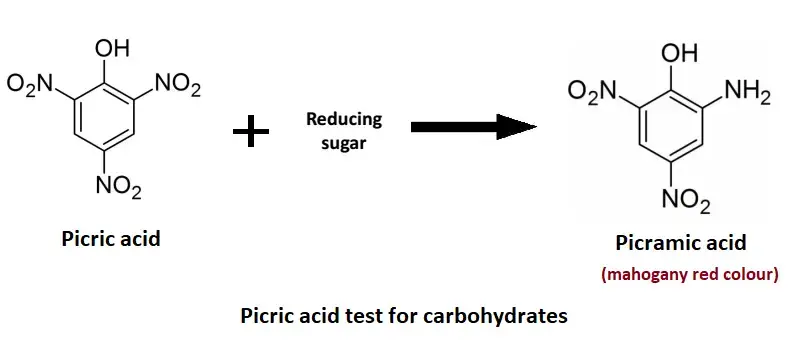
The Picric Acid Test is used to detect the presence of reducing sugars in a solution. It is a general chemical test and it is based on the reduction of picric acid under alkaline condition. Picric acid is a yellow crystalline compound (2,4,6-trinitrophenol) and it is converted into picramic acid when it reacts with a reducing agent in alkaline medium. It is the formation of a red or mahogany-red colour that indicates the presence of reducing sugar in the sample.
It is the process where an alkaline solution of picric acid is heated with the suspected solution. In this step picric acid is reduced due to the electron-donating capacity of reducing sugars like glucose or fructose. The reaction is as follows– the nitro groups of picric acid are partly reduced forming picramic acid (2-amino-4,6-dinitrophenol) which has a distinct red colour. These are the products that help in identifying the reducing nature of the compound. This is referred to as a non-specific reaction because many compounds apart from glucose can also reduce picric acid.
Some of the main features are– the test was used in older clinical methods such as the Lewis and Benedict method for estimating blood glucose. It is also the principle behind the Jaffe reaction for creatinine estimation where alkaline picrate reacts with creatinine. These methods, however, face interference since several other substances like uric acid, ascorbic acid and ketone bodies can also react, so results become higher than the actual value.
The major source of precaution is that picric acid is explosive when dry. So it is always stored moist and contact with metals is avoided because metal picrates are highly sensitive. It is important that the reagent bottle remains tightly closed and handled carefully during heating.
Principle of Picric Acid Test
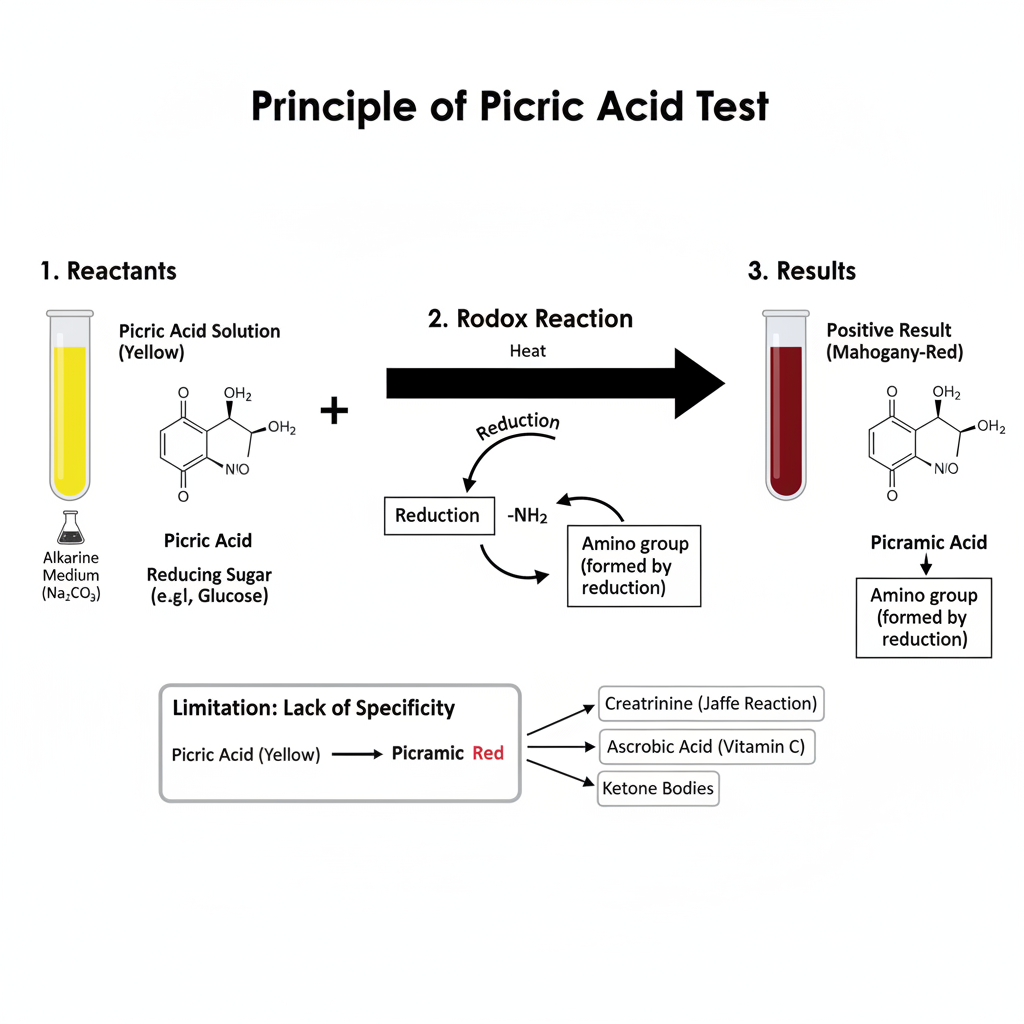
The principle of the Picric Acid Test is based on a redox reaction that occurs in strong alkaline medium where picric acid (2,4,6-trinitrophenol) is reduced by a reducing agent. It is the presence of a free aldehyde or ketone group in the carbohydrate that makes the sugar reactive in this test. In this condition the sugar undergoes enediol formation and this enediol acts as the reducing component. The yellow picric acid is reduced to picramic acid (2-amino-4,6-dinitrophenol) and the colour changes from yellow to a mahogany-red solution which indicates a positive reaction.
