What is Phenol-Sulfuric Acid Method for Total Carbohydrates?
- The Phenol-Sulfuric Acid Method is a straightforward and rapid colorimetric technique designed to determine the total carbohydrates present in a sample. This method is versatile, detecting virtually all classes of carbohydrates, including monosaccharides, disaccharides, oligosaccharides, and polysaccharides.
- In this method, concentrated sulfuric acid breaks down polysaccharides, oligosaccharides, and disaccharides into their constituent monosaccharides. Subsequently, pentoses (five-carbon sugars) are dehydrated to form furfural, while hexoses (six-carbon sugars) dehydrate to yield hydroxymethyl furfural. These furan derivatives then react with phenol, producing a yellow-gold color.
- The intensity of the color formed is directly proportional to the carbohydrate concentration in the sample. For accurate quantification, a spectrophotometer measures the absorbance at specific wavelengths: 480 nm for pentoses and 490 nm for hexoses. Consequently, the concentration of carbohydrates is determined by comparing the sample’s absorbance to a standard curve.
- This method is reliable, with a stability window of several hours for the produced color and an accuracy margin of ±2% under optimal conditions. However, it is essential to construct the standard curve using an appropriate carbohydrate. For samples high in pentoses, such as wheat bran, xylose is used to create the standard curve. In contrast, glucose is commonly used for hexose-rich samples, such as in soft drinks and beer.
- Therefore, the Phenol-Sulfuric Acid Method not only provides a quantitative measure of total carbohydrates but also enables the calculation of caloric content in various beverages. This method remains a crucial tool in both research and industrial applications for carbohydrate analysis.
Objective of Phenol-Sulfuric Acid Method
- Find out the total amount of carbohydrates in the sample.
- Determine the total carbohydrate content of soft drinks and beers.
Principle of Phenol-Sulfuric Acid Method
The principle of the Phenol-Sulfuric Acid Method centers on the reaction of carbohydrates with strong acid and heat, leading to the production of detectable compounds. This method involves the breakdown of carbohydrates—such as simple sugars, oligosaccharides, or polysaccharides—when exposed to concentrated sulfuric acid, resulting in simpler molecular forms.
In an acidic medium, carbohydrates decompose into furan derivatives. Specifically, pentoses are converted into furfural, while hexoses dehydrate to form hydroxymethyl furfural. These furan derivatives subsequently react with phenol, creating a stable yellow-gold color.
The color intensity correlates directly with the carbohydrate concentration in the sample. Utilizing a spectrophotometer, one can measure the absorbance of this color at distinct wavelengths—480 nm for pentoses and 490 nm for hexoses. This provides a quantitative analysis of the total carbohydrates present in the sample.
This method is both reliable and straightforward, making it a preferred choice for carbohydrate analysis. The reaction yields consistent results with minimal variation, ensuring accurate and repeatable measurements. Therefore, the Phenol-Sulfuric Acid Method remains a fundamental technique in carbohydrate quantification.
Requirements for Phenol-Sulfuric Acid Method
The Phenol-Sulfuric Acid Method requires a precise set of reagents, supplies, and equipment to accurately determine total carbohydrate content in samples. Each component plays a crucial role in ensuring the method’s reliability and accuracy.
Reagents:
- Glucose Standard Solution (100 mg/L):
- Prepared by dissolving 100 mg of glucose in 1 liter of distilled water.
- Phenol Solution (80% wt/wt in H2O, 1 ml):
- Created by adding 20 g of deionized distilled water to 80 g of redistilled reagent grade phenol (crystals).
- Concentrated Sulfuric Acid (H2SO4):
- Used to decompose the carbohydrates.
Hazards, Cautions, and Waste Disposal:
- Concentrated H2SO4 is highly corrosive.
- Wear gloves and safety glasses.
- Handle with care to avoid contact with skin or clothing.
- Phenol is toxic and must be disposed of as hazardous waste.
- Follow environmental health and safety protocols for disposal.
- Non-phenol waste can generally be rinsed down the drain with water, adhering to good laboratory practices.
