What is Osazone Test?
The Osazone test is a biochemical method used to identify reducing sugars. This test is also known as the Phenylhydrazine test, referring to the reagent employed in the procedure. It distinguishes reducing sugars based on the time it takes for the osazone complex to form.
Carbohydrates, or sugars, are defined chemically as aldehyde or ketone derivatives of higher polyhydric alcohols, or compounds that yield these derivatives upon hydrolysis. These compounds play several vital roles, including providing energy, storing energy, and acting as components of cell membranes involved in intercellular communication.
Sugars are categorized into simple and complex types. Simple sugars, or monosaccharides, cannot be hydrolyzed into smaller compounds. In contrast, complex sugars, such as disaccharides, oligosaccharides, and polysaccharides, can be broken down into monosaccharides. Reducing sugars, characterized by a free carbonyl group (either aldehyde or ketone), are the primary focus of the Osazone test.
Emil Fischer developed the Osazone reaction to identify aldose sugars that differ only in configuration at the alpha carbon. In this reaction, all reducing sugars react with excess phenylhydrazine when heated, forming osazones. These osazones are insoluble and each type of sugar forms a unique crystal structure, which aids in their identification and differentiation.
The Osazone test is particularly useful in clinical diagnostics. It can detect various sugars in biological fluids such as urine, correlating with conditions like glucosuria in diabetes mellitus, fructosuria in hereditary fructose intolerance, lactose in lactose intolerance, and galactose in galactosemia. Additionally, it can identify arabinose in conditions like autism or candidiasis and xylose in small bowel diseases or malabsorption syndromes.
Definition of Osazone Test
The Osazone test is a biochemical method used to detect and identify reducing sugars by their reaction with phenylhydrazine, forming distinct crystalline osazones.
Objectives of Osazone Test
- Detection of Reducing Sugars
- The primary objective of the Osazone test is to identify the presence of reducing sugars in a sample. Reducing sugars possess a free aldehyde or ketone group capable of reducing phenylhydrazine, leading to the formation of osazones.
- Differentiation from Non-Reducing Sugars
- The Osazone test distinguishes reducing sugars from non-reducing sugars. Non-reducing sugars, such as sucrose, do not react with phenylhydrazine under test conditions, thus not forming osazones.
- Identification of Specific Reducing Sugars
- Besides detecting reducing sugars, the test differentiates among various reducing sugars based on the osazone crystals formed. Each reducing sugar yields a unique crystalline structure when reacted with phenylhydrazine. This characteristic allows for precise identification and differentiation between sugars such as glucose, fructose, and galactose.
- Formation of Distinct Crystals
- Each type of reducing sugar forms osazones with distinctive crystal shapes and sizes. Therefore, analyzing these crystal forms helps in identifying the specific reducing sugar present in the sample.
- Application in Clinical Diagnosis
- The ability to differentiate sugars is useful in diagnosing metabolic disorders. For instance, the test can identify specific sugars in urine, providing valuable information about conditions like diabetes mellitus, fructose intolerance, and galactosemia.
Principle of Osazone Test
- The Osazone test is based on the reaction between reducing sugars and phenylhydrazine, conducted in an acetate buffer. The principle of the test involves several key chemical reactions.
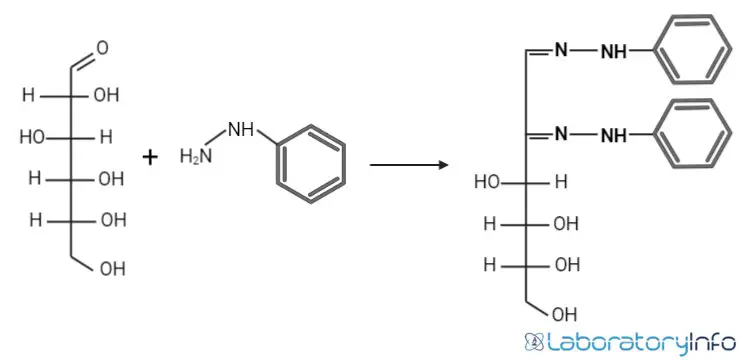
- First, carbohydrates with free or potentially free carbonyl groups react with phenylhydrazine to form osazones. This reaction occurs through a sequence of condensation-oxidation-condensation steps involving three molecules of phenylhydrazine and the carbonyl groups of aldoses or ketoses. The result is the formation of 1,2-diphenylhydrazone, known as osazone.
