What is Mutarotation?
- Mutarotation is a fascinating chemical phenomenon observed in carbohydrate chemistry. It refers to the change in the optical rotation of a solution that occurs due to the equilibrium shift between the α- and β-anomers of a sugar molecule upon dissolution in water. This process is also known as anomerization.
- To grasp the concept of mutarotation, it is essential to understand some key terms associated with it. Optical rotation refers to the ability of a compound to rotate the plane of polarized light. It is denoted by a specific angle, usually measured in degrees, and can be either clockwise (+) or counterclockwise (-) rotation. The magnitude and direction of the rotation provide valuable information about the chemical structure and composition of a substance.
- Isomers are molecules that have the same molecular formula but differ in their arrangement or spatial orientation. In the case of carbohydrates, isomers can exist as α-anomers or β-anomers. These anomers arise due to the stereochemical variability around the carbon atom that forms the linkage between the sugar molecule and the functional group. The α-anomer has the hydroxyl (-OH) group attached to the anomeric carbon in the opposite direction compared to the β-anomer.
- Mutarotation was first discovered by Augustin-Pierre Dubrunfaut, a French chemist, in 1844. He noticed that the specific rotation of sugar solutions changed over time. This observation led to the realization that the equilibrium between the α- and β-anomers of sugars was responsible for this phenomenon.
- The mutarotation process occurs through a mechanism called ring-chain tautomerism. When a cyclic sugar, such as glucose, is dissolved in water, the α- and β-anomers gradually interconvert, forming an equilibrium state. This interconversion involves the opening and closing of the sugar ring, allowing the hydroxyl group to change its position. As a result, the optical rotation of the solution changes over time until it reaches a stable equilibrium value.
- For instance, when α-D-glucose or β-D-glucose is dissolved in water, the specific rotation of the solution gradually shifts to an equilibrium value of +52.7°, which corresponds to approximately 36% α-anomer and 64% β-anomer. The interconversion between these anomers continues until a balance is achieved.
- Mutarotation plays a crucial role in carbohydrate chemistry, as it affects the properties and behavior of sugars in various applications. Understanding this phenomenon is essential for studying sugar metabolism, enzymatic reactions, and the synthesis of carbohydrate-based drugs. By investigating the mutarotation of sugars, scientists can gain insights into their structures, stereochemistry, and interactions with other molecules.
- In summary, mutarotation is the alteration in the optical rotation of a solution caused by the equilibrium shift between α- and β-anomers of a sugar upon dissolution in water. This dynamic process, discovered by Dubrunfaut in 1844, arises from the interconversion of cyclic sugar forms and is a fundamental concept in carbohydrate chemistry.
Properties of Carbohydrates
Carbohydrates exhibit various properties influenced by their structural arrangements. Understanding these properties is essential to grasp concepts like mutarotation and distinguish between different isomers.
- Cyclic Structure: Carbohydrates can exist in both linear and cyclic forms. The formation of cyclic structures occurs due to intermolecular reactions between the carbonyl group and the hydroxyl group. These cyclic arrangements contribute to the diverse properties of carbohydrates.
- Chiral Carbon: Carbohydrates contain chiral carbon, which is a carbon atom bonded to four different atoms or groups. This chiral carbon is crucial for observing mutarotation, a phenomenon characterized by the interconversion between different cyclic forms of carbohydrates.
- Isomers: Isomers are molecules with the same molecular formula but differing chemical properties. In the context of carbohydrates, understanding isomer-related terms is important.
- Structural (Constitutional) Isomers: These isomers possess the same molecular formula but differ in the arrangement of functional groups. They exhibit distinct chemical properties due to their structural variations.
- Stereoisomers: Stereoisomers share the same molecular formula but have different spatial arrangements of functional groups. They can be further classified into two groups:
- Enantiomers: Enantiomers are non-superimposable mirror images of each other. They possess identical physical and chemical properties but differ in their optical activity.
- Diastereomers: Diastereomers are stereoisomers that are neither superimposable nor mirror images of each other. They exhibit different physical and chemical properties due to their distinct arrangements of functional groups.
Anomers, optical rotation, polarized light, optical activity
- Anomers are a specific type of isomers that exhibit differences in configuration at either carbon 1 (for aldoses) or carbon 2 (for ketoses). More precisely, they are epimers, which are diastereomers that vary at only one chiral carbon. The configuration change in these carbons leads to the formation of two distinct anomeric forms.