Supplies:
- Beverage Samples:
- Beer (lite and regular, same brand) and soft drinks (clear-colored, diet and regular, same brand).
- Cuvettes for Spectrophotometer:
- Used for measuring absorbance.
- Erlenmeyer Flasks:
- 100 ml flask for distilled water.
- 2 x 500 ml flasks for beverage samples.
- Mechanical Pipettors:
- Adjustable volume pipettors (1000 ml and 100 ml or 200 ml) with plastic tips.
- Pasteur Pipettes and Bulb:
- For transferring liquids.
- Parafilm®:
- Used to seal containers.
- Pipette Bulb or Pump:
- For accurate liquid measurement.
- Repipettor:
- For fast delivery of 5 ml concentrated H2SO4.
- Test Tubes:
- 20 tubes (16–20 mm internal diameter) for sample reactions.
- Test tube rack to hold tubes.
- Volumetric Flasks:
- 4 x 100 ml or 2 x 1000 ml for accurate liquid measurements.
- Volumetric Pipettes:
- 5 ml and 2 x 10 ml for precise volume measurements.
Equipment:
- Spectrophotometer:
- Essential for measuring the absorbance of the colored reaction product.
- Vortex Mixer:
- Used to thoroughly mix samples.
- Water Bath (maintained at 25°C):
- Ensures consistent reaction conditions.
Preparation of Solutions:
- Phenol 5% Solution:
- Dissolve 50 g of redistilled reagent grade phenol in water and dilute to 1 liter.
- Sulfuric Acid 96%:
- Use reagent grade acid.
- Standard Glucose Solution:
- Stock: Dissolve 100 mg in 100 ml of water.
- Working standard: Dilute 10 ml of stock to 100 ml with distilled water.
Procedure of Phenol-Sulfuric Acid Method
The Phenol-Sulfuric Acid Method is a widely used technique for determining the total carbohydrate content in a variety of samples. This method involves several precise steps to ensure accurate results. Below is a detailed and sequential explanation of the procedure:
1. Preparation of Standard Curve Tubes:
- Aliquots of Glucose Standard Solution:
- Use the glucose standard solution (100 mg/L) and deionized distilled (dd) water to prepare standard curve tubes.
- Pipet aliquots into clean test tubes (in duplicates) to achieve concentrations of 0–100 mg glucose in a total volume of 2 ml.
- Use a 1000 ml mechanical pipettor for accurate measurement.
- The standard curve will include: 0, 20, 40, 60, 80, and 100 mg glucose/2 ml.
- The 0 mg glucose/2 ml sample will serve as the reagent blank.
| mg Glucose/2 ml | ml glucose stock solution | ml dd water |
|---|---|---|
| 0 | 0 | 2.0 |
| 20 | 0.2 | 1.8 |
| 40 | 0.4 | 1.6 |
| 60 | 0.6 | 1.4 |
| 80 | 0.8 | 1.2 |
| 100 | 1.0 | 1.0 |
2. Recording Caloric Content:
- Analyze Beverage Samples:
- Select a regular and diet soft drink of the same brand, or a regular and lite beer of the same brand.
- Record the caloric content from the nutrition label of each sample before proceeding with the analysis.
3. Decarbonate the Beverages:
- Removing Carbonation:
- Pour approximately 100 ml of each beverage into a 500 ml Erlenmeyer flask.
- Shake gently to avoid foaming and continue until no carbon dioxide bubbles are visible.
- Filter the sample if there is any suspended material.
4. Preparation of Sample Tubes:
- Dilution Procedure:
- Prepare samples to contain 20–100 mg glucose/2 ml.
- Dilute as indicated and pipette 1.0 ml of sample into a test tube, adding 1.0 ml of dd water.
- Analyze each diluted sample in duplicate.
| Sample Type | Dilution | Volume Assayed (ml) |
|---|---|---|
| Soft drink | ||
| – Regular | 1:2000 | 1 |
| – Diet | 0 | 1 |
| Beer | ||
| – Regular | 1:2000 | 1 |
| – Lite | 1:1000 | 1 |
- Dilution Schemes:
- 1:2000 Dilution:
- Pipette 5 ml of beverage into a 100 ml volumetric flask and dilute to volume with dd water (1:20 dilution). Seal with Parafilm® and mix well.