It is the process where the reducing sugar is oxidized and at the same time picric acid is reduced. In this step alkalinity is provided by sodium carbonate or similar base because the reaction does not occur in acidic medium. These are the changes that explain the formation of red coloured product in solution. Reducing sugars like glucose, fructose, lactose and maltose usually show this reaction while non-reducing carbohydrates remain unchanged.
This same alkaline picrate principle is used in the Jaffe reaction where creatinine reacts with alkaline picrate and forms a coloured complex. It is also referred to as a non-specific reaction because many compounds like uric acid, ascorbic acid and ketone bodies can interfere and give higher values. The major source of limitation is this lack of specificity, so interpretation needs suitable control conditions.
Requirements for Picric Acid Test
Some of the main reagents used in this test are–
- Picric acid solution (C6H3N3O7). It is used mostly as saturated solution.
- Alkali. Sodium carbonate (Na2CO3) or sodium hydroxide (NaOH) is used because the reaction occur in strong alkaline condition.
- Test sample. These are the solutions containing reducing sugar or creatinine.
It is the process that occur in strongly alkaline medium. The reagents is mixed in proper sequence and then the mixture is heated. The mixture is warmed gently so that the reduction of picric acid is formed. In this step, 5 ml of sample solution is added with 2–3 ml saturated picric acid and almost 1 ml NaOH solution, and then the tube is heated softly.
Sometimes in clinical samples, deproteination is required. This is referred to as removing protein parts from blood before measurement.
The sample must contain reducing substance. These are reducing sugars or creatinine. The reducing group helps in converting picric acid into picramic acid.
Among the important conditions, handling of picric acid is very strict. It is explosive when dried. The major safety rules are–
- It must always be stored wet under water layer. The content is checked after some months to see water is present.
- It is not kept in metal containers. Metal contact form explosive picrates.
- It is handled without metal spatula.
- After using the bottle, the cap and neck are wiped with wet cloth.
- It must be labelled with date and disposed as hazardous waste within the given period.
- If dry crystals is seen outside the bottle, it is not touched or moved. In this step, only trained personnel are allowed to handle the situation.
