What is PAM Sequence (Protospacer adjacent motif)?
Protospacer Adjacent Motif, or PAM, is a type of two-factor authentication that tells Cas to only cut the foreign DNA that is invading.
- A protospacer adjacent motif (PAM) is a 2–6-base pair DNA sequence that comes right after the DNA sequence that the Cas9 nuclease wants to cut out in the CRISPR-based adaptive immune system of bacteria.
- The PAM is part of the virus or plasmid that is invading, but it is not part of the bacterial host genome. Because of this, it is not part of the bacterial CRISPR locus.
- Cas9 won’t be able to bind to or cut the target DNA sequence if the PAM sequence isn’t right after it.
- PAM is an important part of targeting that tells the difference between bacterial self-DNA and non-self DNA. This keeps the CRISPR locus from being targeted by the CRISPR-associated nuclease and destroyed.
- Specifically, it is 2 to 6 nt long and is found on the intruding DNA after the protospacer region.
- Note that the PAM is 3 to 4nt after the site where the nuclease cuts the DNA.
- Jinek M., Chylinski K., et al., Sashital D., and Weidenhelf B. (2021) and Jinek M., Chylinski K., et al., Sashital D., and Weidenhelf B. (2021) wrote that the functional PAM domain can be split into SAM- spacer acquisition motifs and TIM- target interference motifs.
- During the interference process, SAM is involved in the process of putting the spacers together, while TIM is involved in the process of recognising the spacers.
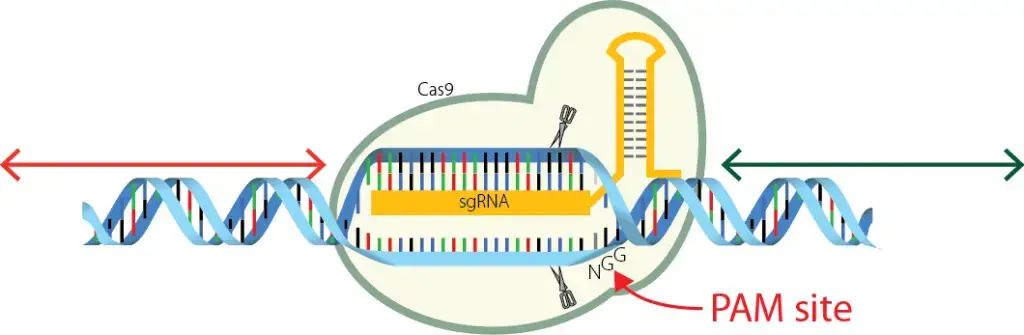
Properties of PAM sequence
- The PAM is a single sequence of a few base pairs long that is found in the protospacer region of the DNA that is trying to take over.
- PAM is on the phage or plasmid that you want to attack.
- PAM is not a part of the immune system of a bacterium.
- The bacterial CRISPR does not have PAM in the spacer.
- The PAM is in the protospacer, which is on the phage or plasmid that you want to attack.
Spacers/protospacers
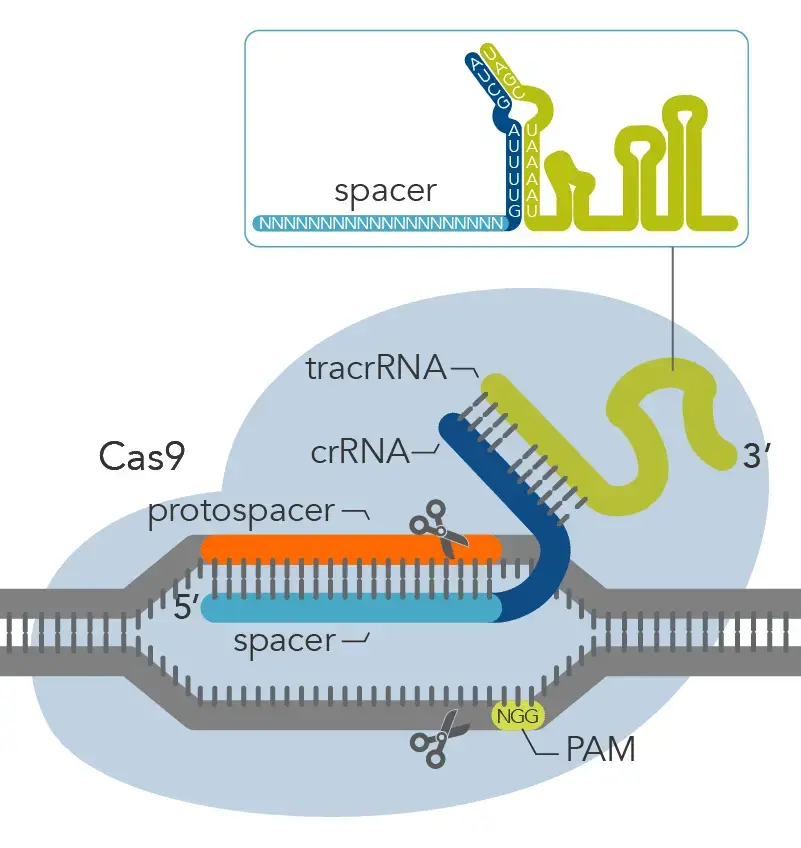
- “Spacers” are pieces of viral DNA that are inserted into a CRISPR locus. In type II adaptive immune systems, these “spacers” were made from viral or plasmid DNA that got into the body (called “protospacers”).