- Optical rotation refers to the phenomenon where the plane of polarized light undergoes a rotation or movement when it traverses a layer of liquid or certain materials. When light travels through specific media, the vibrations of the light waves occur in a single plane. This property is known as polarization. The angle by which the plane of polarized light rotates upon passing through a substance is referred to as the optical rotation.
- Polarized light is the term used to describe light waves that are transmitted through certain materials in a manner where the vibrations of the waves occur in a singular plane. This differs from unpolarized light, where the vibrations occur in various planes. Polarized light can be obtained by passing unpolarized light through a polarizer, which filters out waves oscillating in all planes except one.
- Optical activity is the characteristic exhibited by compounds that have the ability to rotate the plane of polarized light. This property is observed in compounds that possess chirality, meaning they have an asymmetric structure or lack mirror symmetry. The interaction between the electromagnetic radiation of polarized light and the asymmetric arrangement of electrons in chiral compounds results in their optical activity.
When a compound is optically active, it means it has the capability to rotate the plane of linearly polarized light. It is important to note that each optically active compound has its own specific rotation value, which quantifies the extent and direction of the rotation caused by the compound.
In summary, anomers are a type of isomers that differ in configuration at specific carbon atoms. Optical rotation refers to the rotation of the plane of polarized light when it passes through a substance. Polarized light is light waves vibrating in a single plane as they pass through certain materials. Optical activity is the ability of a compound to rotate the plane of polarized light, observed in chiral compounds due to their asymmetric electron arrangements. Optically active compounds possess specific rotation values that define the extent and direction of the rotation.
What does equilibrium between anomers mean?
- The equilibrium between anomers refers to the state of balance or equilibrium between the two forms, namely the alpha (α) and beta (β) forms of a compound, typically observed in a solution.
- When we consider certain compounds, such as sugars, they can exist in different forms due to the stereochemical variability around specific carbon atoms. In the case of anomers, the difference lies in the configuration of either carbon 1 (aldoses) or carbon 2 (ketoses). The alpha and beta anomers are epimers, which means they differ in configuration at only one chiral carbon.
- When a solution contains a compound with anomerism, such as cyclic sugars like glucose, an equilibrium is established between the alpha and beta forms. This equilibrium arises due to the interconversion of the two anomeric forms. The interconversion process occurs through a mechanism called ring-chain tautomerism, where the sugar ring opens and closes, allowing the hydroxyl group to change its position.
- In the solution, the alpha and beta forms of the compound continuously interconvert, but at a certain point, an equilibrium is reached. This equilibrium state represents a balance between the two forms, where the rate of conversion from alpha to beta is equal to the rate of conversion from beta to alpha. The ratio of alpha and beta anomers at equilibrium can vary depending on factors such as temperature, concentration, and solvent properties.
- The equilibrium between anomers is of significance in understanding the behavior and properties of compounds in solution. It impacts various aspects such as the optical rotation, chemical reactivity, and biological activities of the compound. The specific equilibrium ratio between alpha and beta anomers can provide valuable information about the stereochemical preferences and dynamics of the compound.
- In summary, the equilibrium between anomers refers to the balanced state between the alpha and beta forms of a compound in a solution. This equilibrium is achieved through the interconversion of the two forms, resulting in a stable ratio between them. Understanding this equilibrium is important for studying the properties and behavior of compounds, particularly in the context of sugars and other compounds exhibiting anomerism.
History of Mutarotation
The concept of mutarotation, referring to the phenomenon of optical activity change in sugars when dissolved in water, was first discovered by Augustin Pierre Dubrunfaut in 1846. While studying sugars, Dubrunfaut observed that the optical activity of sugar decreased over time after being dissolved in water. He initially termed this phenomenon “birotation,” with the rotation values changing from 110° to 52°.
In 1899, the concept was given a more appropriate name, “mutarotation,” by Lowry, which reflected the underlying concept behind this phenomenon. Following its discovery, numerous scientists became involved in further understanding mutarotation.
These scientists embarked on a series of investigations to explore various aspects of mutarotation, seeking answers to questions such as the mechanisms and reasons behind its occurrence, its presence in different mediums and compounds, and its relationship with different types of sugars.
E. O. Erdmann, in 1855, discovered that lactose exists in two crystalline forms, each demonstrating mutarotation towards the same final rotation value of 52°.
In 1859, Anthon observed that the formation of a saturated solution of glucose in cold water was a slow process even with vigorous mixing.
