What is Pineal Gland?
- The pineal gland, a small endocrine structure located deep within the brain, plays a crucial role in regulating various physiological processes, primarily by releasing the hormone melatonin. This gland, often referred to as the “pineal body,” is situated at the geometric center of the brain, specifically in the posterior area of the cranial fossa. Its unique position and function make it an integral component of the body’s circadian rhythm, which governs sleep-wake cycles.
- The primary function of the pineal gland revolves around its secretion of melatonin. This hormone is instrumental in modulating sleep patterns by influencing cells within the brain and body. The discovery of melatonin dates back to 1958 when American physician Dr. Aaron Lerner first described its properties. Melatonin production is not constant; rather, it occurs in a rhythmic pattern that aligns closely with environmental light conditions. This cyclical release of melatonin acts as a marker for the internal circadian clock, helping to synchronize bodily functions with the solar day.
- The pineal gland’s activity is predominantly regulated by light exposure. When light enters the eyes, it stimulates specific neural pathways that communicate with the pineal gland. Consequently, during periods of darkness, melatonin production is increased, promoting sleep, while exposure to light inhibits this process, fostering wakefulness. This mechanism highlights the gland’s role as a photo-neuro-endocrine organ, integrating visual stimuli to maintain the body’s temporal rhythms.
- Interestingly, the pineal gland has historically been associated with mystical and metaphysical concepts, earning the moniker “the third eye.” This terminology arises from the gland’s structural similarities to the lateral eyes of certain vertebrates, as well as ancient beliefs that it may grant access to higher consciousness or alternate realities. Despite these assertions, scientific investigations have focused on its physiological functions rather than any metaphysical properties.
- In addition to melatonin, the pineal gland also produces other compounds, including serotonin and N,N-dimethyltryptamine (DMT). While serotonin is primarily known for its role in mood regulation, DMT has garnered attention for its potential effects on consciousness, although its specific functions within the pineal gland remain a topic of ongoing research.
- The presence of pineal-like organs in various vertebrates and even some non-vertebrates, such as insects, suggests an evolutionary significance of this gland across species. The understanding of its functions continues to evolve, emphasizing the importance of the pineal gland in maintaining the biological clock that governs sleep and wakefulness, as well as potentially influencing broader aspects of health and well-being.
Definition of Pineal Gland
The pineal gland is a small, pea-shaped endocrine gland located in the brain, responsible for producing the hormone melatonin, which regulates sleep-wake cycles and circadian rhythms. It responds to light exposure, influencing various biological processes in the body.
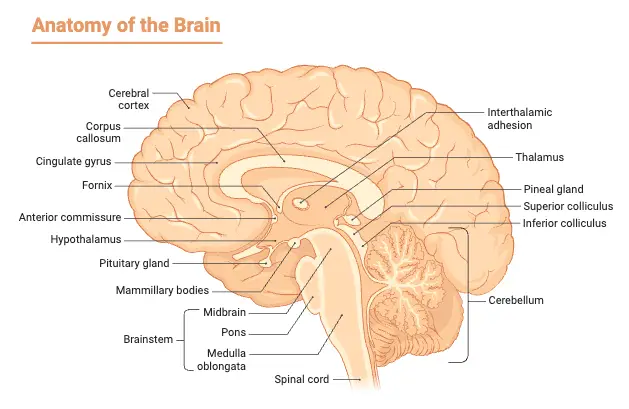
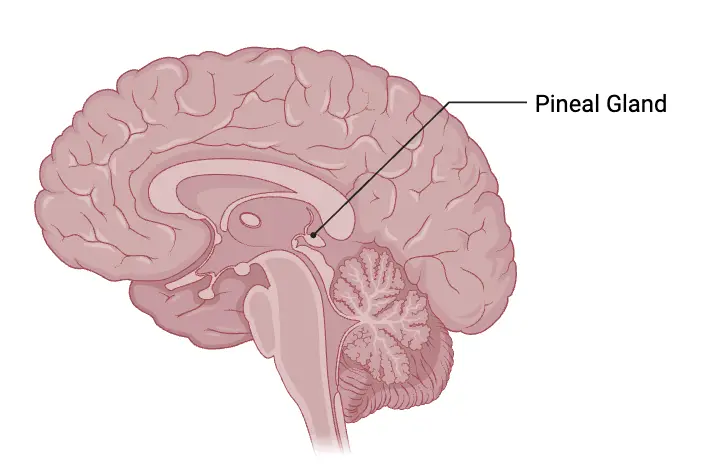
Location of Pineal Gland
- The pineal gland, a small endocrine structure in the brain, occupies a central position within the cranial cavity. It is situated between the left and right hemispheres, approximately resembling the size of a grain of rice. This gland is unique as it is the only midline brain component that is unpaired, emphasizing its singular role in the central nervous system.