- Osazones are characterized by their yellow crystalline appearance, with each type of sugar producing a distinct crystal shape, solubility, and melting point. These unique properties allow for the differentiation of various reducing sugars. For instance, while C-2 epimers produce the same osazone, ketoses with a configuration similar to aldoses below C-2 also yield identical osazones. This means glucose and fructose, despite their different structures, form the same osazone.
- The reaction process can be summarized in three steps: Initially, a reducing sugar reacts with phenylhydrazine hydrochloride to form a phenylhydrazone hydrochloride. This intermediate then reacts with another phenylhydrazine molecule to form a keto derivative. A final reaction with an additional phenylhydrazine molecule yields the osazone. This sequence consumes three moles of phenylhydrazine. In the case of monosaccharides, additional phenylhydrazine is used to oxidize adjacent hydroxyl groups to carbonyl groups, producing bisphenyl hydrazones, or osazones.
- Thus, the Osazone test allows for the identification and differentiation of reducing sugars based on their unique osazone formations.
Requirements for Osazone Test
Reagent
- Osazone Mixture
- Phenylhydrazine Hydrochloride: 0.5 grams is required. This compound reacts with reducing sugars to form osazones.
- Sodium Acetate: 0.1 grams is needed to provide the appropriate buffer environment for the reaction.
- Glacial Acetic Acid
- This acid is used to prepare the reaction mixture and maintain the reaction conditions.
- Test Sample
- The sample should be a solution containing the reducing sugars to be tested.
Materials Required
- Test Tubes
- Test tubes are necessary for mixing reagents and samples, and for observing the reaction.
- Test Tube Stand
- This supports the test tubes during the experiment, keeping them upright and stable.
- Pipettes
- Pipettes are used to accurately measure and transfer liquids, such as reagents and test samples.
Equipment
- Vortex
- A vortex mixer is used to thoroughly mix the test sample and reagents, ensuring uniform reaction.
- Water Bath
- The water bath maintains a constant boiling temperature, essential for the formation of osazones.
- Microscope
- A microscope is required to observe the crystalline osazones formed during the reaction, as they need to be examined at a microscopic level to confirm their identity.
Procedure of Osazone Test
- Preparation of the Test Tube
- Take 5 ml of the test solution in a clean, dry test tube. Ensure the test solution contains the reducing sugar to be analyzed.
- Addition of Reagents
- Add 0.3 grams of the osazone mixture to the test tube. This mixture consists of phenylhydrazine hydrochloride and sodium acetate.
- Add five drops of glacial acetic acid to the test tube. This acid helps to maintain the necessary reaction conditions.
- Mixing and Heating
- Mix the contents of the test tube thoroughly. A vortex can be used to ensure proper mixing.
- Gently warm the test tube in a water bath if needed, to dissolve all components completely.
- Boiling and Observation
- Place the test tube in boiling water. Maintain this temperature to facilitate the formation of osazone crystals.
- Observe the test tube at various time points to monitor crystal formation. The crystals will gradually form as the reaction proceeds.
- Microscopic Examination
- Once crystals have formed, observe their shape using a microscope at low magnification. This step is crucial for identifying the specific osazones formed, as each reducing sugar produces characteristic crystals.
Observations
Osazone crystals formed when examined under the microscope can take on different shapes depending upon the type of carbohydrate.