Mills, Hogarth, and others in 1879 found that the slow formation of a saturated solution was due to a balanced chemical reaction involving the carbonyl group, which is also central to mutarotation. They recognized that this property was not limited to glucose but extended to other aldehyde and ketone sugars.
Further research by Urech in 1882-1885 showed that mutarotation followed the law of unimolecular reactions.
In 1888, Brown, Morris, and Arrhenius determined that the reaction causing mutarotation was not a polymerization or dissociation of the sugar.
In 1895, Charles Tarnet discovered a new crystalline form of glucose with a specific rotation of less than 52°. He also found that regardless of the initial rotating form, the final rotation of the glucose solution remained the same. Tarnet also isolated new crystalline forms of rhamnose, galactose, and arabinose.
Finally, in 1899, Lowry proposed that the mutarotation of glucose occurred due to a balanced reaction between its highest and lowest rotating forms.
The collective efforts of these scientists contributed to our understanding of mutarotation, shedding light on its underlying mechanisms and expanding our knowledge of its occurrence in various sugars and compounds.
In summary, the concept of mutarotation was initially discovered by Dubrunfaut, and subsequent investigations by different scientists provided valuable insights into the mechanisms and properties of mutarotation in sugars. The exploration of this phenomenon continues to deepen our understanding of its intricacies and significance in the field of chemistry.
Mechanism of Mutarotation
- The mechanism of mutarotation is closely linked to the phenomenon of ring-chain tautomerism. In the case of sugars, which exist in cyclic hemiacetal forms, there is an equilibrium established with the linear (straight-chain) form.
- This means that even if a sugar compound initially exists in a pure sample of either the alpha or beta anomer, once it is dissolved in water, it can undergo an equilibrium process through the linear form, interconverting with the other anomer.
- This principle can be explained by the Zeroth Law of Thermodynamics, which states that if P is in equilibrium with Q, and Q is in equilibrium with R, then P is in equilibrium with R.
- At a temperature of 25°C, the equilibrium distribution of isomers for D-glucose in water is typically represented by a ratio of 36:64 between the alpha and beta forms.
- During mutarotation, the mechanism of ring-chain tautomerism comes into play. The different cyclic hemiacetal forms of sugars establish an equilibrium state with the linear form. This means that even if a compound initially exists in a pure form when dissolved in water, it undergoes an equilibrium with its linear pattern. For example, if a 100% pure alpha-glucose form is added to water, it will transform into a linear pattern. Upon reforming, it can convert into either the alpha or beta form. Over time, an equilibrium state is achieved between both forms, following the zeroth law of thermodynamics.
- It is important to note that the alpha and beta anomers of sugars have different specific rotations. A solution containing the pure alpha compound will exhibit a different and opposite direction of rotation compared to a solution of the pure beta compound. The specific optical rotation of each anomer and their ratio in the solution determine the overall optical rotation of the solution.
- To measure the optical rotation of a sample and calculate the ratio of the two forms (anomers) present in the solution, a polarimeter is used. The polarimeter measures the rotation of the sample and enables the determination of the ratio between the two forms of the compound present in the solution. By summing the optical rotation values of each monomer, the optical rotation of the sample can be determined.
- In summary, the mechanism of mutarotation involves the interconversion between the cyclic and linear forms of sugars through ring-chain tautomerism. This equilibrium process follows the zeroth law of thermodynamics. The specific rotations of the alpha and beta anomers determine the optical rotation of the solution, which can be measured using a polarimeter to calculate the ratio between the two forms in the solution.
What’s a reducing sugar?
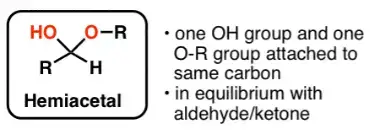
- A reducing sugar refers to any sugar molecule that possesses a free aldehyde or ketone group. These sugars are also known as hemiacetal compounds. In their structure, they exist in equilibrium with the open-ring form of the molecule.
- The distinguishing feature of reducing sugars is the presence of an aldehyde group or a ketone group that can act as a reducing agent. This means that they have the ability to undergo oxidation reactions and donate electrons to other substances. One common application of this property is seen in certain chemical tests.
- For instance, reducing sugars can reduce certain metal salts, such as copper ions (Cu2+), in tests like Benedict’s test and Fehling’s solution. These tests involve the addition of a reagent that contains the metal ion and an alkaline solution. In the presence of a reducing sugar, the aldehyde or ketone group donates electrons to the metal ion, resulting in a color change. The formation of a colored precipitate, such as red copper(I) oxide, indicates the presence of a reducing sugar.