- Anatomically, the pineal gland is positioned in a region known as the epithalamus, which is part of the diencephalon. This strategic location places it behind the third ventricle, contributing to its central placement within the brain’s architecture. The shape of the pineal gland resembles that of a pinecone, which is the origin of its name, symbolizing its unique structure and function.
- Moreover, the presence of the pineal gland is a common characteristic among nearly all existing vertebrates, highlighting its evolutionary significance. Its functionality in regulating circadian rhythms based on light perception underscores its primitive origins, as it plays a crucial role in sleep cycle regulation. However, some species, such as the hagfish, do not possess a distinguishable pineal gland, and certain advanced vertebrates have lost this structure throughout their evolutionary history.
Structure of Pineal Gland
The structure of the pineal gland is relatively simple, lacking complex anatomical layers; however, it comprises several functional elements essential for its role in neuroendocrine activity. The gland is a small, pine cone-shaped organ that is situated at the roof of the third ventricle of the brain. It typically weighs between 100 to 150 milligrams and is characterized by a high degree of vascularization, allowing for effective hormone secretion and regulation.
- Blood Vessels: The pineal gland is integrated into the blood-brain barrier system, featuring a network of blood vessels that facilitate the transport of hormones and nutrients essential for its functions.
- Nervous System Innervation: It receives both sympathetic and parasympathetic innervation, allowing it to regulate its hormonal output in response to environmental stimuli effectively. This dual innervation is crucial for the gland’s ability to adapt to changing light conditions.
- Cell Composition: The primary cell type in the pineal gland is the pinealocyte, which is responsible for the synthesis of melatonin. These cells are organized into compact cords and clusters, providing an efficient arrangement for hormone production.
- Interstitial Cells: In addition to pinealocytes, interstitial cells are present, which support the gland’s structure and function.
- Perivascular Phagocyte Cells: These cells help maintain the gland’s environment, contributing to its immune response and overall health.
- Calcareous Bodies: Within the gland, calcified structures known as acervuli may develop, especially as individuals age. These deposits, formed by the interaction of polypeptides secreted by pinealocytes and accumulated calcium, serve as distinctive radiographic markers for identifying the pineal gland during imaging procedures.
- Cytological Features: Pinealocytes exhibit prominent nuclei and granular cytoplasm, which contains cytoplasmic processes extending into fenestrated capillaries, facilitating the release of melatonin into the bloodstream.
- Extracellular Matrix: The extracellular space of the pineal gland is filled with neuroglial cells that provide support and insulation to the pinealocytes, ensuring optimal functioning.
Histology of Pineal Gland
The pineal gland, often referred to as the “third eye,” plays a crucial role in regulating various physiological functions, primarily through the synthesis of melatonin. Its histological structure provides insights into its functions and the cellular interactions that facilitate hormonal regulation.
- Encapsulation and Structure: The pineal gland is surrounded by a layer of pia mater, which protects it and provides structural support. Within this encapsulation, connective tissue septae extend into the gland, creating a lobulated architecture that organizes the cellular components.
- Cell Types:
- Pinealocytes:
- Comprising approximately 95% of the gland’s cellular composition, pinealocytes are the predominant cell type.