Result and Interpretation of Osazone Test
- Identification by Crystal Shape and Formation Time
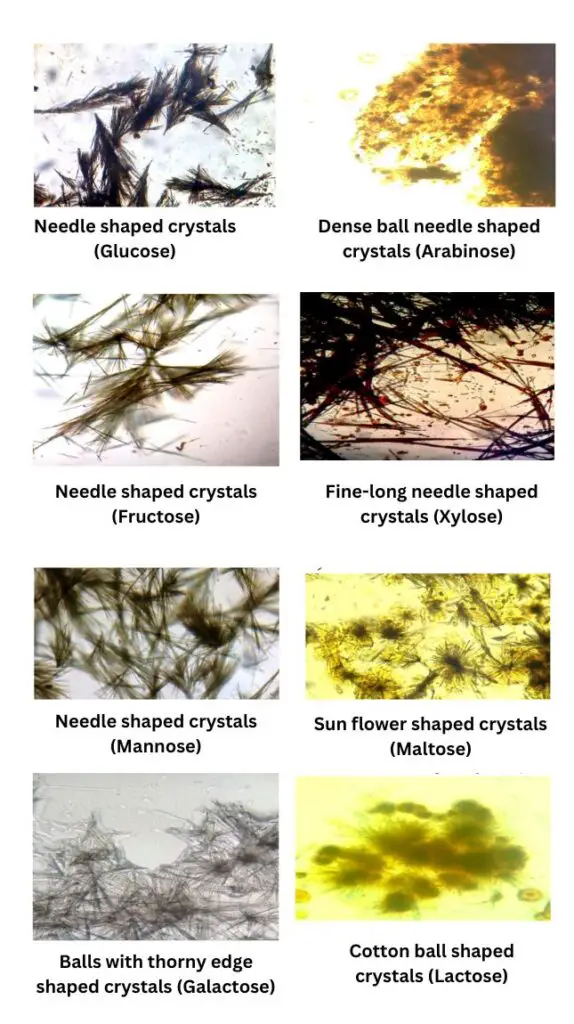
- The results of the Osazone test are determined by the shape and structure of the crystals that form, as well as the time required for their appearance. Each reducing sugar produces a characteristic crystalline form.
- Chart for Sugar Identification
- Fructose
- Time of Formation: Approximately 2 minutes.
- Crystalline Structure: Needle-shaped crystals.
- Glucose
- Time of Formation: Approximately 5 minutes.
- Crystalline Structure: Needle-shaped crystals.
- Galactose
- Time of Formation: Approximately 20 minutes.
- Crystalline Structure: Thorny ball-shaped crystals.
- Maltose
- Time of Formation: Approximately 30 to 45 minutes.
- Crystalline Structure: Sunflower or star-shaped crystals.
- Lactose
- Time of Formation: Approximately 30 to 45 minutes.
- Crystalline Structure: Cotton ball or powder puff-shaped crystals.
- Fructose
- Interpretation
- By comparing the observed crystal shapes and their formation times with the chart, different reducing sugars can be identified. Each sugar’s unique crystal morphology and the duration of crystal formation provide a basis for differentiation. This allows for the accurate identification of sugars in various samples.
| Sugar | Time of Formation | Crystalline Structure |
|---|---|---|
| Fructose | Approximately 2 minutes | Needle-shaped crystals |
| Glucose | Approximately 5 minutes | Needle-shaped crystals |
| Galactose | Approximately 20 minutes | Thorny ball-shaped crystals |
| Maltose | Approximately 30 to 45 minutes | Sunflower or star-shaped crystals |
| Lactose | Approximately 30 to 45 minutes | Cotton ball or powder puff-shaped crystals |
Uses of the Osazone Test
- Distinguishing Lactose from Maltose
- The Osazone test is uniquely effective for differentiating lactose from maltose.
- Lactose forms cotton ball or powder puff-shaped crystals.
- Maltose produces sunflower or star-shaped crystals.
- This distinction is crucial when identifying unknown sugars in various samples.
- The Osazone test is uniquely effective for differentiating lactose from maltose.
- Identification of Reducing Sugars
- This test offers a straightforward, cost-effective method for identifying and differentiating reducing sugars.
- It is particularly useful in clinical settings for analyzing biological fluids.
- The simplicity and relatively quick execution of the test make it practical for routine use.
- This test offers a straightforward, cost-effective method for identifying and differentiating reducing sugars.
- Detection in Plant Tissues
- The Osazone test can also be applied to locate sugars in plant tissues.