- Similarly, reducing sugars can also reduce silver ions (Ag+) in Tollen’s test. In this test, a solution containing a reducing sugar is mixed with an ammoniacal silver nitrate solution. The aldehyde or ketone group of the sugar reduces the silver ions, leading to the formation of a silver mirror or silver precipitate.
- It is important to note that not all sugars are reducing sugars. For example, non-reducing sugars, such as sucrose, lack a free aldehyde or ketone group and therefore cannot act as reducing agents in these tests.
- In summary, a reducing sugar is a sugar molecule that possesses a free aldehyde or ketone group. These sugars are in equilibrium with the open-ring form of the molecule and can undergo oxidation reactions, acting as reducing agents. They demonstrate the ability to reduce metal ions, such as copper and silver ions, in tests like Benedict’s test, Fehling’s solution, and Tollen’s test, resulting in characteristic color changes or precipitate formations.
What is a non-reducing sugar and why don’t they show mutarotation?
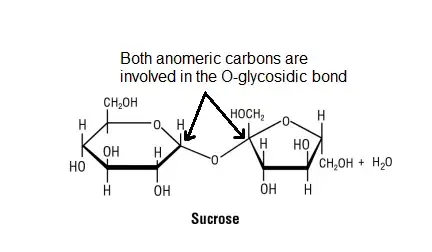
- Non-reducing sugars are a type of sugar molecule that lacks free aldehyde or ketone groups. This absence of a free functional group is what distinguishes them from reducing sugars. Non-reducing sugars, such as sucrose and trehalose, do not exhibit the properties of reducing agents and are not oxidized by weak oxidizing agents.
- To understand why non-reducing sugars do not show mutarotation, let’s consider the example of sucrose. Sucrose is formed through a condensation reaction between a glucose molecule and a fructose molecule. During this reaction, the anomeric carbons of glucose and fructose come together, resulting in the formation of an O-glycosidic bond between the two molecules.
- For mutarotation to occur, a compound must have a free anomeric carbon that can interconvert between the alpha and beta forms. However, in the case of sucrose, both anomeric carbons of glucose and fructose are involved in the formation of the glycosidic linkage. As a result, they are not free to undergo the interconversion between alpha and beta forms, and thus mutarotation does not take place.
- Since non-reducing sugars lack free aldehyde or ketone groups, they do not exhibit the characteristics of reducing sugars. They do not possess reducing power and are not capable of reducing weak oxidizing agents. This is due to the absence of the functional groups that are necessary for the oxidation-reduction reactions observed in reducing sugars.
- In summary, non-reducing sugars do not possess free aldehyde or ketone groups, which are required for mutarotation and the reducing properties exhibited by reducing sugars. Examples like sucrose and trehalose lack the ability to undergo mutarotation and do not show reducing power due to the absence of these functional groups.
Mutarotation Occurrence and Measurement
Mutarotation, which involves the interconversion between the alpha (α) and beta (β) anomeric forms of sugars, occurs due to the mechanism of ring-chain tautomerism. The cyclic hemiacetal forms of sugars establish an equilibrium with the linear form, even if the compound is initially 100% pure when dissolved in water.
When a pure alpha-glucose form is added to water, it undergoes ring-opening, revealing a linear chain structure. Upon reforming, it can convert into either the alpha or beta form. Over time, an equilibrium state is reached between these two forms, adhering to the principles of the zeroth law of thermodynamics.
The alpha and beta anomers of sugars exhibit distinct specific rotations. A liquid solution of pure alpha compound will rotate plane-polarized light in a different angle and opposite direction compared to a solution of pure beta compound. The optical rotation of a solution depends on the individual optical rotations of each anomer and their respective ratio in the solution.
To measure the occurrence of mutarotation, a polarimeter is employed to determine the rotation of a sample. The optical rotation is calculated by summing the optical rotations of each monomer present in the solution.