Procedure of Picric Acid Test
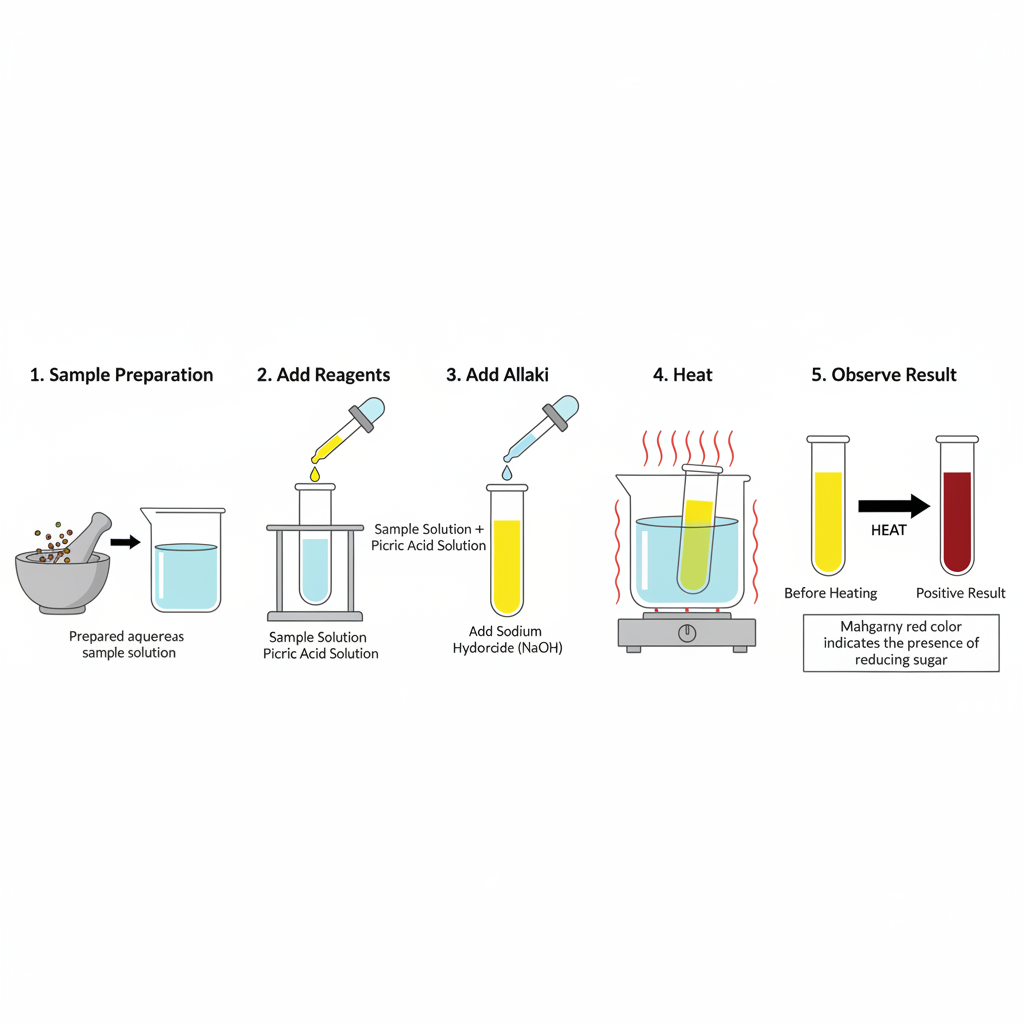
The picric acid test is carried out in alkaline medium, and it is the process where picric acid is reduced to picramic acid giving the red colour. The general steps are as follows–
- Preparation of sample– The sample solution is first taken. If the material is solid (food sample), it is crushed and mixed with distilled water so that a liquid portion is obtained for testing. This is the solution used in the reaction.
- Addition of reagents– In this step, around 1 ml to 2 ml of sample solution is placed in a clean test tube. An equal volume of saturated picric acid solution is added with the sample. The contents are allowed to mix.
- Addition of alkali -To this mixture, 1 ml of sodium hydroxide solution or sodium carbonate solution is added. It is the alkaline reagent that is needed because the reaction occur in strong basic condition and helps in forming the enediol form of reducing sugar.
