Oxygen Toxicity
- Oxygen is both beneficial and poisonous to living organisms.
- It is beneficial because its strong oxidizing ability makes it an excellent terminal electron acceptor for the energy-yielding process known as respiration. However, oxygen is also a toxic substance.
- Aerobic and facultative organisms have developed protective mechanisms that greatly mitigate this toxicity, but microaerophiles and anaerobes are deficient in these mechanisms and are restricted to habitats where little or no oxygen is present.
- Toxic O2 derivatives are formed when cellular proteins such as flavoproteins transfer electrons to O2.
- These toxic O2 derivatives are called reactive oxygen species (ROS), and they can damage proteins, lipids, and nucleic acids.
- ROS include the superoxide radical, hydrogen peroxide, and the most dangerous hydroxyl radical.
Formation of reactive oxygen species (ROS)
Various cellular enzymes catalyze chemical reactions involving molecular oxygen; some of these reactions can result in addition of a single electron to an oxygen molecule, thereby forming superoxide radical.
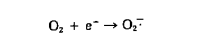
Superoxide radicals can inactivate vital cell components. However, recent studies suggest that their greatest detrimental action is through the production of even more toxic substances such as hydrogen peroxide (H2O2) and hydroxyl radicals by means of the following reactions:
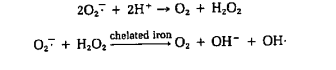
Damages caused by Oxygen Toxicity
- Hydroxyl radicals are among the most reactive free radicals known to organic chemistry and can damage almost every kind of molecule found in living cells.
- Molecular oxygen can directly oxidize certain essential reduced groups, such as thiol (-SH) groups, or enzymes, resulting in enzyme inactivation. For instance, the enzyme complex known as nitrogenase, responsible for nitrogen fixation, is irreversibly destroyed by even small amounts of oxygen.
- Even molecules that have evolved to function aerobically can be damaged by O2. This is because the unpaired electrons in the outer shell of oxygen make it inherently unstable.
- Hydrogen peroxide is not a free radical, but it is a powerful oxidizing agent that is highly toxic to many kinds of cells.
- Another toxic derivative of oxygen is an energized form known as singlet oxygen. which is produced in biological systems by certain photochemical reactions.
Protective Mechanism Against Oxygen Toxicity
- Many microorganisms possess enzymes that protect against toxic O2 products. Obligate aerobes and facultative anaerobes usually contain the enzymes superoxide dismutase (SOD) and catalase, which catalyze the destruction of superoxide radicals and hydrogen peroxide, respectively.
- Peroxidase also can be used to destroy hydrogen peroxide.
- Strict anaerobes lack these enzymes or have them in very low concentrations and therefore cannot tolerate O2.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.