known as pines, possess a wide array of uses that extend beyond their ecological significance. These uses range from construction materials to food sources, showcasing the versatility and importance of this genus. Below is a detailed overview of some of the primary applications of Pinus:
- Construction and Timber:
- Pine wood is highly esteemed in commercial lumber industries due to several key characteristics:
- Strength and Manipulation: The wood’s structural integrity makes it suitable for framing, furniture production, plywood, and various other construction applications.
- Lightness: Compared to certain hardwoods, pine is relatively light, facilitating easier handling and transportation.
- Pine wood is highly esteemed in commercial lumber industries due to several key characteristics:
- Pulpwood and Paper:
- Several species of Pinus grow rapidly and serve as a significant source of pulpwood, which is essential for paper production. The quick growth rate of these trees makes them an economically viable choice for the industry.
- Resins and Chemicals:
- Pine trees produce resin, a sticky sap that can be harvested and processed into various products:
- Turpentine: This solvent is commonly used in paints, varnishes, and thinners, making it a crucial component in the manufacturing and painting industries.
- Rosin: Extracted from pine resin, rosin is utilized in numerous applications, including adhesives, soap, cosmetics, and other products.
- Pine trees produce resin, a sticky sap that can be harvested and processed into various products:
- Food:
- The seeds of certain Pinus species, such as the Korean pine (Pinus koraiensis) and Italian stone pine (Pinus pinea), are collected and consumed as pine nuts. These nuts are not only a nutritious food source but also add flavor to various culinary dishes.
- Fuelwood:
- Pine trees are often used as a source of firewood for heating and cooking, particularly in specific regions where they are abundant. Their accessibility and burn efficiency make them a popular choice for firewood.
- Ecological Benefits:
- Pine trees contribute significantly to their ecosystems by:
- Providing Shelter and Sustenance: They offer habitat and food for various wildlife species, promoting biodiversity.
- Soil Erosion Prevention: The root systems of pine trees help anchor soil, reducing erosion and maintaining soil health.
- Enhancing Air Quality: Pine trees play a role in improving air quality by absorbing carbon dioxide, which contributes to a healthier environment.
- Pine trees contribute significantly to their ecosystems by:
- Ornamental Purposes:
- Many pine species are favored as ornamental trees in parks, gardens, and landscapes due to their attractive evergreen foliage and distinctive shapes. Their aesthetic appeal enhances the visual quality of outdoor spaces.
- Additional Uses:
- Pine needles are employed in various crafting activities, while pine bark is often used as garden mulch, offering both functional and decorative applications in gardening.
- https://www.sscollegejehanabad.org/study-material/1981601304Punya%20morphology.pdf
- https://gacbe.ac.in/pdf/ematerial/18BBO33C-U4.pdf
- https://www.biologydiscussion.com/essay/gymnosperms/essay-on-the-life-cycle-of-pinus-class-coniferopsida-gymnosperms-botany/76971
- https://asutoshcollege.in/new-web/Study_Material/PINUS_for_students.pdf
- https://bncollegebgp.ac.in/wp-content/uploads/2020/04/Pinus.pdf
- https://ramsadaycollege.com/upload/eclassroom/Botany/GDM_Botany_Pinus_sem2_01.pdf
- https://adpcollege.ac.in/online/attendence/classnotes/files/1624597770.pdf
- https://www.pw.live/exams/neet/pinus/
- https://www.geeksforgeeks.org/life-cycle-of-pinus/
- https://www.egyankosh.ac.in/bitstream/123456789/16717/1/Unit-3.pdf