- Pipette 1.0 ml of this 1:20 dilution into another 100 ml volumetric flask and dilute to volume with dd water. Seal with Parafilm® and mix well.
- Alternatively, pipette 1.0 ml of beverage into a 1000 ml volumetric flask and dilute to volume with dd water. Mix well.
- 1:1000 Dilution:
- Pipette 10 ml of beverage into a 100 ml volumetric flask and dilute to volume with dd water (1:10 dilution). Seal with Parafilm® and mix well.
- Pipette 1.0 ml of this 1:10 dilution into another 100 ml volumetric flask and dilute to volume with dd water. Mix well.
- Alternatively, pipette 1.0 ml of beverage into a 1000 ml volumetric flask and dilute to volume with dd water. Mix well.
- 1:2000 Dilution:
5. Addition of Phenol:
- Phenol Reaction:
- Add 0.05 ml of 80% phenol to each tube containing a total volume of 2 ml.
- Use a 100 or 200 ml mechanical pipettor for accuracy.
- Mix the contents using a Vortex mixer.
6. Addition of Sulfuric Acid:
- Sulfuric Acid Reaction:
- Add 5.0 ml of concentrated H2SO4 to each tube.
- Direct the stream of acid against the liquid surface for good mixing.
- Mix using a Vortex mixer and allow the tubes to stand for 10 minutes.
- Place tubes in a 25°C water bath for another 10 minutes to cool to room temperature.
- Vortex the tubes again before reading the absorbance.
7. Reading Absorbance:
- Spectrophotometric Analysis:
- Pour samples from test tubes into cuvettes, wearing gloves.
- Zero the spectrophotometer with the reagent blank (0 mg glucose/2 ml).
- Read absorbances at 490 nm for standard curve tubes from low to high concentration.
- Read absorbance of beverage samples.
- Ensure cuvettes are clean and dry before each reading.
8. Absorbance Spectra:
- Determining Absorbance Spectra:
- Use a standard curve sample with an absorbance of 0.5–0.8.
- Determine the absorbance spectra from 450 to 550 nm at 10 nm intervals.
- Zero the spectrophotometer with the blank at each interval.
Simple Protocol of Phenol-Sulfuric Acid Method
The Phenol-Sulfuric Acid Method is a widely used analytical procedure for quantifying carbohydrates in a sample. This method leverages the reaction between phenol, sulfuric acid, and carbohydrates to produce a colorimetric response that can be measured spectrophotometrically. Here’s a step-by-step guide to the protocol:
- Sample Preparation
- Weigh exactly 100 mg of the sample and place it into a boiling tube.
- Hydrolysis
- Add 5 mL of 2.5 N hydrochloric acid (HCl) to the boiling tube.
- Hydrolyze the sample by heating it in a boiling water bath for 3 hours.
- After hydrolysis, cool the tube to room temperature.
- Neutralization
- Neutralize the acidic solution by adding solid sodium carbonate (Na2CO3) gradually until the effervescence stops.
- Volume Adjustment and Centrifugation
- Adjust the final volume of the solution to 100 mL with distilled water.
- Centrifuge the solution to remove any particulate matter.
- Standard Curve Preparation
- Prepare a series of test tubes with the working standard solution in volumes of 0.2, 0.4, 0.6, 0.8, and 1.0 mL, respectively.
- Sample Aliquots
- Pipette 0.1 mL and 0.2 mL of the prepared sample solution into two separate test tubes.
- Adjust the volume in each test tube to 1 mL with distilled water.
- Blank Preparation
- Set up a blank by pipetting 1 mL of distilled water into an additional test tube.
- Reaction with Phenol and Sulfuric Acid
- Add 1 mL of phenol solution to each test tube containing the standards, samples, and blank.
- Carefully add 5 mL of 96% sulfuric acid to each test tube. The addition should be slow and done with caution due to the exothermic nature of the reaction.
- Mix the contents of each tube thoroughly by shaking.
- Incubation
- Allow the reaction mixture to stand for 10 minutes.