- These cells exhibit an irregular shape with multiple peripheral processes and possess large, round nuclei that stain lightly.
- Pinealocytes are essential for the production of melatonin, which is regulated by light exposure.
- This hormone is instrumental in managing circadian rhythms, which are closely controlled by the suprachiasmatic nucleus located in the hypothalamus.
- Besides regulating sleep-wake cycles, melatonin may also influence sexual maturation, thermoregulation, and various metabolic processes within the body.
- Neuroglia Cells:
- Present alongside pinealocytes, neuroglia serve supportive and protective roles within the gland.
- These glial cells contribute to the maintenance of the extracellular environment, provide structural support, and facilitate communication between pinealocytes.
- Pinealocytes:
- Corpora Arenacea (Brain Sand):
- Within the pineal gland, there exist structures known as corpora arenacea, commonly referred to as “brain sand.”
- These calcified bodies become more prominent with age and are considered a normal histological feature of the pineal gland.
- As individuals age, the accumulation of calcified material in these structures results in increased radiopacity, making them visible on radiographic imaging. This characteristic is utilized as a landmark in diagnostic procedures.
- Age-Related Changes:
- The calcification of corpora arenacea is a typical occurrence associated with aging.
- This process can be significant in clinical imaging, as it can help delineate the pineal gland’s location and assess for age-related changes or potential pathologies.
Microanatomy of Pineal Gland
The microanatomy of the pineal gland is crucial for understanding its physiological roles, primarily centered around the production and secretion of melatonin. The structure of the gland reveals a complex organization of various cell types that contribute to its functions. The pineal body is primarily composed of a lobular parenchyma made up of pinealocytes, which are surrounded by connective tissue spaces. This arrangement creates a distinctive architecture that supports the gland’s activities.
- Pinealocytes: The dominant cell type within the pineal gland, pinealocytes are characterized by their cell bodies from which 4 to 6 processes extend. These cells are responsible for synthesizing and secreting melatonin, a hormone integral to regulating circadian rhythms. When stained using specialized silver impregnation techniques, pinealocytes exhibit light basophilic cytoplasm and extensive branched processes that connect with surrounding connective septa and blood vessels, facilitating their function.
- Interstitial Cells: Nestled between pinealocytes, interstitial cells feature elongated nuclei and possess a cytoplasm that stains darker than that of pinealocytes. Their specific role in the gland’s physiology is not fully understood, but they likely contribute to the overall cellular architecture.
- Perivascular Phagocytes: Numerous capillaries are present within the pineal gland, and perivascular phagocytes reside near these blood vessels. These cells function as antigen-presenting cells, suggesting a role in the immune response and maintenance of the local environment.
- Pineal Neurons: In higher vertebrates, the presence of neurons within the pineal gland is noted; however, this is not the case in rodents. These neurons may be involved in modulating gland activity, although their exact functions require further elucidation.
- Peptidergic Neuron-like Cells: Certain species possess peptidergic neuron-like cells within the pineal gland. These cells are believed to have a paracrine regulatory role, influencing the activity of surrounding cells and contributing to the gland’s overall functionality.
The pineal gland is enveloped by a pial capsule that provides structural support and protection. Its cellular density can be quite pronounced, leading to potential misinterpretations as neoplastic growth in histological evaluations. Thus, the microanatomy of the pineal gland is characterized by a specialized organization of diverse cell types, each playing a vital role in the gland’s function and, by extension, in regulating various physiological processes such as sleep-wake cycles and hormonal balance.
Hormones of Pineal Gland – Melatonin
The pineal gland is primarily recognized for producing the hormone melatonin, which plays a significant role in regulating various physiological processes, particularly those associated with sleep and circadian rhythms. While melatonin is the main hormone secreted, the gland also produces several hormone precursors and chemicals, though their specific functions remain less understood.