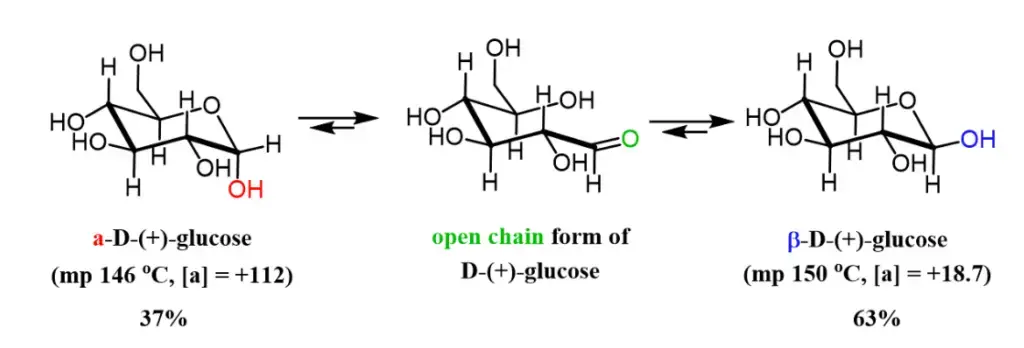
For example, if a solution of β-D-glucopyranose is dissolved in water, it initially exhibits a specific optical rotation of +18.7°. Over time, some of the β-D-glucopyranose undergoes mutarotation to transform into α-D-glucopyranose, which possesses an optical rotation of +112.2°. As a result, the rotation of the solution increases from +18.7° to an equilibrium value of +52.7° as the β form converts to the α form. The equilibrium mixture typically consists of approximately 64% β-D-glucopyranose and 36% α-D-glucopyranose, although there may also be traces of other forms, including furanoses and the open-chain form.
Examples of Mutarotation
1. Mutarotation in Glucose
- Mutarotation in glucose refers to the interconversion between its two diastereomeric forms, alpha (α) and beta (β) glucose. The study of this process was first proposed by Bronsted and Guggenheim, who suggested that mutarotation in glucose can occur through acid or base catalysis.
- Glucose exists in two distinct forms, alpha-D-glucose and beta-D-glucose, which differ in their physical properties. When methanol is added to glucose, it promotes the crystallization of alpha-D-glucose, which has a melting point of 46 °C. On the other hand, the addition of acetic acid leads to the formation of beta-D-glucose, which melts at a higher temperature of 150 °C. These different forms also exhibit specific rotations, with the alpha form having a specific rotation of +112.2° and the beta form having a specific rotation of +18.7°.
- When a cyclic glucose molecule is dissolved in water, it undergoes reversible epimerization through the linear open-chain form. Over time, the specific rotation of the glucose solution gradually changes until it reaches an equilibrium state at +52.7°.
- The equilibrium mixture of glucose in water consists of approximately 36% alpha-D-glucose, 64% beta-D-glucose, and a small amount (0.02%) of the open-chain form of glucose. The interconversion of glucose between its pyranose form (cyclic) and the aldehyde form (linear) involves three steps: protonation of O5, breaking of the O1-H bond through intramolecular transfer, and breaking of the O5-C1 bond.
- Overall, mutarotation in glucose refers to the dynamic equilibrium between its alpha and beta forms, facilitated by the interconversion between the cyclic and linear forms of the molecule.

Cause and Occurrence of Mutarotation of Glucose
- Mutarotation of glucose occurs due to the presence of different forms of glucose in solution, which are in equilibrium with each other. One of the well-known forms of glucose is the Fischer projection representation of “2,3,4,5,6-pentahydroxyhexanal.” However, it’s important to note that this representation should not be used with sugars. The hydroxyl (-OH) and aldehyde (-CHO) groups in glucose can combine to form a hemiacetal, and considering the various hydroxyl groups available, different hemiacetal forms can be conceptualized.
- In solution, the most stable form of glucose is a six-membered ring structure. When a glucose crystal dissolves, it initially releases a large amount of glucose in a specific form, which exhibits its own unique optical activity. Each form of glucose present in the solution has its own optical activity as well. As a result, the optical activity of the glucose solution is essentially an average of the individual components’ optical activities.
- Over time, the optical activity of the glucose solution gradually changes from the initial form found in the crystal to the optical activity of the equilibrium mixture. This change occurs because the different forms of glucose interconvert, leading to a dynamic equilibrium between them. As the equilibrium is established, the optical activity of the solution reflects the combined influence of the various forms of glucose present.
- In summary, the cause of mutarotation in glucose is the equilibrium between different forms of the molecule in solution. The initial optical activity of the glucose solution, determined by the predominant form in the crystal, gradually shifts to the optical activity of the equilibrium mixture as interconversion between different forms takes place.

