What are Neurotransmitters?
- Neurotransmitters are vital chemical messengers that play a key role in the functioning of the nervous system. They consist of various types of molecules such as amino acids, amines, purines, and neuropeptides. These molecules are synthesized within neurons and are released upon stimulation, allowing them to transmit signals across synapses, the gaps between neurons or between neurons and other target cells like muscle or glandular cells.
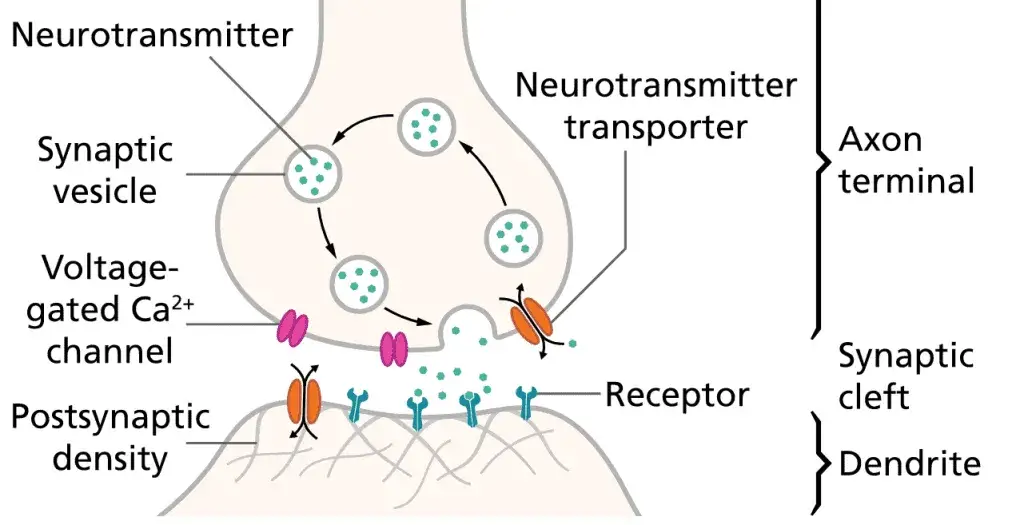
- The production of neurotransmitters occurs endogenously within the neuron, primarily involving the endoplasmic reticulum (ER) and Golgi apparatus. The ER generates active precursor molecules, while the Golgi packages these neurotransmitters into synaptic vesicles. These vesicles then travel to the edge of the axon, where neurotransmitters are stored until the neuron is activated. Upon stimulation, the synaptic vesicles merge with the plasma membrane, releasing neurotransmitters into the synaptic cleft.
- Once released, neurotransmitters bind to specific receptors on the postsynaptic cell. This binding can either excite or inhibit the postsynaptic neuron depending on the type of neurotransmitter and its receptor. Excitatory neurotransmitters promote the creation of an action potential in the receiving neuron, which allows the nerve signal to continue down the chain. In contrast, inhibitory neurotransmitters work to suppress action potentials, preventing the transmission of a signal. The exact effect is determined by the receptor type the neurotransmitter binds to.
- Besides excitatory and inhibitory functions, neurotransmitters also play a regulatory role. These chemical messengers are not limited to neurons alone; they can also affect muscle cells and gland cells by controlling muscle contraction or gland secretion. This versatility is critical for maintaining various bodily functions, from muscle movements to hormonal regulation.
- The release process of neurotransmitters is highly organized. They are stored in synaptic vesicles located at the end of the axon and are positioned near the synaptic cleft, where the signal transmission occurs. Neurotransmitters move from one neuron to another by crossing this cleft and binding to receptors on the target cell. Synaptic vesicles in chemical synapses typically have a spherical shape, while those in electrical synapses may take on pleomorphic forms, indicating structural variability based on the type of synapse.
- Overall, neurotransmitters are fundamental to how neurons communicate and coordinate functions in the body. Their ability to either stimulate or inhibit nerve signals ensures the balance and regulation necessary for proper neural function.
Definition of Neurotransmitters
Neurotransmitters are chemical messengers that transmit signals between neurons or from neurons to other target cells, such as muscle or gland cells, across synapses, helping regulate various functions in the nervous system.
Properties of Neurotransmitter
Below are key properties that define neurotransmitters and their role in neural communication.
- Synthesis and Storage in the Presynaptic Neuron
Neurotransmitters must be synthesized within the presynaptic neuron. This process relies heavily on the cell’s organelles, particularly the endoplasmic reticulum and the Golgi apparatus:- Endoplasmic Reticulum: The smooth endoplasmic reticulum plays a crucial role in synthesizing enzymes and active precursors that are necessary for neurotransmitter production.
- Golgi Apparatus: Once neurotransmitters are synthesized, the Golgi body packages them into vesicles. These vesicles, which are surrounded by a protein coat, protect the neurotransmitters from degeneration before they are released into the synaptic cleft.
- Release upon Stimulation
For neurotransmission to occur, neurotransmitters must be released by the presynaptic neuron. This release happens when the presynaptic neuron is stimulated, typically by an action potential reaching the axon terminal. At this point, the vesicles containing neurotransmitters fuse with the presynaptic membrane, allowing the neurotransmitters to diffuse into the synaptic cleft. - Binding to Postsynaptic Receptors
After release, neurotransmitters must travel across the synaptic cleft and bind to specific receptors on the postsynaptic neuron:- The postsynaptic neuron contains specialized receptors that recognize and bind only to certain neurotransmitters. This binding initiates a response in the postsynaptic neuron, leading to either excitation or inhibition, depending on the type of neurotransmitter and receptor involved.
- For a neurotransmitter to be functional, its interaction with the receptor on the postsynaptic neuron must mimic the natural physiological response that would occur during normal neurotransmission.
- Reuptake and Inactivation
Once neurotransmission occurs, it is essential that the neurotransmitter’s action is terminated to prevent continuous stimulation of the postsynaptic neuron. This happens through two primary mechanisms:- Reuptake Systems: After neurotransmitters bind to receptors and trigger a response, they are often reabsorbed back into the presynaptic neuron through reuptake systems. This ensures that neurotransmitters do not remain in the synaptic cleft, which would lead to their depletion and disrupt further signaling.
- Enzymatic Breakdown: Some neurotransmitters, after being released, are broken down by enzymes in the synaptic cleft. For example, the enzyme acetylcholinesterase breaks down acetylcholine after it has transmitted a signal.
- Consistency of Response in the Postsynaptic Neuron
A defining property of a neurotransmitter is its ability to consistently produce a response in the postsynaptic neuron. When experimentally applied to a postsynaptic neuron, the neurotransmitter should evoke the same response as when it is naturally released from the presynaptic neuron. This feature ensures that neurotransmission remains predictable and efficient across different neurons.
Types of Neurotransmitters
Neurotransmitters are chemical messengers that enable communication between neurons. Based on their functional role in the nervous system, neurotransmitters are classified into several types. Below is a detailed explanation of the different types of neurotransmitters:
- Excitatory Neurotransmitters
These neurotransmitters promote the generation of an action potential, leading to the activation of the postsynaptic neuron.- They facilitate the transmembrane flow of ions such as sodium (Na+) and calcium (Ca2+), resulting in depolarization of the neuron.