- Subsequently, place the test tubes in a water bath set to a temperature range of 25-30°C for 20 minutes.
- Measurement
- After incubation, measure the absorbance of each tube at 490 nm using a spectrophotometer.
- Calculation
- Use the absorbance readings from the standard solutions to plot a standard curve.
- Determine the concentration of total carbohydrates in the sample solution by comparing its absorbance to the standard curve.
Data and Calculations
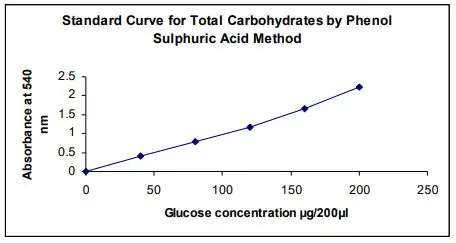
The Phenol-Sulfuric Acid Method is an essential protocol in biochemical assays to quantify carbohydrates. This section focuses on the data handling and calculations involved in the method. Accurate recording of the procedures and results is crucial for reliable analysis.
1. Summary of Procedures and Results
Table of Standards and Samples
| Tube # | Sample Identity | Dilution Scheme | mL Diluted Std or Unknown | A490 | Glucose Equivalent (mg in tube) | g/L in Original Sample |
|---|---|---|---|---|---|---|
| 1 | Blank | – | – | – | – | – |
| 2 | Std. 20mg | – | 0.2 | 0.243 | 20 | 0.10 |
| 3 | Std. 20mg | – | 0.2 | 0.243 | 20 | 0.10 |
| 4 | Std. 40mg | – | 0.4 | – | 40 | 0.20 |
| 5 | Std. 40mg | – | 0.4 | – | 40 | 0.20 |
| 6 | Std. 60mg | – | 0.6 | – | 60 | 0.30 |
| 7 | Std. 60mg | – | 0.6 | – | 60 | 0.30 |
| 8 | Std. 80mg | – | 0.8 | – | 80 | 0.40 |
| 9 | Std. 80mg | – | 0.8 | – | 80 | 0.40 |
| 10 | Std. 100mg | – | 1.0 | – | 100 | 0.50 |
| 11 | Std. 100mg | – | 1.0 | – | 100 | 0.50 |
| 12 | Soft drink, reg. | 1:2000 | 1.0 | 0.648 | 79 | 158 |
| 13 | Soft drink, reg. | 1:2000 | 1.0 | 0.648 | 79 | 158 |
| 14 | Soft drink, diet | 1:2000 | 1.0 | – | – | – |
| 15 | Soft drink, diet | 1:2000 | 1.0 | – | – | – |
| 16 | Beer, reg. | 1:2000 | 1.0 | – | – | – |
| 17 | Beer, reg. | 1:2000 | 1.0 | – | – | – |
| 18 | Beer, lite | 1:2000 | 1.0 | – | – | – |
| 19 | Beer, lite | 1:2000 | 1.0 | – | – | – |
2. Standard Curve Construction
To determine the concentration of glucose, a standard curve is constructed using absorbance (A490) versus known glucose concentrations. The standard curve is critical for translating absorbance values into glucose concentrations.
Equation of the Line
An example standard curve equation is:
y=0.011x+0.1027
where y represents the absorbance (A490), and x represents the glucose concentration in mg/2 mL.