- Heating– The tube is now heated softly in a boiling water bath. The heating is carried for few minutes so that the reduction of picric acid occur completely. Six test tubes can also be heated together if many samples are tested.
- Observation– After heating, the change in colour is observed. The solution turning from yellow to red indicates positive result. In positive case, a mahogany red colour is formed which is because picric acid is reduced into picramic acid (2-amino-4,6-dinitrophenol). The red coloration is the main indication for reducing sugar presence.
This is the common procedure followed for picric acid test in laboratory.
Result of Picric Acid Test

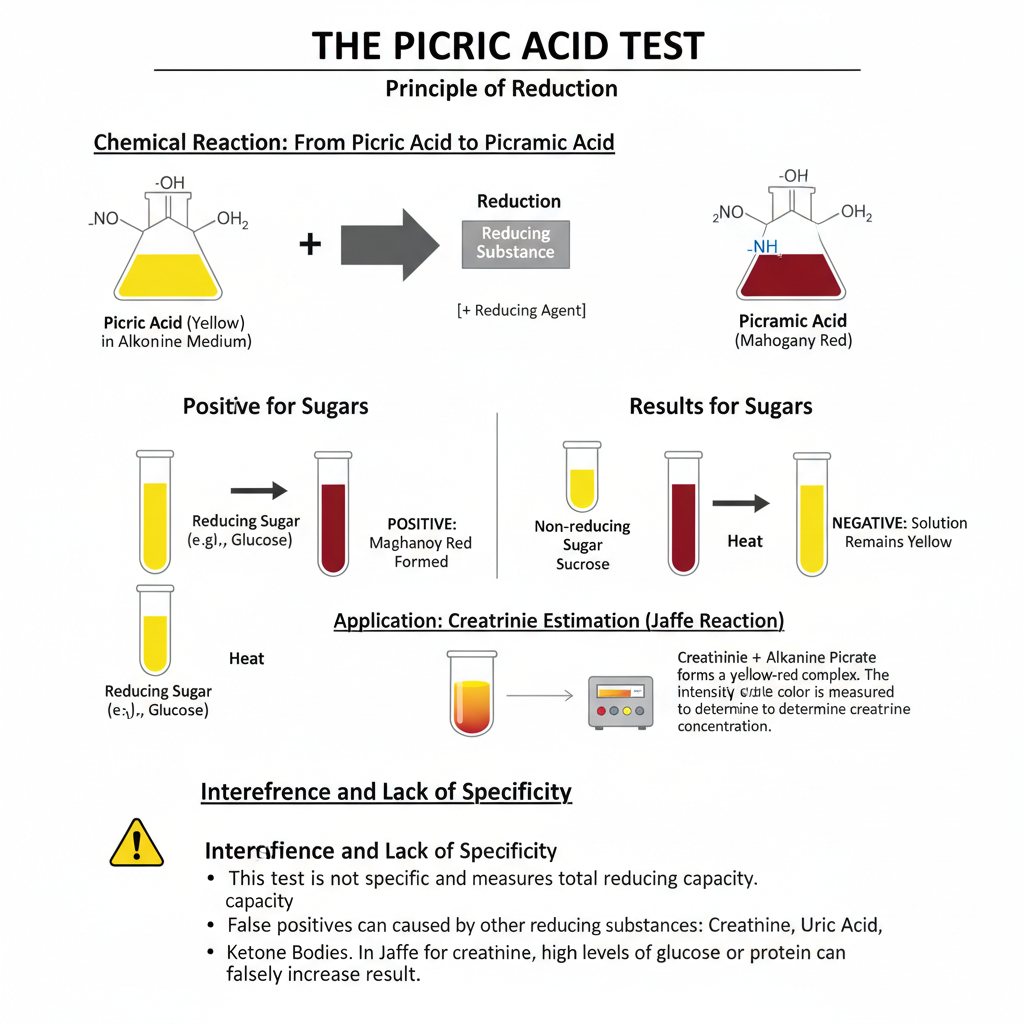
The result of the picric acid test depends on the reducing nature of the substance. It is the process where yellow picric acid is reduced in alkaline medium to picramic acid, and this produce the characteristic colour.
Positive Result
When reducing sugar is present, the colour of the solution changes from yellow to red. The formation of mahogany red solution is the main indication. This red colour appear because picric acid (2,4,6-trinitrophenol) is converted into picramic acid (2-amino-4,6-dinitrophenol).