- Melatonin: The principal hormone synthesized by the pineal gland, melatonin is derived from the amino acid tryptophan. Its production involves a sequence of enzymatic reactions where tryptophan is first converted into serotonin and then further processed to form melatonin. The synthesis of melatonin is primarily stimulated by darkness, utilizing post-ganglion beta-adrenergic sympathetic fibers originating from the cervical sympathetic ganglia.
- Regulation of Sleep-Wake Cycles: Melatonin levels typically rise in the evening as darkness sets in, promoting sleep and aiding in the establishment of the body’s circadian rhythm. Conversely, exposure to light during the day inhibits its production, signaling the body to be alert and awake. This diurnal pattern underscores melatonin’s critical role in sleep regulation.
- Seasonal Rhythms: Melatonin secretion is influenced by the duration of daylight, which affects seasonal behaviors in various animals, including reproduction and hibernation. In humans, variations in melatonin levels may help adapt to changes in daylight exposure across different seasons.
- Antioxidant Properties: Melatonin functions as a potent antioxidant, effectively neutralizing free radicals and protecting cells from oxidative stress. It not only scavenges harmful radicals but also stimulates the activity of other antioxidant enzymes, contributing to cellular restoration.
- Mood Regulation: Maintaining appropriate melatonin levels is associated with mood stabilization. Disruptions in melatonin production may increase the risk of mood disorders, including depression.
- Immune System Support: Melatonin plays a role in enhancing immune function, supporting the body’s defense mechanisms against infections and inflammation. It is believed to act as an anti-inflammatory agent, particularly during acute inflammatory responses.
- Hormonal Interactions: Melatonin’s effects extend to various hormonal systems. It has been observed to exert antigonadotrophic effects in children, downregulate thyroid secretion, and induce sleep while influencing norepinephrine levels, resulting in potential hypotensive effects.
- Receptors for Melatonin: The body contains multiple melatonin receptors, with the highest concentrations found in the suprachiasmatic nucleus (SCN) and the pituitary gland. These receptors are critical for melatonin’s role in regulating circadian rhythms. Other receptors are located in the ovaries, where melatonin levels can influence menstrual cycle dynamics, including the timing, duration, and frequency of cycles.
- Gut Health: Melatonin receptors are also present in the walls of blood vessels and the intestinal tract. In the gastrointestinal system, melatonin contributes to mucosal protection by mitigating free radical damage, thereby reducing the risk of conditions such as esophagitis and gastritis.
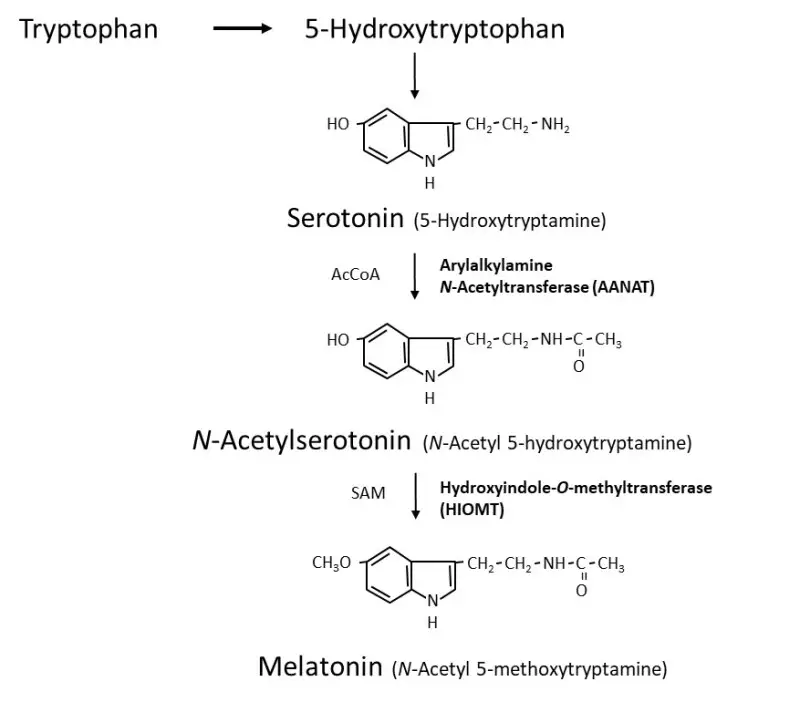
Melatonin Synthesis
Melatonin, chemically known as N-acetyl-5-methoxytryptamine, is a hormone predominantly synthesized in the pineal gland. Its production is intricately linked to the light-dark cycle, with synthesis peaking during periods of darkness. Understanding the synthesis process of melatonin involves exploring the biochemical pathways, enzymatic activities, and regulatory mechanisms involved.