3. Calculation of Glucose Concentration
Example Calculation for Soft Drink (Regular)
Using the standard curve equation and the measured absorbance for a regular soft drink sample:
- Measured Absorbance (A490): 0.648
- Equation of the Line: y=0.011x+0.1027
Solving for x:

4. Caloric Content Calculation
Soft Drink (Regular)
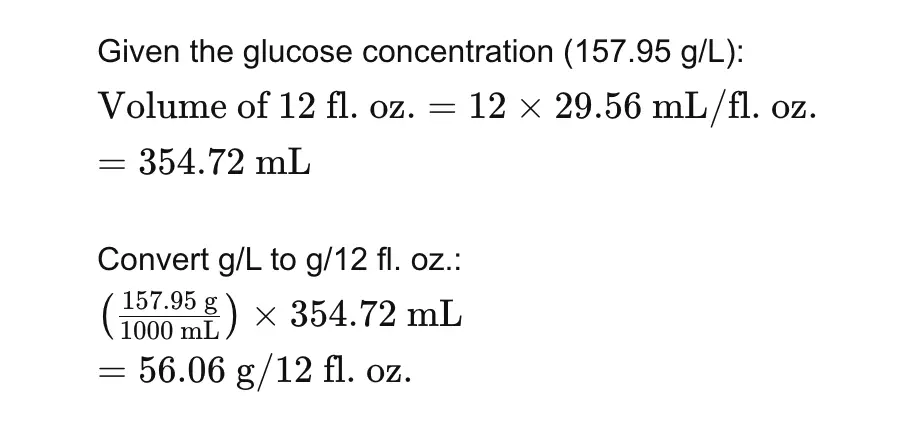
Caloric Content (Based on Carbohydrate Content)
Each gram of carbohydrate provides approximately 4 calories:
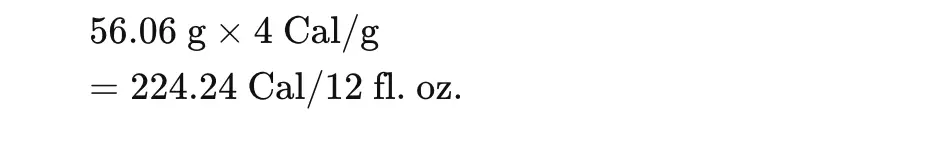
5. Absorbance Spectra
To validate the results and ensure accurate readings, it’s crucial to measure absorbance across a spectrum, typically between 450 and 550 nm. This step helps confirm that the peak absorbance is correctly recorded at 490 nm, ensuring the reliability of the carbohydrate quantification.
Example Absorbance Data
| nm | 450 | 460 | 470 | 480 | 490 | 500 | 510 | 520 | 530 | 540 | 550 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abs. | 0.10 | 0.12 | 0.18 | 0.22 | 0.65 | 0.58 | 0.50 | 0.43 | 0.38 | 0.34 | 0.30 |
Plotting this data provides a visual representation of the absorbance peak, ensuring accurate quantification.
By following these steps meticulously, the Phenol-Sulfuric Acid Method allows for precise determination of carbohydrate content in various samples, facilitating further nutritional and biochemical analyses.

Interpretation
The method of phenol sulfuric acids involves mixing a set solutions that have known glucose concentrations and the method’s phenol sulfuric acid reagent. A standard curve may be made, and the sugar concentrations of untested samples can be determined.
Advantages of Phenol-Sulfuric Acid Method
- Simplicity and Accessibility:
- Straightforward Procedure: The method involves simple steps that are easy to perform, making it accessible even for beginners in biochemical analysis.
- Minimal Equipment Requirement: Requires basic laboratory equipment, reducing the barrier to implementation in various settings.
- High Sensitivity:
- Detection of Low Concentrations: The method is highly sensitive, capable of detecting even trace amounts of carbohydrates in samples.
- Quantitative Analysis: Provides accurate quantification of carbohydrates, crucial for precise measurements in research and quality control.
- Wide Applicability:
- Versatile in Sample Types: Suitable for a wide range of sample types, including biological fluids, food products, and environmental samples.
- Compatibility with Various Carbohydrates: Can be applied to different types of carbohydrates, from simple sugars to complex polysaccharides.
- Cost-Effectiveness:
- Economical Reagents: Relies on affordable reagents such as phenol and sulfuric acid, making it a cost-effective choice for routine carbohydrate analysis.
- Batch Analysis: Allows for batch processing of samples, optimizing laboratory workflow and reducing overall costs per analysis.
- Quantitative Analysis:
- Precise Quantification: Offers precise quantification of carbohydrates through calibration with standard curves, ensuring reliable and reproducible results.
- High Accuracy: Provides accurate measurements, crucial for scientific research, nutritional labeling, and quality assurance in industries like food and pharmaceuticals.