These sugars give positive result– glucose, fructose, galactose, maltose, lactose. The presence of free aldehyde or ketone group help in reducing the picric acid.
Negative Result
Non-reducing sugars do not change the colour. In this case, the solution remain yellow. Sucrose, starch, glycogen are some common examples which do not produce the red colour.
Result in Creatinine Test (Jaffe reaction)
The same principle is used in creatinine estimation. In this step, creatinine form a yellow-red complex with alkaline picrate. This colour is measured colorimetrically and indicate the amount of creatinine in serum.
Interference
It is noted that the test is not very specific. Total reducing capacity of the sample affect the final colour. Substances like creatinine, uric acid, ascorbic acid, ketone bodies, and some drugs can also reduce picric acid and produce higher colour.
In creatinine estimation, glucose and proteins may increase the colour, while bilirubin and hemoglobin reduce the colour intensity.
Uses of Picric Acid Test
Some of the main uses of picric acid test are–
- It is used earlier for measuring blood glucose level by Lewis and Benedict method. The reaction produce mahogany red colour which is measured colorimetrically.
- This method was simple but not specific because many non-glucose reducing substances also reduce picric acid.
- It is used for measuring creatinine by Jaffe reaction. In this step, creatinine react with alkaline picrate to form yellow-red complex.
- Modern clinical laboratories use modified kinetic forms to reduce the interference from glucose, proteins or ketone bodies.
- It is used as general qualitative test for detecting reducing sugars in biochemical samples. The formation of red picramic acid indicate reducing property.
- It is used in estimation of hydrogen cyanide (HCN) by forming red isopurpurate which is measured photometrically.
- It is also used in some forensic laboratories for urine detection and for special staining methods.
Advantages of Picric Acid Test
Some of the main advantages of picric acid test are–
- It is a sensitive method for detecting proteins in urine. Picric acid form visible yellow precipitate with albumin which help in early detection of proteinuria.
- It show clear reaction with proteins, so it is used for both qualitative and quantitative study in clinical samples.
- It act as good fixative in histology. The tissues remain soft after fixation which help in sectioning without disturbing the morphology.
- It has strong affinity for basic proteins which improve staining with acid dyes and preserve biochemical features of tissues.
- It is used for detecting different analytes like reducing sugars, alkaloids, creatinine, and also used for testing picrate ions in soil and water.
- The colour formed in the reaction is easily seen. This colourimetric nature make it suitable for handling many samples in less time.
- The colour changes formed with picric acid are stable and distinct, so the test mixture become easy to read and reliable in routine laboratory use.
- It preserve glycogen in tissue sections by trapping it within precipitated proteins, which is important when glycogen study is required.
Limitations of Picric Acid Test
Some of the main limitations of picric acid test are–
Analytical limitations
- It is a non-specific reaction, so the test measure the total reducing property of the sample and not a single compound alone.
- In glucose estimation, the test cannot separate glucose from other reducing sugars like fructose, galactose, lactose, and maltose. All these sugars reduce picric acid in alkaline condition.
- In creatinine measurement by Jaffe reaction, picric acid also react with many other compounds and form coloured products, so the result become uncertain.
- Many non-glucose reducing substances present in blood or urine give positive interference. These include creatinine, uric acid, ascorbic acid, ketone bodies, and some amino acids.
- High glucose concentration can also interfere in creatinine estimation and increase the colour.
- Pigments like bilirubin and hemoglobin reduce the final colour in alkaline medium, giving negative interference.
- Many drugs and their metabolites interfere with the test. Substances like salicylates, isoniazid, streptomycin, penicillin, and some contrast media can give false positive results.
- The quantitative value depend on strict control of alkali strength, heating time and temperature. Small variations give large error because the reaction is not completely stoichiometric.
- It can give only approximate semi-quantitative estimation of reducing sugar.
Safety and handling limitations
- Picric acid is explosive when dry and is very sensitive to heat, shock and friction.
- It form metal picrates with metals like copper or iron. These metal picrates are more explosive and increase the hazard.
- It must be stored always wet under water layer. Metal caps or metal lids cannot be used because of picrate formation.
- If dry crystals appear near the cap or inside the bottle, opening the container may cause friction and explosion.