- Source of Precursors: The synthesis of melatonin begins with the amino acid tryptophan, which serves as the fundamental building block. Tryptophan is converted into serotonin (5-hydroxytryptamine, 5HT), a crucial precursor in the melatonin synthesis pathway.
- Role of Serotonin-N-Acetyltransferase (AA-NAT):
- The enzyme serotonin-N-acetyltransferase, also referred to as arylalkylamine N-acetyltransferase (AA-NAT), is pivotal in the transformation of serotonin into N-acetylserotonin (NAS).
- This enzymatic reaction predominantly occurs during the dark phase, marked by a significant increase in AA-NAT activity. The elevated levels of AA-NAT facilitate the conversion of serotonin to NAS, a direct precursor of melatonin.
- Conversion to Melatonin:
- The final step in the melatonin synthesis involves the conversion of N-acetylserotonin to melatonin, catalyzed by the enzyme acetylserotonin O-methyltransferase. This step further solidifies the role of NAS as an essential intermediate in melatonin production.
- Influence of Light and Proteolysis:
- Melatonin synthesis is negatively impacted by light exposure, particularly during nighttime. The presence of light appears to trigger proteasomal proteolysis, resulting in a rapid decline in melatonin production. Therefore, the synthesis of melatonin is tightly regulated by environmental light conditions.
- Role of Norepinephrine (NE):
- Norepinephrine acts as a primary neurotransmitter influencing melatonin synthesis. It primarily activates β-1 adrenergic receptors and, to some extent, α-1b adrenergic receptors, enhancing AA-NAT activity.
- Notably, norepinephrine levels are elevated at night, occurring in a rhythmic pattern that is approximately 180 degrees out of phase with serotonin levels. Both NE and serotonin are essential stimulants for melatonin synthesis, ensuring that production aligns with the physiological requirements of the organism.
- Additional Sites of Synthesis:
- Although the pineal gland is the main site for melatonin production, evidence indicates that melatonin can also be synthesized in various other tissues, including the skin, gastrointestinal tract, retina, and bone marrow. In these locations, melatonin may function in an autocrine or paracrine manner, influencing local physiological processes.
- Recent studies have demonstrated that in the mouse brain, melatonin synthesis occurs within the mitochondrial matrix. Following synthesis, melatonin is released into the cytoplasm, where it activates mitochondrial MT1 receptors, subsequently inhibiting stress-induced pathways that lead to cell death and inflammation. This mechanism, termed automitocrine signaling, suggests that locally synthesized melatonin may play a protective role against neurodegeneration.
- Pinealectomy and Melatonin Levels:
- It is essential to note that while other tissues can synthesize melatonin, the contribution to overall circulating levels in mammals is minimal compared to that from the pineal gland. Following pinealectomy (surgical removal of the pineal gland), circulating levels of melatonin are often undetectable, emphasizing the pineal gland’s predominant role in melatonin production.
Factors Influencing Human Melatonin Secretion and Production
Melatonin, a hormone primarily secreted by the pineal gland, plays a critical role in regulating sleep-wake cycles and other biological rhythms in humans. Various factors influence the secretion and production of melatonin, which can have significant implications for health and well-being. Understanding these factors is essential for recognizing how lifestyle and environmental changes can affect melatonin levels.