- Examples include glutamate, acetylcholine, histamine, epinephrine, and norepinephrine. These neurotransmitters play a crucial role in stimulating nerve signals, which in turn lead to functions like muscle contractions or alertness.
- Inhibitory Neurotransmitters
Inhibitory neurotransmitters prevent the firing of action potentials, thus reducing neuronal activity.- They promote hyperpolarization by allowing the influx of anions like chloride (Cl−), making it harder for the neuron to reach the threshold needed for activation.
- Common inhibitory neurotransmitters include GABA, taurine, glycine, and dopamine. These neurotransmitters are essential for calming neural circuits, controlling anxiety, and preventing overexcitation of neurons.
- Modulatory Neurotransmitters (Neuromodulators)
These neurotransmitters regulate the activity of other neurons and do not directly participate in neurotransmission.- They are not reabsorbed or broken down after release and influence overall brain activity.
- Examples include dopamine, histamine, and acetylcholine, which modulate various functions, including attention, emotion, and motor control.
Classifications of Neurotransmitters
- Monoamines
Monoamine neurotransmitters are involved in regulating mood, emotional response, and cognitive functions.- Serotonin: An inhibitory neurotransmitter that regulates mood, anxiety, and sleep. It is primarily found in the gut and central nervous system. Low levels of serotonin are linked to depression, while excess can lead to serotonin syndrome, characterized by agitation and hallucinations.
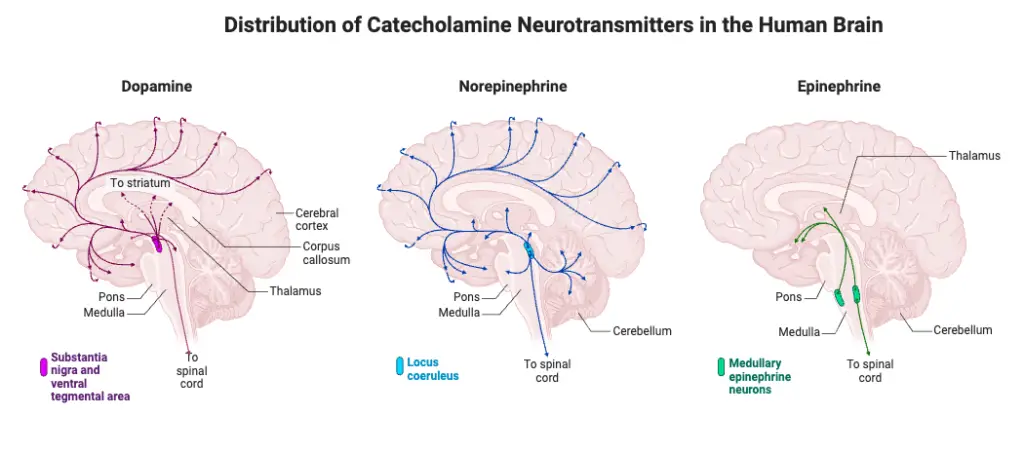
- Epinephrine (Adrenaline): An excitatory neurotransmitter that stimulates the fight-or-flight response. Excess epinephrine can lead to anxiety and hypertension, while a deficit may reduce stress responses.
- Norepinephrine (Noradrenaline): Functions in activating the brain during stress. It is vital for alertness and focus during dangerous situations. High levels can lead to anxiety and high blood pressure, whereas low levels may cause fatigue and depression.
- Dopamine: Both excitatory and inhibitory, dopamine regulates reward, motivation, and motor functions. Excess dopamine is linked to addiction and compulsive behavior, while a deficit is associated with Parkinson’s disease and depression.
- Amino Acids
Amino acid neurotransmitters are essential for both excitatory and inhibitory functions in the central nervous system.- Gamma-Aminobutyric Acid (GABA): The brain’s primary inhibitory neurotransmitter, responsible for regulating anxiety and motor control. Insufficient GABA can result in seizures, while an excess may cause excessive drowsiness.
- Glutamate: The most abundant excitatory neurotransmitter, crucial for learning and memory. However, excess glutamate can lead to excitotoxicity, which may cause neuronal damage and contribute to diseases like Alzheimer’s and epilepsy.
- Peptides
Peptide neurotransmitters are involved in pain regulation and emotional responses.- Endorphins: Inhibitory neurotransmitters that reduce pain and induce feelings of euphoria. They are released during physical activity and stress. Low levels can cause chronic pain and depression, while excess might result in addiction to high-intensity activities.
- Purines
Purines play a significant role in regulating sleep and energy transfer in the brain.- Adenosine: A neuromodulator that suppresses arousal and promotes sleep. High levels can cause drowsiness, while low levels lead to sleep disturbances. Caffeine blocks adenosine receptors, resulting in increased wakefulness.
- Adenosine Triphosphate (ATP): Functions as both an energy carrier and an excitatory neurotransmitter. ATP facilitates communication between neurons and glial cells and influences sensory processing.
- Acetylcholine
Acetylcholine is the primary neurotransmitter in both the central and peripheral nervous systems.- It is critical for muscle movement, memory formation, and learning. High levels may cause muscle paralysis and excessive salivation, while low levels are associated with memory impairment and conditions like Alzheimer’s disease.
Neurotransmitter systems
Neurotransmitter systems are fundamental components of the brain’s communication network, facilitating the transmission of signals that regulate various physiological and psychological functions. Each system operates through specific neurotransmitters, influencing behavior, mood, cognition, and bodily functions. Understanding these systems provides valuable insights into their roles and interconnections within the central nervous system.
- Acetylcholine System:
- This system is critical for both the central nervous system (CNS) and peripheral nervous system (PNS).
- Acetylcholine (ACh) is involved in muscle activation, learning, memory, and attention.
- It regulates REM sleep and is essential for cognitive processes.
- Dysregulation of the acetylcholine system is implicated in conditions such as Alzheimer’s disease, characterized by memory loss and cognitive decline.
- Dopamine System:
- The dopamine system plays a vital role in reward, motivation, and motor control.
- Dopamine acts as a primary mediator of the brain’s reward pathways, influencing behaviors related to pleasure and reinforcement.
- It is crucial for cognitive functions such as attention and executive control.
- Abnormalities in dopamine levels are associated with several disorders, including Parkinson’s disease, schizophrenia, and addiction.
- Noradrenaline (Norepinephrine) System:
- The noradrenaline system is integral to the body’s response to stress, regulating arousal, alertness, and mood.
- Norepinephrine is released during stressful situations, contributing to the “fight or flight” response.
- It influences attention, response times, and emotional regulation.
- Dysregulation in this system can lead to mood disorders, such as depression and anxiety.