- Old containers must be disposed safely as hazardous waste because drying occur with time.
- When dryness or crystallization is seen, the bottle must not be touched or moved. Only special emergency personnel are allowed to handle such cases.
Precautions
Some of the main precautions of picric acid test are–
- Picric acid must always be stored in wet condition. A water layer is kept above the crystals so that it remain hydrated.
- The bottle is checked regularly to see the moisture level, and distilled water is added if needed.
- It is stored in cool and well-ventilated place away from heat. The container is labelled with date of receiving and opening.
- After two years, the bottle is disposed as hazardous waste because drying may occur during long storage.
- Picric acid is kept separate from other chemicals to avoid unwanted contact.
- Metal spatula is not used for handling solid picric acid. It must not touch metal caps or metal lids because metal picrates are highly explosive.
- Contact with metals like copper, iron, lead or zinc must be avoided.
- After every use, the neck and cap of the bottle are wiped with damp cloth to remove any crystal formation. The cloth is discarded as hazardous waste.
- If dry crystals are seen near the cap or threads, the bottle is not touched or moved. In this step, only trained emergency personnel are allowed to handle it.
- Proper protective equipment is used which include lab coat, safety glasses and chemical-resistant gloves. Closed shoes and long trousers must be worn.
- Picric acid solutions are handled inside fume hood.
- The volume of sample and reagents are measured accurately before mixing.
- The mixture is heated slowly. Sudden boiling is avoided because it may cause splashing and error in colour formation.
- Test tube holder is used while heating the tube in water bath.
- If the test is used quantitatively, the same heating time, alkali strength and temperature must be maintained strictly for all tubes.
- The results are interpreted carefully because many non-glucose reducing substances also reduce picric acid and give positive colour.
- Brilliant Biology Student. (2015). Benedict’s test for reducing sugars.
- El-Khateeb, A. Y. (2020). Practical biochemistry principles and techniques approach. Progress in Chemical and Biochemical Research, 3(3), 180–193. https://doi.org/10.33945/SAMI/PCBR.2020.3.1
- Erbach, M., Freckmann, G., Hinzmann, R., Kulzer, B., Ziegler, R., Heinemann, L., & Schnell, O. (2016). Interferences and limitations in blood glucose self-testing: An overview of the current knowledge. Journal of Diabetes Science and Technology, 10(5), 1161–1168.
- Flinn Scientific, Inc. (n.d.). Benedict’s quantitative solution.
- GeeksforGeeks. (2025, July 23). Benedict’s test.
- Gencheva, I. I., & Ruseva, A. (2015). Effects of glucose and bilirubin on the kinetic Jaffe’s and the enzymatic methods for serum creatinine assay. Journal of Biomedical and Clinical Research, 8(1), 35–39. https://doi.org/10.1515/jbcr-2015-0149
- Hortin, G. L., Goolsby, K., Slickers, K. A., & Sine, H. E. (1997). Editor’s note:. Clinical Chemistry, 43(2), 408–410. https://doi.org/10.1093/clinchem/43.2.408
- Jahiz, A. (n.d.). Picric acid test (Riz’s report) [Report]. Scribd.
- Makaroni, H. (n.d.). Picric acid test [Report]. Scribd.
- North West London Pathology. (2025, August 27). Reducing substances (urine).
- Rotblatt, M. D., & Koda-Kimble, M. A. (1987). Review of drug interference with urine glucose tests. Diabetes Care, 10(1), 103–110. https://doi.org/10.2337/diacare.10.1.103
- Saleem, A., Rafi, N., Qasim, S., Ashraf, U., & Virk, N. H. (2019). Synthesis of picric acid at domestic scales. International Journal of Innovations in Science and Technology, 1(2), 62–78. https://doi.org/10.33411/IJIST/2019010202
- Stanford Environmental Health & Safety. (n.d.). Information on Picric Acid.
- The Picric Acid Test for Glucose (Lewis and Benedict Method): A Retrospective Analytical Critique. (n.d.).
- University of Pittsburgh. (2010). University of Pittsburgh safety manual EH&S guideline number: 04-011—Subject: Picric acid.
- Wiener, K. (2003, September). Principles and problems of blood glucose measurement. Acutecaretesting.org.
- Wikipedia. (n.d.). Picric acid.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.