- Light Exposure: Light is one of the most significant external factors influencing melatonin secretion. Bright light exposure, particularly light at wavelengths of 460-480 nm (blue light), has a suppressive effect on melatonin production. Specifically, light levels exceeding 30 lux can inhibit melatonin synthesis, making light management crucial for sleep health. Conversely, reduced light exposure during the evening allows for increased melatonin secretion, facilitating the onset of sleep.
- Sleep Timing: The timing of sleep has a secondary effect on melatonin levels, primarily influenced by light exposure. Misalignment of sleep timing, such as staying awake later in brightly lit environments, can disrupt the normal melatonin secretion pattern, leading to phase shifts in circadian rhythms.
- Posture: Research indicates that body posture can also impact melatonin production. Standing, particularly at night, may elevate melatonin levels, although the physiological mechanisms behind this are not fully understood.
- Exercise: Physical activity, especially strenuous exercise, can induce phase shifts in melatonin secretion. This relationship suggests that regular exercise may enhance the synchronization of circadian rhythms, promoting better sleep quality.
- Pharmacological Influences: Several pharmacological agents affect melatonin secretion:
- Beta-adrenergic antagonists (e.g., anti-hypertensives) generally decrease melatonin synthesis, demonstrating the impact of adrenergic signaling on hormonal regulation.
- Selective serotonin reuptake inhibitors (SSRIs) like fluvoxamine may elevate melatonin levels due to metabolic effects on serotonin pathways.
- Antidepressants can induce changes in melatonin timing; for instance, norepinephrine uptake inhibitors can alter the secretion schedule, and monoamine oxidase inhibitors may also influence melatonin phase shifts.
- Hormonal Factors: Hormonal changes can significantly impact melatonin levels:
- Testosterone appears to decrease melatonin secretion, suggesting a possible regulatory interaction between these hormones.
- Oral contraceptives (OCs) may increase melatonin production, while the effects of estradiol remain ambiguous, requiring further investigation.
- The menstrual cycle exhibits inconsistent effects on melatonin, with potential increases in amenorrhea contributing to variations in secretion patterns.
- Lifestyle Factors: Lifestyle choices also play a role in melatonin production:
- Smoking may lead to unclear changes in melatonin levels, reflecting a complex interaction with the hormonal environment.
- Alcohol consumption has a dose-dependent effect, generally resulting in decreased melatonin levels.
- Caffeine has been shown to delay the clearance of exogenous melatonin, potentially disrupting sleep patterns.
- Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): Medications like aspirin and ibuprofen may lower melatonin levels, highlighting the interaction between pain management and hormonal regulation.
- Chlorpromazine: This antipsychotic drug may increase melatonin production through metabolic pathways, underscoring the need to consider medications when evaluating melatonin levels.
Melatonin’s mechanisms of action
Melatonin is a hormone produced by the pineal gland, known for its role in regulating circadian rhythms and various physiological processes. The mechanisms of action for melatonin are intricate and encompass both receptor-mediated and non-receptor-mediated pathways, impacting multiple systems throughout the body. Understanding these mechanisms is crucial for comprehending how melatonin affects sleep, mood, and overall health.
- Target Sites and Receptors: Melatonin exerts its effects at various target sites, both central and peripheral. Notable sites in the brain include the pars tuberalis, hypothalamus, suprachiasmatic nucleus (SCN), and other regions involved in circadian regulation. Additionally, melatonin receptors are present in the immune system, gonads, kidneys, and cardiovascular tissues, highlighting its widespread influence.
- Receptor-Mediated Actions: Melatonin primarily acts through two types of G protein-coupled receptors—MT1 and MT2—both of which have distinct distribution patterns and functions.
- MT1 Receptor: Predominantly located in the pars tuberalis and SCN, MT1 receptor activation leads to acute suppression of neuronal firing, which plays a role in regulating circadian rhythms.
- MT2 Receptor: Found mainly in the SCN and retina, this receptor is involved in phase shifting, facilitating the adjustment of the biological clock in response to environmental changes.