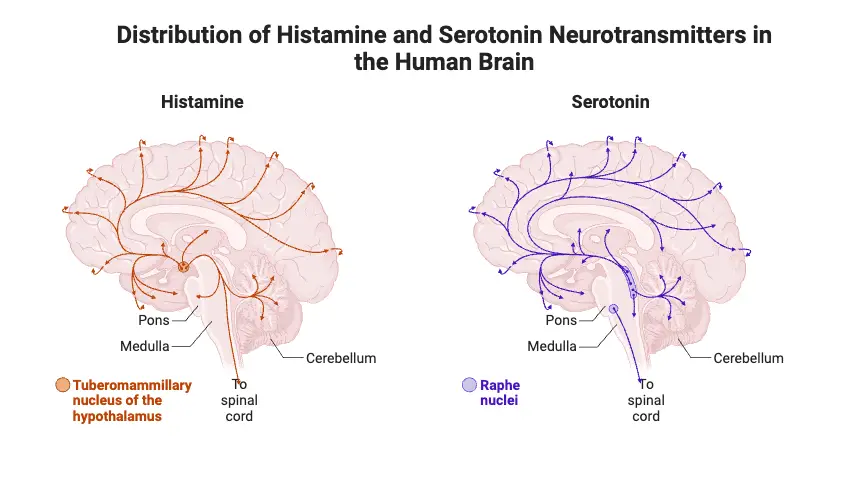
- Histamine System:
- Histamine is primarily known for its role in immune responses, but it also functions as a neurotransmitter in the brain.
- It plays a significant role in regulating arousal, sleep-wake cycles, and appetite control.
- The histamine system is involved in modulating cognitive functions and memory.
- Alterations in histamine levels can affect sleep patterns and have been linked to disorders like narcolepsy and certain allergies.
- Serotonin System:
- The serotonin system is essential for mood regulation, social behavior, sleep, and appetite.
- Serotonin is often referred to as the “feel-good” neurotransmitter, influencing feelings of well-being and happiness.
- It plays a role in various bodily functions, including gastrointestinal motility.
- Imbalances in serotonin levels are commonly associated with mood disorders, such as depression and anxiety.
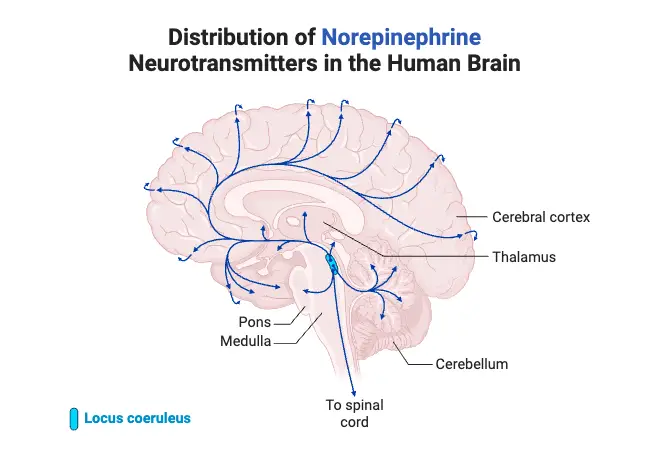
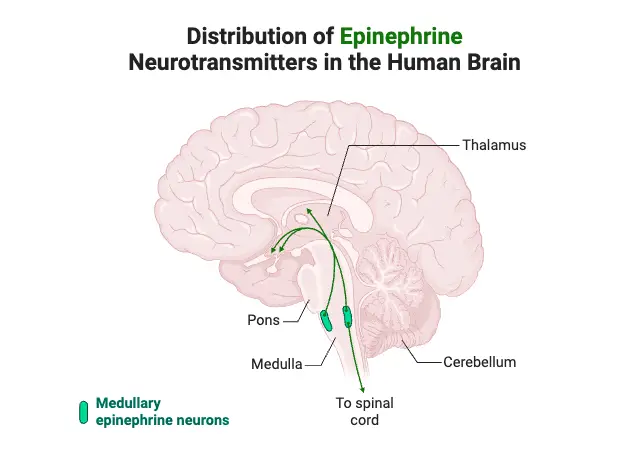
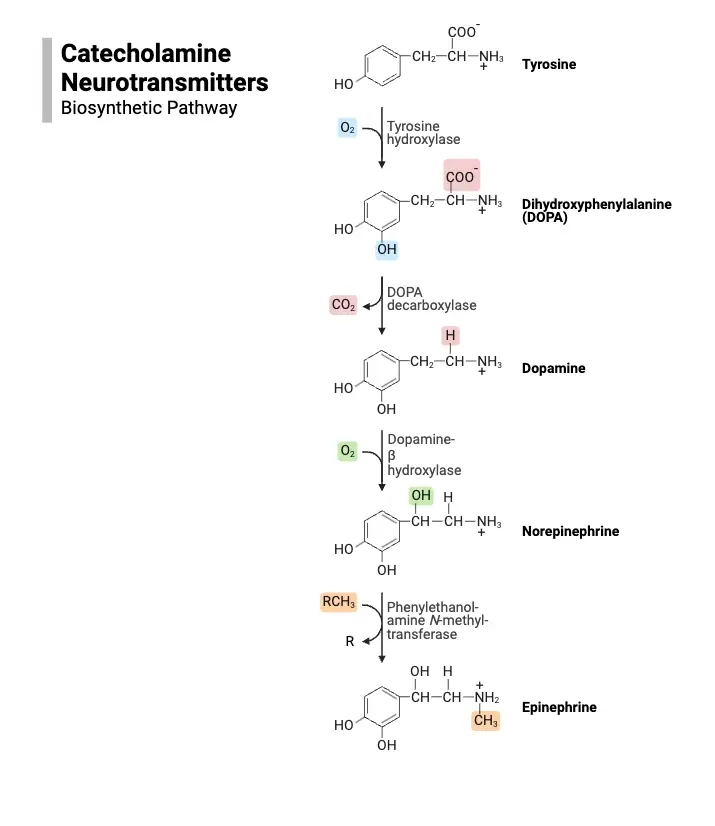
How Neurotransmitters Work
Their mechanism of action involves a series of well-coordinated biochemical events that ensure effective signal transmission and termination. Understanding this process is essential for comprehending how neurotransmitters influence both physiological and psychological functions.
- Release Mechanism:
- Neurotransmitters are stored in synaptic vesicles located at the presynaptic terminal of neurons.
- When an action potential reaches the presynaptic terminal, it induces depolarization of the membrane, leading to the activation of voltage-gated calcium (Ca²⁺) channels.
- The influx of Ca²⁺ ions into the neuron triggers conformational changes that facilitate the fusion of synaptic vesicles with the plasma membrane.
- Consequently, neurotransmitters are released into the synaptic cleft, the space between the presynaptic and postsynaptic neurons.
- Diffusion and Binding:
- Once released, neurotransmitters diffuse across the synaptic cleft.
- They bind to specific receptors located on the postsynaptic membrane, leading to a series of cellular responses.
- This binding can result in either excitatory or inhibitory effects on the postsynaptic neuron, influencing its likelihood of generating an action potential.
- Excitatory Effects: Increase the chances of action potential generation.
- Inhibitory Effects: Decrease the chances of action potential generation.
- Signal Propagation:
- If the postsynaptic neuron is sufficiently depolarized, an action potential is generated at the axon hillock, propagating the signal along the neuron.
- The neuron that releases neurotransmitters is termed the presynaptic neuron, while the receiving neuron is known as the postsynaptic neuron.
- Termination of Signal:
- After neurotransmission, neurotransmitters must be cleared from the synaptic cleft to terminate the signal and allow the postsynaptic neuron to return to a resting state.