Limitations of Phenol-Sulfuric Acid Method
- Specificity:
- Non-Specific Detection: The method detects total carbohydrates but does not differentiate between different types of carbohydrates (e.g., glucose, fructose, sucrose), which can be a limitation in certain applications requiring specific analysis.
- Interference from Impurities:
- Effect of Impurities: Presence of impurities in samples can interfere with the reaction between phenol and carbohydrates, potentially leading to inaccurate results.
- Sample Preparation: Requires careful sample preparation to minimize interference and ensure accurate carbohydrate quantification.
- Labor Intensive:
- Manual Handling: The method involves multiple manual steps, including preparation of reagents and standards, which can be labor-intensive and time-consuming.
- Precision in Handling: Requires precise handling of reagents and samples to maintain reproducibility and accuracy.
- Limited Dynamic Range:
- Detection Sensitivity: While sensitive, the method may have a limited dynamic range for quantification, particularly for samples with very low or very high carbohydrate concentrations.
- Standard Curve Adjustments: Requires careful calibration and adjustment of standard curves to accurately quantify carbohydrates across different concentration ranges.
- Environmental Considerations:
- Use of Hazardous Reagents: Involves the use of concentrated sulfuric acid, which is hazardous and requires proper handling and disposal protocols to ensure laboratory safety and environmental compliance.
- Application Scope:
- Suitability for Specific Samples: May not be suitable for all sample matrices or types of carbohydrates, limiting its applicability in certain research or industrial settings.
- Alternative Methods: Depending on the specific requirements, alternative methods with higher specificity or automation may be preferred.
Questions
1. What are the advantages, disadvantages, and sources of error for this method to determine total carbohydrates?
Advantages:
- Simplicity: The procedure is straightforward and does not require sophisticated equipment.
- Sensitivity: It can detect low concentrations of carbohydrates.
- Versatility: Applicable to a wide range of sample types, including food products, biological samples, and environmental samples.
Disadvantages:
- Destructive: The method is destructive to the sample.
- Interferences: Certain substances may interfere with the reaction, leading to inaccurate results.
- Handling: The use of strong acids and phenol requires careful handling and safety precautions.
Sources of Error:
- Impurities in Reagents: Impure reagents can introduce errors.
- Improper Mixing: Insufficient mixing of samples and reagents can lead to non-uniform reactions.
- Inconsistent Temperature: Variations in temperature during the reaction can affect the results.
- Contaminated Glassware: Residues from previous experiments can lead to erroneous readings.
2. Your lab technician performed the phenol–H2SO4 analysis on food samples for total carbohydrates but the results showed low precision, and the values seemed a little high. The technician had used new test tubes (they had never been used, and were taken right from the cardboard box). What most likely caused these results? Why? Describe what happened.
Cause of Results:
- The low precision and high values are likely due to contamination from the new test tubes.
Explanation:
- New test tubes, even when taken directly from the box, can contain residues from manufacturing processes, such as oils or plasticizers. These residues can interact with the reagents, causing higher absorbance readings and thus elevated carbohydrate values. Additionally, the lack of thorough cleaning or pre-rinsing of the new test tubes before use can introduce inconsistencies, leading to low precision in the results.
3. If you started with a glucose standard solution of 10 g glucose/liter, what dilution of this solution would be necessary such that you could pipette 0.20, 0.40, 0.60, 0.80, 1.0 mL of the diluted glucose standard solution into test tubes and add water to 2 ml for the standard curve tubes (20–100 mg/2 ml)? Show all calculations.
Dilution Calculation:
- Original glucose concentration: 10 g/L (10,000 mg/L).
- Desired concentrations: 20 mg/2 ml, 40 mg/2 ml, 60 mg/2 ml, 80 mg/2 ml, 100 mg/2 ml.
- Desired concentration per ml: 10 mg/ml, 20 mg/ml, 30 mg/ml, 40 mg/ml, 50 mg/ml.
To achieve these concentrations from 10,000 mg/L:
- For 20 mg/2 ml (10 mg/ml):
- Dilution factor = 10 mg/ml / 10,000 mg/L = 1/1000.
- Volume of standard solution needed = 0.2 ml.