- When the invader comes back, a CRISPR-associated nuclease like Cas9 attaches to a tracrRNA–crRNA complex, which directs Cas9 to the invader’s protospacer sequence.
- But Cas9 won’t cut the protospacer sequence if there isn’t a PAM sequence next to it.
- The Cas9 nuclease will not cut the spacer in the bacterial CRISPR loci because it does not have a PAM sequence. However, the protospacer in the invading virus or plasmid will have the PAM sequence, so the Cas9 nuclease will cut it.
- In genome editing, a short oligonucleotide called a guide RNA (gRNA) is made to take the place of the tracrRNA–crRNA complex in recognising gene sequences with a PAM sequence at the 3′-end. This “guides” the nuclease to a specific sequence that the nuclease can cut.
PAM sequences
- The standard PAM is the sequence 5′-NGG-3′, where “N” can be any nucleobase and “G” is guanine.
- Guide RNAs can take Cas9 to any place in the genome where a gene needs to be edited, but editing can only happen at sites where Cas9 recognises PAM.
- The Cas9 nuclease of Streptococcus pyogenes (called SpCas9) is linked to the canonical PAM. The Cas9 proteins of Neisseria meningitidis, Treponema denticola, and Streptococcus thermophilus are linked to different PAMs.
- 5′-NGA-3′ can be a very effective non-canonical PAM for human cells, depending on where it is in the genome.
- People have tried to make Cas9s recognise different PAMs to make it easier for CRISPR-Cas9 to edit genes at any place in the genome.
- Francisella novicida’s Cas9 recognises the standard PAM sequence 5′-NGG-3′, but it has been modified to also recognise 5′-YG-3′, where “Y” is a pyrimidine. This expands the range of targets that Cas9 can go after. Francisella novicida’s Cpf1 nuclease recognises the PAM 5′-TTTN-3′ or 5′-YTN-3′.
- Aside from CRISPR-Cas9 and CRISPR-Cpf1, there are likely many nucleases and PAMs that have not yet been found.
- CRISPR/Cas13a (formerly C2c2) comes from the bacterium Leptotrichia shahii. It is an RNA-guided CRISPR system that targets sequences in RNA instead of DNA. PAM doesn’t matter for RNA-targeting CRISPR, but a guanine on either side of the target decreases its effectiveness and has been called a “protospacer flanking site” (PFS).
Functions of PAM Sequence
1. Role of PAM in spacer acquisition
- When you think about how CRISPR works in bacteria, you might remember that invading DNA gets added to the CRISPR locus through a process called “spacer acquisition.”
- Previous progress in the CRISPR field suggests that the Cas, the leader sequence, the repeat region, and the PAM sequence all play a big part in acquisition (Shah A, Hansen N & Garrett R, 2009 and Shah A & Edder P et al., 2009).
- The detailed analysis shows that PAM gives Cas a place to find protospacer to cut it. Loss of mutation studies show that when a PAM is lost or can’t be found, the process of getting something new stops.
- Also, protospacers show PAM within their own sequences for other protospacers (for other CRISPR loci). The whole integration process and what PAM does haven’t been well explained yet.
- Simply put, PAM is a marker that helps Cas find the protospacer, cut it in half, and add it to CRISPR.
2. Role of PAM in interference
- In a bacterial defence system, the interference process works together to use a nucleophilic attack to kill the foreign DNA. Several works show that PAM doesn’t play a very important part in interference.
- Insertional mutagenic studies, on the other hand, show that the mechanism can’t work without a specific PAM or a mutant PAM. This means that the virus can easily skip the mechanism.
- Different studies also show that, except for a few bases, the interference still works when a mutation happens in a non-conserved spot on PAM (Gracia-Heredia I & Marin-Cuadrado AB, 2012; Lopez-Sanchez MJ, Sauvage E).