- The fate of neurotransmitters can include:
- Diffusion: Some neurotransmitters may simply diffuse away from the synaptic cleft.
- Enzymatic Breakdown: Enzymes in the synaptic cleft can metabolize neurotransmitters, rendering them inactive.
- Reuptake: Transporter proteins on the presynaptic neuron can reabsorb neurotransmitters, allowing them to be repackaged into synaptic vesicles for future use.
- Role of Calcium Ions:
- Calcium ions play an essential role in neurotransmitter release; their entry into the presynaptic neuron is critical for triggering the exocytosis of vesicles containing neurotransmitters.
- If Ca²⁺ channels are blocked, neurotransmitter release is inhibited, demonstrating the importance of calcium in this signaling process.
- Neurotransmitter Action:
- Upon binding to receptors on the postsynaptic neuron, neurotransmitters initiate a cascade of events that can modify neuronal activity.
- This modulation can lead to various physiological responses, depending on the type of neurotransmitter and the receptors involved.
Synthesis and Storage of Small Molecule Transmitters
The synthesis and storage of small molecule neurotransmitters are vital processes that ensure efficient communication between neurons. These neurotransmitters are primarily synthesized by specific enzymes located in the presynaptic terminals, allowing for quick and effective packaging for release. Below is a detailed exploration of the synthesis and storage mechanisms of various small molecule neurotransmitters.
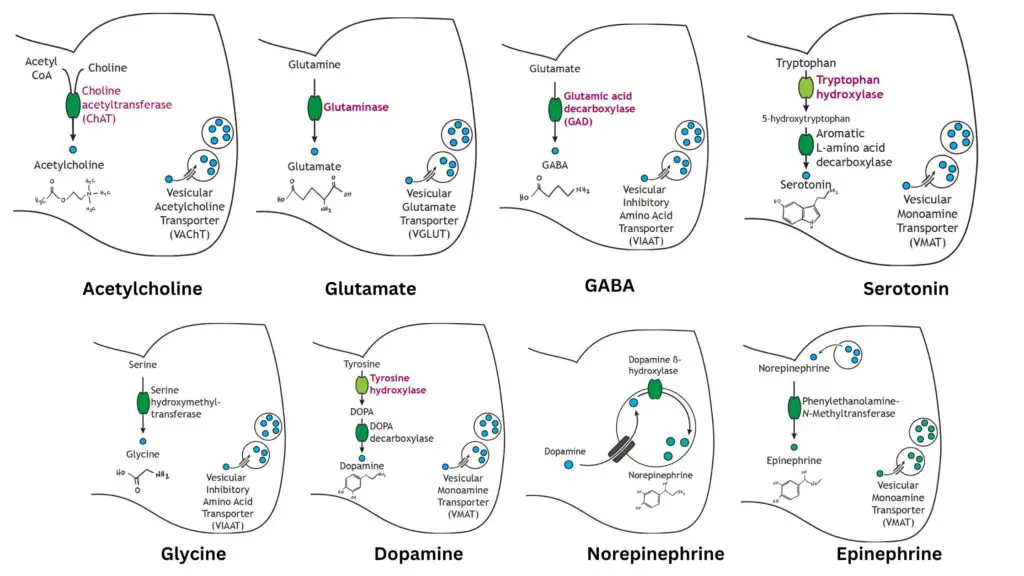
- General Mechanism:
- Small molecule neurotransmitters are synthesized by enzymatic reactions that primarily occur in the cytoplasm of presynaptic terminals.
- The synthesized neurotransmitters are subsequently packaged into vesicles for storage until they are needed for synaptic transmission.
- Acetylcholine:
- Synthesized from acetyl coenzyme A (acetyl CoA) and choline through the enzyme choline acetyltransferase.
- This step is the rate-limiting factor in acetylcholine synthesis.
- Packaged into vesicles for storage via the vesicular acetylcholine transporter (VAChT).
- This ensures a ready supply for neurotransmission at neuromuscular junctions.
- Synthesized from acetyl coenzyme A (acetyl CoA) and choline through the enzyme choline acetyltransferase.
- Glutamate:
- An amino acid neurotransmitter and the primary excitatory neurotransmitter in the central nervous system.
- Synthesized from glutamine by the enzyme glutaminase, which serves as the rate-limiting step.
- Packaged into vesicles through the vesicular glutamate transporter, allowing for efficient release during synaptic transmission.
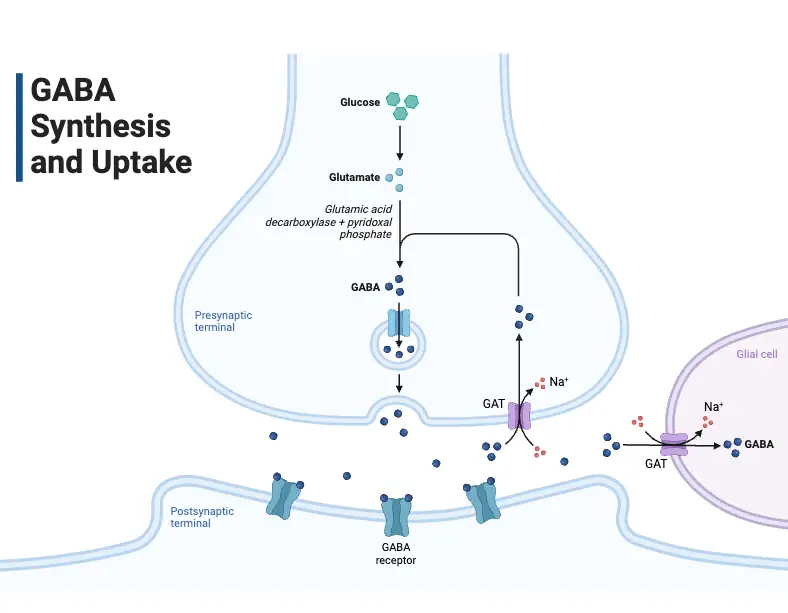
- Gamma-Aminobutyric Acid (GABA):
- Synthesized from glutamate via the enzyme glutamic acid decarboxylase.
- This conversion is crucial, as GABA acts as the primary inhibitory neurotransmitter in the brain.
- GABA is packaged into vesicles for storage using the vesicular inhibitory amino acid transporter.
- Synthesized from glutamate via the enzyme glutamic acid decarboxylase.
- Glycine:
- Another inhibitory neurotransmitter, more prevalent in the spinal cord.
- Synthesized from the amino acid serine through the action of serine hydroxymethyltransferase.
- The rate-limiting step occurs before the synthesis of serine.
- Packaged into vesicles by the vesicular inhibitory amino acid transporter, similar to GABA.
- Dopamine:
- A catecholamine neurotransmitter, crucial for functions such as reward and movement.
- Synthesized from the amino acid tyrosine in a two-step process:
- Tyrosine is first converted into DOPA by tyrosine hydroxylase, the rate-limiting step.
- DOPA is then converted to dopamine by DOPA decarboxylase.
- Packaged into synaptic vesicles via the vesicular monoamine transporter.
- Norepinephrine:
- Another catecholamine transmitter, synthesized from dopamine.