- Affinity and Interaction: Melatonin has a higher affinity for the MT1 receptor, approximately five times greater than that for the MT2 receptor. However, the exact functional roles of these receptors in various tissues remain somewhat unclear due to the overlapping distribution of the receptors.
- Non-Receptor-Mediated Actions: Beyond receptor interactions, melatonin exhibits non-receptor-mediated actions owing to its amphipathic properties. These properties allow melatonin to cross cellular and nuclear membranes freely. As a result, it can act as an effective antioxidant, scavenging free radicals and mitigating oxidative stress within cells.
- Chronobiotic Effects: Melatonin is recognized for its chronobiotic properties, which allow it to synchronize and reset biological oscillations. It follows a phase-response curve (PRC) that defines its influence on circadian rhythms:
- Phase-Advance Zone: Early evening administration promotes an advance in the biological clock, encouraging earlier sleep onset.
- Phase-Delay Zone: Late-night administration leads to a delay in circadian rhythms, extending wakefulness.
- Non-Responsive Zone: High melatonin levels result in a period during which further melatonin administration has little effect.
- Seasonal Synchronization: Besides daily rhythms, melatonin also functions as a circannual synchronizer. The hormone’s levels fluctuate with seasonal changes, increasing during winter months and decreasing in summer. This seasonal production aligns with changes in daylight duration, influencing reproductive cycles and other physiological adaptations.
- Interaction with Circadian and Clock Genes: Melatonin’s influence on clock genes, especially in the pars tuberalis, suggests a complex interplay between melatonin and circadian regulation. While the SCN is known as the primary circadian pacemaker, melatonin may modulate gene expression and, therefore, biological rhythms, although the specifics of this modulation are still under investigation.
Diseases and Disorders of Pineal Gland
Below are some key disorders linked to the pineal gland, detailing their characteristics, causes, and potential implications.
- Pineal Gland Cysts:
- These are fluid-filled sacs that may develop within the pineal gland.
- Most cysts remain asymptomatic and benign; however, larger cysts may exert pressure on surrounding brain structures, leading to symptoms such as headaches, visual disturbances, or sleep issues.
- Surgical intervention is typically reserved for symptomatic cases.
- Pineal Tumors:
- Tumors can be either benign or malignant and are relatively rare, accounting for about 1% of all brain tumors.
- Common types include gliomas, pineal cell tumors, and germ cell tumors.
- Symptoms often manifest as headaches, nausea, seizures, visual impairments, and memory issues due to increased intracranial pressure or blockage of the cerebrospinal fluid (CSF) pathway.
- Diagnosis usually involves MRI scans, with treatment options including surgery, radiation therapy, or chemotherapy, depending on tumor type and severity.
- Melatonin Deficiency:
- A decrease in melatonin production can lead to sleep disorders, including insomnia and circadian rhythm disruptions.
- Factors contributing to melatonin deficiency include aging, shift work, and excessive exposure to artificial light, particularly at night.
- Treatment often involves melatonin supplementation and lifestyle modifications to enhance sleep hygiene.
- Melatonin Excess:
- Although rare, excessive melatonin production can occur due to certain tumors or overuse of melatonin supplements.
- Symptoms may include excessive daytime sleepiness, decreased alertness, and hormonal imbalances.
- Management typically involves addressing the underlying cause of excess melatonin.
- Seasonal Affective Disorder (SAD):
- This form of depression is linked to seasonal changes, particularly during winter months when daylight hours are reduced.
- Fluctuations in melatonin and serotonin levels, regulated by the pineal gland, are believed to contribute to the onset of SAD.
- Treatments often include light therapy and melatonin regulation to improve mood and restore normal circadian rhythms.
- Calcification of the Pineal Gland:
- Calcification involves the deposition of calcium and phosphate within the gland, a process that becomes more common with age.
- While often asymptomatic, excessive calcification can interfere with melatonin production and disrupt circadian rhythms.
- Research is ongoing to fully understand the implications of calcification on pineal function and overall health.