- Almendras et al. (2012) backs up the current work by saying that interference activity goes up a lot when the Cytosine nucleotide is at positions 2 and 3 in the PAM.
- Simply put, the role of PAM alone can’t be explained or denied in the interference process. Either statement needs more research to back it up.
- People say that PAM is an important part of the CRISPR system and that it was set up on purpose. It is an important marker-like region for Cas to do endonuclease action, either in acquisition or interference.
3. Role of PAM in gene editing
- CAS can work with the spacer sequence, but the PAM sequence adds an extra level of precision and accuracy to the editing process. So, both in theory and in practise, the need for PAM weakens gene editing.
- Yes, it creates a problem. Because a PAM sequence isn’t always present in a gene of interest, which is what we are testing. If so, that would just be a chance. But unlike the bacteria’s own system, the PAM adds another level of accuracy.
- So it makes gene therapy even more accurate than PAMless editing. But the question is, what order we choose to get the PAM is impossible. As we’ve already talked about, different Cas have different PAM sequences. The list has already been given.
- It depends on the Cas that the bacteria gave off. And may vary. So, the first step in gene therapy is for researchers to find the PAM-like sequence, analyse it, and match it up with one of the Cas options.
- At least for now, that’s the only way out. And it means that the technology is accurate but not very useful. Scientists made Cas protein from scratch and changed proteins that already existed to meet the needs.
- The moral of the story is that using the PAM is a smart choice if you want to get very accurate editions. In a few situations, using PAM can also be a better choice.
- Reverse engineering shows that adding the PAM to the DNA sequence of gRNA is helpful in the process of differentiating cells and barcoding cells.
- Here, a guided RNA made from the PAM-containing DNA destroys the cells’ own DNA. This is useful for studying the lineage of cells and is called a “homing sgRNA.” Homing gRNA is an interesting subject, so we’ll talk about it in another article.
- Walton et al., 2020, recently explained almost a PAMless system that targets the whole genome instead of a single locus. Their research shows that when SpRY and the SpG-like system work together, there is no need for PAM.
- And because of this, a single CAS can target many places, genes, or sequences without needing the PAM. Even so, these kinds of systems haven’t been tested yet to see how well they work and how accurate they are.
PAM sequence Examples
References
- Almendros C, Guzmán NM, Díez-Villaseñor C, García-Martínez J, Mojica FJ. Target motifs affecting natural immunity by a constitutive CRISPR-Cas system in Escherichia coli. PLoS One. 2012;7(11):e50797. doi: 10.1371/journal.pone.0050797. Epub 2012 Nov 26. PMID: 23189210; PMCID: PMC3506596.
- Lopez-Sanchez MJ, Sauvage E, Da Cunha V, Clermont D, Ratsima Hariniaina E, Gonzalez-Zorn B, Poyart C, Rosinski-Chupin I, Glaser P. The highly dynamic CRISPR1 system of Streptococcus agalactiae controls the diversity of its mobilome. Mol Microbiol. 2012 Sep;85(6):1057-71. doi: 10.1111/j.1365-2958.2012.08172.x. Epub 2012 Jul 27. PMID: 22834929.
- Shah SA, Hansen NR, Garrett RA. Distribution of CRISPR spacer matches in viruses and plasmids of crenarchaeal acidothermophiles and implications for their inhibitory mechanism. Biochem Soc Trans. 2009 Feb;37(Pt 1):23-8. doi: 10.1042/BST0370023. PMID: 19143596.
- Lillestøl RK, Shah SA, Brügger K, Redder P, Phan H, Christiansen J, Garrett RA. CRISPR families of the crenarchaeal genus Sulfolobus: bidirectional transcription and dynamic properties. Mol Microbiol. 2009 Apr;72(1):259-72. doi: 10.1111/j.1365-2958.2009.06641.x. Epub 2009 Feb 23. PMID: 19239620.
- Garcia-Heredia I, Martin-Cuadrado AB, Mojica FJ, et al. Reconstructing viral genomes from the environment using fosmid clones: the case of haloviruses. PLoS One. 2012;7(3):e33802. doi:10.1371/journal.pone.0033802.
- https://en.wikipedia.org/wiki/Protospacer_adjacent_motif
- https://geneticeducation.co.in/importance-of-pam-sequence-protospacer-adjacent-motif-in-crispr-system/
- https://sg.idtdna.com/pages/support/faqs/what-is-a-pam-sequence-and-where-is-it-located
- https://www.synthego.com/guide/how-to-use-crispr/pam-sequence#:~:text=The%20protospacer%20adjacent%20motif%20(or,downstream%20from%20the%20cut%20site.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.