- Once dopamine is stored in vesicles, it is converted into norepinephrine by the enzyme dopamine beta-hydroxylase.
- This synthesis occurs within the vesicles, distinguishing it from other neurotransmitters.
- The synthesis pathway’s rate-limiting step remains the activity of tyrosine hydroxylase.
- Epinephrine:
- Commonly regarded as a hormone, it also functions as a neurotransmitter in limited neuronal contexts.
- Synthesized from norepinephrine in the cytoplasm by phenylethanolamine-N-methyltransferase.
- Following its synthesis, epinephrine is repackaged into vesicles via the vesicular monoamine transporter.
- Serotonin:
- Known for its influence on mood regulation, serotonin is synthesized from tryptophan.
- The conversion occurs in two steps:
- Tryptophan is first transformed into 5-hydroxytryptophan by tryptophan hydroxylase, a rate-limiting step.
- 5-Hydroxytryptophan is then decarboxylated to form serotonin by aromatic L-amino acid decarboxylase.
- Serotonin is packaged into vesicles by the vesicular monoamine transporter.
- Histamine:
- Synthesized from histidine via histidine decarboxylase, which is the rate-limiting step in the pathway.
- Histamine is also packaged into synaptic vesicles through the vesicular monoamine transporter.
Studying neurotransmitter systems
Studying neurotransmitter systems is a fundamental aspect of neuroscience, as neurotransmitters play a crucial role in neuronal communication and the overall functioning of the nervous system. The process of identifying and characterizing neurotransmitter systems is intricate and involves several critical steps.
- The initial step in studying a neurotransmitter system is the identification of the neurotransmitter itself. This task is complex, given the vast array of chemicals present in the brain. To determine whether a molecule qualifies as a neurotransmitter, specific criteria must be fulfilled:
- Synthesis and Storage: The molecule must be synthesized and stored within the presynaptic neuron.
- Release Upon Stimulation: The molecule must be released from the presynaptic axon terminal upon neuronal stimulation.
- Mimicking Postsynaptic Response: When the molecule is experimentally applied, it should produce a postsynaptic response that closely resembles the response generated by the naturally released neurotransmitter.
- To establish whether a molecule meets these criteria, scientists employ various methodologies:
- Localization Techniques:
- Immunocytochemistry: This technique is utilized to localize specific molecules within cells. After purifying the candidate neurotransmitter, it is injected into an animal to elicit an immune response, generating antibodies that bind specifically to the neurotransmitter. These antibodies can be labeled with detectable markers and applied to brain tissue sections, revealing which cells contain the neurotransmitter. This method can also localize synthesizing enzymes associated with the neurotransmitter, providing evidence for its production within specific neurons.
- In Situ Hybridization: This technique detects specific mRNA molecules that encode proteins within neurons. By creating labeled probes that bind to the target mRNA, researchers can visualize which neurons produce the neurotransmitter candidate. Methods such as autoradiography or fluorescence allow for the identification of these labeled cells.
- Localization Techniques:
- Following the confirmation of a molecule’s synthesis and localization in the presynaptic terminal, the next phase involves demonstrating its release upon stimulation. Techniques include:
- Electrophysiological Measurements: Researchers can stimulate specific neurons or axons and analyze the fluids surrounding their synaptic targets. The biological activity of these fluids can then be tested for characteristics indicative of neurotransmitter release, similar to those observed in intact synapses.
- In Vitro Brain Slice Experiments: Using living brain slices, researchers can induce neurotransmitter release by bathing the slices in a high-potassium solution, which depolarizes the membrane and triggers transmitter release. It is crucial to establish that this release is calcium-dependent, as calcium ions are essential for neurotransmitter secretion.
- Optogenetics: A newer technique allows for the precise activation of specific neuron populations using light-sensitive proteins. By stimulating these neurons with light, researchers can confirm which neurotransmitters are released from targeted synapses.
- After confirming the release of the neurotransmitter candidate, the third criterion must be evaluated: the ability of the molecule to produce a postsynaptic response that mirrors the effect of the natural neurotransmitter. This can be assessed through:
- Microiontophoresis: This method involves applying the neurotransmitter candidate directly to the postsynaptic neuron using a finely tipped pipette. By measuring the resulting changes in membrane potential, researchers can determine if the effects are consistent with those of naturally released neurotransmitters.
- The study of neurotransmitter receptors is equally important. Neurotransmitters exert their effects by binding to specific receptors, which can exhibit subtype diversity. No two neurotransmitters typically bind to the same receptor, yet a single neurotransmitter may interact with multiple receptor subtypes. Research methods employed to analyze receptor subtypes include:
- Neuropharmacological Analysis: This approach examines the differing effects of various drugs on receptor activity. For instance, the identification of nicotinic and muscarinic receptors for acetylcholine illustrates how receptor subtypes can be distinguished based on their pharmacological properties.
- Ligand-Binding Methods: Researchers use labeled ligands to identify receptor sites on neuronal membranes. The binding of these ligands provides insights into receptor distribution and function, helping to elucidate the roles of neurotransmitter systems.
- Molecular Analysis: Advances in molecular biology have allowed for detailed studies of neurotransmitter receptor structure. This research has revealed significant diversity among receptor subtypes, particularly in G-protein-coupled receptors and ion channels, suggesting a complex interplay of receptor types within the nervous system.
Transmitter-gated channels
Structure of Transmitter-Gated Channels
Transmitter-gated ion channels play a pivotal role in synaptic transmission, converting chemical signals from neurotransmitters into electrical signals in neurons. The structural complexity of these channels is crucial for their specific functions in various physiological processes. This exposition aims to elucidate the structural characteristics of these channels, focusing on the well-studied nicotinic acetylcholine (ACh) receptor as a prototype.
- The nicotinic ACh receptor is a pentameric protein complex, consisting of five distinct protein subunits that form a central ion-conducting pore.
- Subunit Composition: The receptor is comprised of two alpha (α) subunits and one each of beta (β), gamma (γ), and delta (δ) subunits, denoted as α₂βγδ. This specific combination is critical for its functionality at the neuromuscular junction.
- Binding Sites: Each α subunit contains a binding site for ACh, necessitating the simultaneous occupancy of both sites for channel activation, which allows ion flux across the membrane.
- Each subunit features four transmembrane segments that adopt an alpha-helical conformation.
- Hydrophobic Properties: The amino acid composition of these segments is predominantly hydrophobic, facilitating their integration into the lipid bilayer and creating the transmembrane structure.
- Pore Formation: The arrangement of these helices enables the formation of a central pore, similar to the structures observed in voltage-gated sodium and potassium channels.
- Beyond the nicotinic ACh receptor, various transmitter-gated channels in the central nervous system exhibit comparable structural motifs.
- GABA_A and Glycine Receptors: These receptors also adopt a pentameric configuration and include the characteristic four hydrophobic segments, emphasizing a shared evolutionary lineage among these receptor types.
- Glutamate Receptors: In contrast to the pentameric structure of ACh and GABA receptors, glutamate receptors are formed from four subunits, resulting in a tetrameric assembly. The M2 region of these subunits adopts a hairpin structure, which enters and exits the membrane, distinguishing them from other transmitter-gated channels.