- It helps in the analysis of carbohydrate content and distribution within plant samples.
- This application is valuable in both research and agricultural contexts.
- The Osazone test can also be applied to locate sugars in plant tissues.
Advantages of the Osazone Test
- Distinguishing Reducing and Non-Reducing Sugars
- The Osazone test effectively differentiates between reducing and non-reducing sugars.
- Reducing sugars react to form osazones, while non-reducing sugars do not.
- This capability simplifies the identification of sugars in various samples.
- The Osazone test effectively differentiates between reducing and non-reducing sugars.
- Cost-Effective and Easy to Perform
- The test is economical, requiring minimal equipment and materials.
- It utilizes readily available reagents like phenylhydrazine and sodium acetate.
- The procedure is straightforward, making it accessible for routine laboratory use.
- The test is economical, requiring minimal equipment and materials.
- Identification of Sugars in Plant Tissues
- The Osazone test is applicable for detecting sugars in plant tissues.
- It aids in analyzing carbohydrate content and distribution within plant samples.
- This application is valuable for both research and agricultural practices.
- The Osazone test is applicable for detecting sugars in plant tissues.
- Effective Separation of Reducing Sugars
- Different reducing sugars produce distinct osazone crystals.
- For example, glucose and fructose both form needle-shaped crystals, but with different formation times.
- This characteristic allows for effective separation and identification of various reducing sugars.
- Different reducing sugars produce distinct osazone crystals.
Limitations of the Osazone Test
- False Positives for Non-Reducing Sugars
- The Osazone test can yield a false-positive result for sucrose if it is boiled for 30 minutes or longer.
- Sucrose, a non-reducing sugar, may form osazone-like crystals under prolonged heating.
- This limitation can lead to misidentification of non-reducing sugars as reducing sugars.
- The Osazone test can yield a false-positive result for sucrose if it is boiled for 30 minutes or longer.
- Ineffectiveness with Sugar Mixtures
- The test becomes less effective when the sample contains a mixture of different sugars.
- Distinguishing between multiple reducing sugars in a complex mixture is challenging.
- Therefore, the test may not provide clear results for samples with diverse sugar compositions.
- The test becomes less effective when the sample contains a mixture of different sugars.
- Requirement for Large Quantities
- A significant amount of sugar is needed to achieve a positive result in the Osazone test.
- This requirement can be a limitation when dealing with samples that have low sugar concentrations.
- For accurate detection, reducing sugars must be present in substantial quantities within the sample.
- A significant amount of sugar is needed to achieve a positive result in the Osazone test.
- Potential for False Positives
- Heating the sample for more than 30 minutes may produce false-positive results for sucrose.
- Extended heating can lead to the formation of osazone-like structures, misinterpreting non-reducing sugars as reducing sugars.
- This aspect can obscure the accurate identification of sugar types.
- Heating the sample for more than 30 minutes may produce false-positive results for sucrose.
References
- Tiwari A. (2015). Practical Biochemistry. LAP Lambert Academic Publishing.
- Shah, Tejas. (2016). Utility of Osazone Test to Identify Sugars. Journal of Medical Science And clinical Research. 04. 14361-14365. 10.18535/jmscr/v4i12.14.
- Mester, L.; El Khadem, H.; Horton, D. (1970). “Structure of saccharide osazones.” Journal of the Chemical Society C: Organic (18): 2567. doi:10.1039/J39700002567.
- https://www.topperlearning.com/answer/what-is-osazone-test-please-explain-with-the-help-of-example%20/d1yd9w11
- https://pubs.acs.org/doi/10.1021/ja01971a011
- http://ecoursesonline.iasri.res.in/mod/page/view.php?id=53489
- https://idswater.com/2019/12/08/what-is-osazone-formation-and-its-significance/
- https://pdfcoffee.com/osazone-test-pdf-pdf-free.html
- https://aklectures.com/lecture/reactions-and-disaccharides/osazone-formation-from-d-glucose
- https://www.jaypeedigital.com/book/9788184482591/chapter/ch2
- https://alevelbiology.co.uk/notes/tests-for-carbohydrates/#64-osazone-test