- Total volume = 2 ml, so add 1.8 ml of water.
- For 40 mg/2 ml (20 mg/ml):
- Dilution factor = 20 mg/ml / 10,000 mg/L = 2/1000 = 1/500.
- Volume of standard solution needed = 0.4 ml.
- Total volume = 2 ml, so add 1.6 ml of water.
- For 60 mg/2 ml (30 mg/ml):
- Dilution factor = 30 mg/ml / 10,000 mg/L = 3/1000 = 1/333.3.
- Volume of standard solution needed = 0.6 ml.
- Total volume = 2 ml, so add 1.4 ml of water.
- For 80 mg/2 ml (40 mg/ml):
- Dilution factor = 40 mg/ml / 10,000 mg/L = 4/1000 = 1/250.
- Volume of standard solution needed = 0.8 ml.
- Total volume = 2 ml, so add 1.2 ml of water.
- For 100 mg/2 ml (50 mg/ml):
- Dilution factor = 50 mg/ml / 10,000 mg/L = 5/1000 = 1/200.
- Volume of standard solution needed = 1 ml.
- Total volume = 2 ml, so add 1 ml of water.
4. If you had not been told to do a 2000-fold dilution of a soft drink sample, and if you know the approximate carbohydrate content of regular soft drinks (U.S. Department of Agriculture Nutrient Database for Standard Reference indicates ca. 3 g carbohydrate/fl. oz.), how could you have calculated the 2000-fold dilution was appropriate if you wanted to use 1 ml of diluted soft drink in the assay. Show all calculations.
Soft Drink Carbohydrate Content:
- Regular soft drinks: approximately 3 g carbohydrate per fluid ounce.
- Convert to ml: 1 fl. oz. ≈ 29.5735 ml.
- Therefore, 3 g/fl. oz. ≈ 3 g/29.5735 ml ≈ 101.42 g/L.
Desired Dilution:
- Assay requires 20-100 mg/2 ml.
- Target concentration for assay: 10-50 mg/ml.
- Starting concentration: 101.42 g/L (101,420 mg/L).
Calculating Dilution:
- Target concentration (10 mg/ml) from 101,420 mg/L.
- Dilution factor = 10 mg/ml / 101,420 mg/L = 1/10,142.
- Approximately, 2000-fold dilution is sufficient.
5. How does your calculated value compare to the caloric content on the food label? Do the rounding rules for Calories explain any differences? (See Tables 3–5 of Nielsen, Food Analysis textbook) Does the alcohol content (assume 4–5% alcohol at 7 Cal/g) of beer explain any differences?
Caloric Content Comparison:
- Calculate total carbohydrates from the standard curve and determine the caloric value.
- Compare calculated value to food label.
- Rounding rules for calories can cause minor differences.
Alcohol Content in Beer:
- Assuming 4-5% alcohol, with 7 Cal/g.
- Calculate alcohol contribution to total calories.
6. Was it best to have read the absorbance for the standard curve and other samples at 490 nm? Explain why a wavelength in this region is appropriate for this reaction.
Reason for 490 nm Wavelength:
- The Phenol-Sulfuric Acid method produces a colored complex with maximum absorbance around 490 nm.
- This wavelength is optimal for detecting the colored complex, ensuring accurate measurement of carbohydrate concentration.
References
- Viel, Marie & Collet, Florence & Lanos, Christophe. (2018). Chemical and multi-physical characterization of agro-resources’ by-product as a possible raw building material. Industrial Crops and Products. 120. 214-237. 10.1016/j.indcrop.2018.04.025.
- Nielsen, S. Suzanne (2010). [Food Science Texts Series] Food Analysis Laboratory Manual || Phenol-Sulfuric Acid Method for Total Carbohydrates. , 10.1007/978-1-4419-1463-7(Chapter 6), 47–53. doi:10.1007/978-1-4419-1463-7_6
- https://biocyclopedia.com/index/plant_protocols/carbohydrates/phenol_suplhuric_acid_method_for_total_carbohydrate.php
- https://noteshippo.com/phenol-sulfuric-acid-method/
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.