- Structural similarities suggest evolutionary relationships among different types of channels.
- Common Ancestry Hypothesis: The resemblance between glutamate receptors and certain potassium channels has led to hypotheses that they may have evolved from a common ancestral ion channel.
- Purinergic Receptors: These receptors exhibit a unique structural arrangement, comprising three subunits with only two transmembrane segments each, further illustrating the diversity in transmitter-gated channel structures.
- Functional diversity among these channels is attributable to variations in their binding sites and structural configurations.
- Specificity of Action: Differences in binding site characteristics enable channels to selectively respond to specific neurotransmitters such as glutamate or GABA, underscoring the precision of synaptic transmission.
- Functional Implications: The structural diversity directly influences channel permeability, gating mechanisms, and overall responsiveness to neurotransmitters, thereby affecting neuronal signaling and communication.
Amino Acid-Gated Channels
Amino acid-gated channels are integral to fast synaptic transmission within the central nervous system (CNS), playing essential roles in various functions, including sensory processing, memory formation, and the pathophysiology of neurological disorders. These channels respond to specific neurotransmitters, with distinct pharmacological properties that define their interactions and functions. The following points provide a detailed overview of the structural and functional characteristics of amino acid-gated channels.
- General Characteristics:
- Amino acid-gated channels are primarily activated by neurotransmitters, which are amino acids or their derivatives.
- They facilitate rapid excitatory or inhibitory synaptic transmission depending on the specific channel type and its permeability to ions.
- Pharmacological Profiles:
- The binding sites of these channels dictate which neurotransmitters can activate them and how various pharmacological agents interact with the receptors.
- This pharmacology is crucial for understanding how drugs can modify synaptic transmission and impact behavior.
- Kinetics and Gating:
- The kinetics of neurotransmitter binding and subsequent channel gating influence the duration and intensity of synaptic responses.
- Fast kinetics are characteristic of excitatory channels, allowing for quick signal propagation in neural networks.
- Ion Selectivity:
- The selectivity of amino acid-gated channels determines whether they generate excitatory or inhibitory postsynaptic potentials.
- For example, channels that allow the influx of sodium (Na⁺) ions typically produce excitatory effects, whereas those permeable to chloride (Cl⁻) ions mediate inhibitory responses.
- Glutamate-Gated Channels:
- Glutamate receptors are a primary subset of amino acid-gated channels, with three main subtypes: AMPA, NMDA, and kainate.
- AMPA Receptors:
- They mediate fast excitatory synaptic transmission and are permeable to both Na⁺ and potassium (K⁺) ions.
- The rapid depolarization associated with AMPA receptor activation is essential for conveying excitatory signals in the CNS.
- NMDA Receptors:
- NMDA receptors also facilitate excitatory transmission but have unique properties: they are permeable to calcium (Ca²⁺), Na⁺, and K⁺, making them critical for synaptic plasticity and long-term potentiation.
- Their activation is contingent upon both glutamate binding and membrane depolarization, due to a magnesium (Mg²⁺) block that occurs at resting membrane potentials.
- This voltage-dependent property allows NMDA receptors to integrate synaptic signals, playing a key role in learning and memory.
- Kainate Receptors:
- These receptors are less understood but are distributed throughout the CNS and may contribute to both pre- and postsynaptic modulation.
- AMPA Receptors:
- Glutamate receptors are a primary subset of amino acid-gated channels, with three main subtypes: AMPA, NMDA, and kainate.
- GABA and Glycine-Gated Channels:
- Gamma-aminobutyric acid (GABA) and glycine are primary inhibitory neurotransmitters in the CNS, acting on their respective receptors to mediate inhibitory synaptic transmission.
- GABAA Receptors:
- Structurally similar to nicotinic ACh receptors, GABAA receptors gate Cl⁻ channels and consist of multiple subunits, with binding sites for GABA and various modulatory compounds, including benzodiazepines and barbiturates.
- The modulation of GABAA receptors by these drugs enhances inhibitory currents, resulting in stronger inhibitory postsynaptic potentials (IPSPs).
- The physiological implications of this enhancement are significant, as it can influence behaviors such as anxiety and sedation.
- Glycine Receptors:
- Like GABAA receptors, glycine receptors are selective for Cl⁻ and contribute to inhibitory neurotransmission, primarily in the spinal cord and brainstem.
- GABAA Receptors:
- Gamma-aminobutyric acid (GABA) and glycine are primary inhibitory neurotransmitters in the CNS, acting on their respective receptors to mediate inhibitory synaptic transmission.
- Clinical Relevance:
- Dysregulation of amino acid-gated channels is implicated in various neurological disorders, including epilepsy and anxiety disorders.
- Understanding the molecular mechanisms and structural nuances of these channels can inform therapeutic strategies targeting synaptic transmission and neuronal excitability.
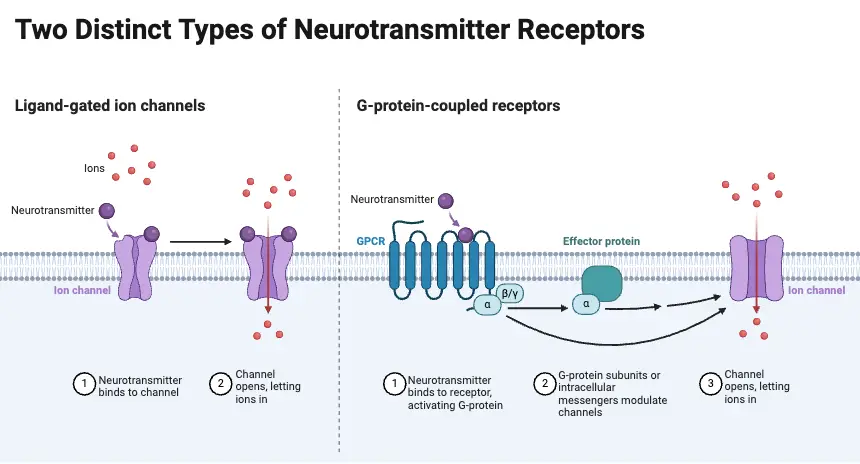
G-protein-coupled receptors and effectors
Structure of G-Protein-Coupled Receptors
G-protein-coupled receptors (GPCRs) represent a fundamental class of membrane proteins that facilitate communication between extracellular signals and intracellular responses. These receptors play a critical role in numerous physiological processes by transmitting signals from various ligands, including neurotransmitters and hormones. The basic structure of GPCRs can be described through several key points, each highlighting essential aspects of their function and organization.
- Core Structure:
- GPCRs are characterized by a single polypeptide chain that traverses the cell membrane seven times, forming seven membrane-spanning α-helices.
- This unique structure contributes to their classification as seven-transmembrane (7TM) receptors.
- Transmitter Binding Sites:
- Two of the extracellular loops of the GPCR form the sites for ligand binding, where neurotransmitters, agonists, or antagonists interact with the receptor.
- Variations in the amino acid composition of these loops determine the specificity of ligand binding, which influences the receptor’s pharmacological properties.
- Two of the extracellular loops of the GPCR form the sites for ligand binding, where neurotransmitters, agonists, or antagonists interact with the receptor.