- Disorders Affecting Sleep and Mood:
- Given the pineal gland’s role in regulating sleep-wake cycles, disorders affecting its function can also lead to mood disorders.
- Chronic stress and poor dietary habits have been implicated in decreased melatonin levels, potentially exacerbating conditions such as depression and anxiety.
- There is evidence suggesting that low melatonin output may correlate with disturbances in cortisol levels, further influencing mood stability.
Functions of Pineal Gland
The following points provide a detailed overview of the key functions of the pineal gland:
- Secretion of Melatonin:
- The primary function of the pineal gland is the production of the hormone melatonin, which is integral to the regulation of circadian rhythms.
- Melatonin secretion is closely tied to light exposure; during periods of darkness, the gland increases melatonin production, which induces drowsiness and facilitates the onset of sleep.
- Conversely, exposure to light inhibits melatonin secretion, promoting wakefulness and alertness. This light-sensitive regulation enables the synchronization of internal biological rhythms with the external light-dark cycle, thus maintaining a healthy sleep-wake pattern.
- Regulation of Seasonal Biological Rhythms:
- Beyond daily rhythms, the pineal gland is involved in the modulation of seasonal biological processes.
- It helps organisms adapt to varying day lengths, which can influence behaviors related to reproduction and hibernation.
- In humans, this function is particularly relevant to conditions like Seasonal Affective Disorder (SAD), where fluctuations in light exposure affect mood and emotional well-being.
- Modulation of Mood:
- The pineal gland, via its production of melatonin, indirectly affects mood and emotional states.
- Adequate melatonin levels are correlated with improved mood and a lower risk of mood disorders such as depression and anxiety.
- Disruptions in melatonin production can therefore contribute to mood dysregulation, highlighting the gland’s importance in mental health.
- Influence on Reproductive Hormones:
- The pineal gland exerts influence over reproductive hormone secretion, particularly luteinizing hormone (LH) and follicle-stimulating hormone (FSH).
- This effect is more pronounced in animals, where the gland helps regulate breeding cycles based on seasonal changes, thus coordinating reproductive activities with environmental conditions.
- In humans, while the relationship is less direct, it still suggests that the pineal gland may play a role in reproductive health.
- Mediator Between Nervous and Endocrine Systems:
- The pineal gland acts as a critical mediator between the nervous and endocrine systems.
- It converts photic input from the retina into neural output, allowing the brain to respond appropriately to changes in light and dark conditions.
- This interaction underscores the gland’s role in maintaining homeostasis within the body, balancing both hormonal and neural influences.
- Influence on Drug Metabolism:
- In various mammals, including rodents, the pineal gland has been shown to influence the action of certain drugs, such as antidepressants and cocaine.
- This effect highlights the gland’s potential involvement in pharmacological responses and its broader implications for health and disease management.
- Ilahi S, Beriwal N, Ilahi TB. Physiology, Pineal Gland. [Updated 2023 Apr 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK525955/
- Arendt J, Aulinas A. Physiology of the Pineal Gland and Melatonin. [Updated 2022 Oct 30]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK550972/
- https://www.kenhub.com/en/library/anatomy/pineal-gland
- https://www.verywellhealth.com/pineal-gland-anatomy-4774967
- https://rnlkwc.ac.in/pdf/study-material/zoology/Pineal_Gland.pdf
- https://biologydictionary.net/pineal-gland/
- https://www.geeksforgeeks.org/pineal-gland/
- https://www.physio-pedia.com/Pineal_Gland
- https://pressbooks-dev.oer.hawaii.edu/anatomyandphysiology/chapter/the-pineal-gland/
- https://en.wikipedia.org/wiki/Pineal_gland
- https://anatomyinfo.com/pineal-gland-function/
- https://teachmeanatomy.info/neuroanatomy/structures/pineal-gland/
- https://geekymedics.com/the-pineal-gland/#Histology
Fantastic post! The clarity and organization of your content made it really enjoyable to read. I’m sure this will help many others as well!