- Intracellular Loop Functions:
- Two intracellular loops are involved in binding and activating G-proteins, which are essential for signal transduction.
- Structural differences in these loops dictate the specific G-proteins that are activated upon ligand binding, leading to varied intracellular signaling pathways.
- Two intracellular loops are involved in binding and activating G-proteins, which are essential for signal transduction.
- Diversity of GPCRs:
- The human genome encodes approximately 800 different GPCRs, which are categorized into five major families based on structural similarities.
- This diversity enables a wide range of physiological responses, with GPCRs involved in processes such as vision, taste, olfaction, immune response, and neurotransmission.
- The human genome encodes approximately 800 different GPCRs, which are categorized into five major families based on structural similarities.
- Widespread Biological Importance:
- While GPCRs are well-known for their roles in neurons, they are also crucial in virtually all cell types throughout the body.
- Their involvement in numerous physiological systems underscores their significance in health and disease.
- While GPCRs are well-known for their roles in neurons, they are also crucial in virtually all cell types throughout the body.
- Role in Pharmacology:
- The understanding of GPCR structure and function has profound implications for drug development.
- Many pharmaceuticals target GPCRs, leveraging their signaling pathways to elicit therapeutic effects for various conditions, including cardiovascular diseases, mental health disorders, and metabolic syndromes.
- The understanding of GPCR structure and function has profound implications for drug development.
The Ubiquitous G-Proteins
G-proteins serve as crucial intermediaries in the majority of signaling pathways initiated by neurotransmitter receptors, connecting the receptors to various effector proteins. These guanosine triphosphate (GTP)-binding proteins are integral to cellular communication and signaling, with a diverse family consisting of approximately 20 different types. The interplay between neurotransmitter receptors and G-proteins is essential for a multitude of physiological responses.
- Basic Structure and Function:
- Each G-protein comprises three subunits, namely α (alpha), β (beta), and γ (gamma).
- The α subunit is the key player in signal transduction, being responsible for binding GTP or GDP.
- In its resting state, the α subunit is bound to GDP, while the β and γ subunits remain associated with it.
- Each G-protein comprises three subunits, namely α (alpha), β (beta), and γ (gamma).
- Activation Mechanism:
- When a neurotransmitter binds to its receptor, the associated G-protein undergoes a conformational change.
- This change facilitates the release of GDP from the α subunit, allowing GTP to bind in its place, thus activating the G-protein.
- The binding of GTP causes the G-protein to dissociate into two functional components: the GTP-bound α subunit and the βγ complex.
- Both of these components can then interact with various effector proteins, influencing downstream signaling pathways.
- When a neurotransmitter binds to its receptor, the associated G-protein undergoes a conformational change.
- Inactivation Process:
- The α subunit acts as an enzyme, hydrolyzing GTP back into GDP after a certain time, which leads to its inactivation.
- This self-termination mechanism is crucial for regulating the duration of the signaling response.
- Following this hydrolysis, the α subunit re-associates with the βγ complex, reinstating the G-protein to its original inactive state, thus preparing for another cycle of activation.
- The α subunit acts as an enzyme, hydrolyzing GTP back into GDP after a certain time, which leads to its inactivation.
- Types of G-proteins:
- G-proteins can be categorized based on their functional roles:
- Stimulatory G-proteins (Gs): These promote the activation of effector proteins, enhancing the signaling pathways.
- Inhibitory G-proteins (Gi): These inhibit the activity of effector proteins, reducing the signaling output.
- The existence of both stimulatory and inhibitory G-proteins allows for nuanced regulation of cellular responses, ensuring that signaling pathways can be finely tuned based on physiological needs.
- G-proteins can be categorized based on their functional roles:
- Diversity and Distribution:
- There are more types of neurotransmitter receptors than there are G-proteins, resulting in some G-proteins being activated by multiple receptors.
- This redundancy allows for versatility in signaling and responsiveness to a wide range of extracellular signals.
- There are more types of neurotransmitter receptors than there are G-proteins, resulting in some G-proteins being activated by multiple receptors.
G-Protein-Coupled Effector Systems
G-protein-coupled effector systems represent critical pathways through which activated G-proteins mediate cellular responses following neurotransmitter receptor activation. These systems utilize two primary mechanisms: the shortcut pathway, involving direct interaction with ion channels, and second messenger cascades, which initiate a series of enzymatic reactions. Understanding these pathways is essential for comprehending how neurotransmitters exert their effects on cellular function.
- Shortcut Pathway:
- This pathway is characterized by its direct engagement between neurotransmitter receptors, G-proteins, and ion channels, enabling rapid signaling.
- An example is the action of acetylcholine (ACh) on muscarinic receptors in cardiac tissue, which are coupled to specific potassium channels via G-proteins.
- When ACh binds, the βγ subunits of the G-protein migrate within the membrane and activate potassium channels, resulting in a slowing of the heart rate.
- The shortcut pathway is noted for its speed, with responses typically initiated within 30 to 100 milliseconds after neurotransmitter binding.
- Despite being faster than second messenger cascades, it does not achieve the immediacy of transmitter-gated channels that operate without intermediaries.
- This pathway is highly localized due to the restricted diffusion of G-proteins, meaning only nearby ion channels are influenced, enhancing specificity in signaling.
- This pathway is characterized by its direct engagement between neurotransmitter receptors, G-proteins, and ion channels, enabling rapid signaling.
- Second Messenger Cascades:
- G-proteins can activate specific enzymes that trigger a series of biochemical reactions, known as second messenger cascades.
- For instance, the activation of norepinephrine’s β-adrenergic receptor stimulates the Gs protein, which in turn activates adenylyl cyclase.
- Adenylyl cyclase converts ATP to cyclic adenosine monophosphate (cAMP), a pivotal second messenger that subsequently activates protein kinase A (PKA).
- Many signaling pathways employ a push-pull mechanism to balance stimulation and inhibition.
- For example, the activation of the inhibitory α2-adrenergic receptor engages Gi, which suppresses adenylyl cyclase activity, thereby counteracting the stimulatory effects.
- G-proteins can activate specific enzymes that trigger a series of biochemical reactions, known as second messenger cascades.
- Phospholipase C Pathway:
- Certain G-proteins can activate phospholipase C (PLC), leading to the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2).
- This reaction generates two important second messengers: diacylglycerol (DAG) and inositol trisphosphate (IP3).
- DAG remains in the membrane and activates protein kinase C (PKC), while IP3 diffuses into the cytosol and triggers calcium release from intracellular stores, facilitating a wide range of cellular responses.
- The release of calcium ions can activate additional kinases, including calcium-calmodulin-dependent protein kinase (CaMK), which is essential for various processes, including those involved in memory formation.
- This reaction generates two important second messengers: diacylglycerol (DAG) and inositol trisphosphate (IP3).
- Certain G-proteins can activate phospholipase C (PLC), leading to the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2).
- Phosphorylation and Dephosphorylation:
- A critical aspect of second messenger cascades is the role of protein kinases, such as PKA, PKC, and CaMK, which phosphorylate target proteins, altering their activity.
- Phosphorylation typically enhances or modifies the functionality of proteins, influencing processes such as ion channel activity and metabolic pathways.
- Conversely, protein phosphatases serve to remove phosphate groups, ensuring that the signaling can be turned off and preventing the saturation of proteins with phosphates.
- The interplay between kinases and phosphatases maintains a dynamic balance that is essential for proper cellular signaling.
- A critical aspect of second messenger cascades is the role of protein kinases, such as PKA, PKC, and CaMK, which phosphorylate target proteins, altering their activity.
- Advantages of Signal Cascades:
- The complexity and length of G-protein-coupled signaling pathways allow for significant signal amplification.
- For example, one activated receptor can lead to the activation of numerous G-proteins, resulting in the production of multiple cAMP molecules, which can then activate various downstream kinases.
- This amplification effect permits a single neurotransmitter to exert profound and widespread effects on cellular physiology.
- Furthermore, the use of small, diffusible second messengers enables signaling over longer distances within the cell, facilitating coordinated responses across large areas of the cell membrane.
- Signal cascades also offer multiple regulatory points, allowing for fine-tuning of responses and integration with other signaling pathways, which is vital for the adaptability and plasticity of cellular responses.
- The complexity and length of G-protein-coupled signaling pathways allow for significant signal amplification.
Diseases Related to Neurotransmitters
Understanding these diseases provides insights into the complex roles neurotransmitters play in both mental and physical health.
- Alzheimer’s Disease:
- A neurodegenerative condition primarily characterized by progressive memory loss and cognitive decline.
- The pathophysiology involves a significant deficiency of acetylcholine, a neurotransmitter essential for memory and learning.
- This reduction leads to the impairment of synaptic function and neuronal communication, ultimately affecting cognitive processes.
- Parkinson’s Disease:
- This disorder arises due to the depletion of dopamine, a neurotransmitter crucial for regulating motor functions.
- The loss of dopaminergic neurons in the substantia nigra results in the hallmark symptoms of Parkinson’s disease, which include muscle rigidity, bradykinesia, and uncontrollable tremors.
- The imbalance in neurotransmitter activity disrupts normal motor control, significantly impacting the quality of life.
- Depression:
- Depression is often linked to a deficiency of several key neurotransmitters, notably dopamine, norepinephrine, and serotonin.
- These neurotransmitters are involved in mood regulation, emotional response, and overall psychological well-being.
- Low levels of these chemicals contribute to symptoms such as persistent sadness, loss of interest, and fatigue, indicating a disruption in the brain’s chemical signaling pathways.
- Schizophrenia:
- Schizophrenia is a severe mental disorder associated with an excess of dopamine activity, particularly in the frontal lobes.
- This hyperactivity can lead to symptoms such as hallucinations, delusions, and cognitive disturbances.
- The precise relationship between dopamine dysregulation and the emergence of psychotic symptoms highlights the complexity of neurotransmitter interactions in the brain.
- Epilepsy:
- Epilepsy is characterized by recurrent seizures, which can arise from imbalances in neurotransmitter systems.
- A deficiency of inhibitory neurotransmitters, such as gamma-aminobutyric acid (GABA), or an excess of excitatory neurotransmitters, such as glutamate, can lead to increased neuronal excitability and seizure activity.
- This disruption in neurotransmitter balance emphasizes the importance of proper inhibition and excitation in maintaining neural stability.
- Other Disorders:
- Various other neurological and psychiatric disorders can also be linked to neurotransmitter dysregulation, including anxiety disorders, bipolar disorder, and autism spectrum disorders.
- Each of these conditions may involve alterations in specific neurotransmitter systems, further underscoring the intricate relationship between neurotransmitter function and mental health.
Functions of Neurotransmitter
Neurotransmitters are essential chemical messengers in the nervous system, responsible for transmitting signals across synapses and facilitating communication between neurons. They play crucial roles in various physiological and psychological functions, influencing behavior, mood, cognition, and overall bodily functions. Understanding the functions of neurotransmitters provides insights into their complex roles within the nervous system.
- Carrier Agent:
- Neurotransmitters function as carrier molecules released across the axolemma during nerve signaling.
- After release, they bind to specific receptors on the postsynaptic neuron, propagating the nerve signal and facilitating communication between neurons.
- Booster:
- These molecules enhance nerve signal conduction from one neuron to another or from a neuron to a target cell.
- The release of excitatory neurotransmitters increases the likelihood of action potentials in the postsynaptic neuron, promoting further signaling.
- Modulatory Factor:
- Beyond merely facilitating or inhibiting action potentials, neurotransmitters play a role in balancing the signals between neurons.
- They modulate synaptic transmission, influencing the overall excitability and responsiveness of neural circuits.
- Norepinephrine:
- As the primary neurotransmitter of the sympathetic nervous system, norepinephrine mobilizes the brain and body for action.
- It regulates wakefulness, circadian rhythms, and feeding behavior, while also playing a role in cognitive control and memory.
- An imbalance can lead to conditions such as attention deficit hyperactivity disorder (ADHD) and depression.
- Acetylcholine:
- This neurotransmitter is crucial for muscle contraction and is involved in movement, pain responses, and REM sleep regulation.
- It influences emotional regulation, motivation, and short-term memory. Deficiencies are linked to conditions like myasthenia gravis and Alzheimer’s disease.
- Dopamine:
- Dopamine plays a pivotal role in motivation, pleasure, and reward, acting as a key mediator of reinforcement.
- It influences motor function, sexual arousal, and cognitive control.
- Abnormal levels are associated with disorders such as Parkinson’s disease and schizophrenia.
- GABA (Gamma-Aminobutyric Acid):
- GABA is the primary inhibitory neurotransmitter, essential for regulating neuronal excitability and anxiety.
- It plays a significant role in motor function and the modulation of cortical activities.
- Low levels can lead to seizures and mood disorders, while excessive levels may result in daytime sleepiness.
- Serotonin:
- Often referred to as the “feel-good” neurotransmitter, serotonin regulates mood, social behavior, and sleep.
- It also plays a role in bowel function and blood clotting following injury.
- Low serotonin levels are linked to anxiety and depression, whereas excess levels can lead to serotonin syndrome, characterized by various physiological symptoms.
- Glutamate:
- As the most abundant excitatory neurotransmitter, glutamate is vital for cognitive functions, including memory and learning.
- It also plays a role in muscle function and energy production.
- Imbalances in glutamate levels can be associated with neurological disorders such as schizophrenia and Alzheimer’s disease.
- Endorphins:
- Endorphins are neuropeptides that act as natural pain relievers, providing a sense of euphoria.
- They are crucial for modulating stress and pain responses.
- Deficiencies in endorphins can lead to conditions like depression, while excess can result in heightened feelings of well-being and increased appetite.
- https://biologyreader.com/neurotransmitters.html
- https://human-memory.net/neurotransmitters/#Mechanism_of_action
- https://www.simplypsychology.org/neurotransmitter.html#Classification
- https://www.brainkart.com/article/Neurotransmitters_29217/
- https://openbooks.lib.msu.edu/neuroscience/chapter/neurotransmitter-synthesis-and-storage/
- https://www.physio-pedia.com/Neurotransmitters
- https://collegedunia.com/exams/neurotransmitter-science-articleid-6215