2. Mutarotation in Lactose
- Mutarotation is also observed in lactose, which is a disaccharide commonly known as milk sugar. Lactose consists of one glucose molecule and one galactose molecule linked together by a beta (1-4) glycosidic linkage. In an aqueous solution, lactose undergoes mutarotation due to the presence of the anomeric carbon (C1) of the glucose residue.
- The equilibrium mixture of lactose in solution is composed of approximately 62.7% beta-lactose and 37.3% alpha-lactose. This indicates that the configuration at the anomeric carbon of the glucose residue changes during mutarotation.
- The mutarotation of lactose follows a first-order reaction kinetics. However, the rate of mutarotation is relatively slow due to the higher solubility of lactose. The presence or absence of other sugars and salts in the solution can also affect the rate of mutarotation. For example, when the concentration of sucrose in the solution exceeds 40%, the mutarotation of lactose decreases to half the normal rate of specific rotation.
- Several factors influence the mutarotation of lactose, including the temperature of the solution (with slower mutarotation observed at lower temperatures), the wavelength of the light source used for measurement, and the concentration of the lactose solution.
- In solid-state lactose, mutarotation can be observed after heating monohydrate crystalline samples. The heating process induces changes in the crystal structure, leading to the interconversion between the two structural forms of lactose achieved after mutarotation.
- In summary, lactose, as a reducing sugar, undergoes mutarotation in an aqueous solution due to the presence of the anomeric carbon in the glucose residue. The equilibrium mixture of lactose consists of alpha-lactose and beta-lactose. The rate of mutarotation is influenced by factors such as temperature, the presence of other sugars or salts, and the concentration of the lactose solution.

3. Mutarotation in Fructose
- Mutarotation is a phenomenon observed in fructose, a monosaccharide that can exist in different forms. Unlike glucose, fructose contains a ketone carbonyl group, which gives it unique characteristics in terms of mutarotation.
- The mutarotation of fructose occurs when the fructose molecule undergoes a change from one form to another. This change can be induced by factors such as changes in pH (presence of acidic protons) or thermal excitations (temperature change).
- The open-chain form of fructose can form two different cyclic structures: β-fructopyranose and β-fructofuranose. These two forms have distinct structural differences, with β-fructopyranose having a six-membered ring structure and β-fructofuranose having a five-membered ring structure.
- Due to these structural differences, β-fructopyranose and β-fructofuranose exhibit different optical activities. The specific rotation of each form is unique, and their ratio in a fructose solution determines the overall optical rotation of the solution.
- Similar to other sugars undergoing mutarotation, the interconversion between β-fructopyranose and β-fructofuranose in fructose occurs through the open-chain form. The equilibrium mixture achieved during mutarotation contains a certain proportion of each form, resulting in a balanced optical rotation for the fructose solution.
- In summary, mutarotation in fructose involves the conversion between its different cyclic forms, β-fructopyranose and β-fructofuranose. These forms have distinct optical activities due to their structural differences. Factors such as pH and temperature can induce mutarotation in fructose, leading to an equilibrium mixture of the two forms and resulting in a balanced optical rotation for the fructose solution.

Techniques Used to Study the Mutarotation of Compounds
Two commonly used techniques for studying the mutarotation of compounds are polarimetry and dielectric spectroscopy. These methods provide valuable insights into the optical activity and dielectric properties of substances undergoing mutarotation.
- Polarimetry is an analytical technique that measures the angle of rotation or optical activity of a compound. It involves passing polarized light through an optically active substance and observing the change in the plane of polarization. By measuring the angle of rotation, the extent of mutarotation and the ratio of different forms of a compound can be determined. Polarimeters are widely used in chemistry, biochemistry, and pharmaceutical industries to analyze the optical properties of chiral compounds. The discovery of polarimetry is credited to Etienne-Louis Malus in 1808.
- Dielectric spectroscopy, on the other hand, is a technique used to measure the dielectric properties of a sample in response to an applied electric field of fixed or varying frequency. It provides information about the electrical polarization and relaxation processes occurring in a material. In the context of mutarotation, dielectric spectroscopy can be utilized to investigate the changes in the dielectric properties of a compound as it undergoes interconversion between different forms. This technique finds applications in various scientific fields, including fuel cell testing, biomolecular interactions, and microstructural characterization.
By employing polarimetry and dielectric spectroscopy, researchers can gain insights into the structural dynamics and equilibrium states of compounds undergoing mutarotation. These techniques provide valuable data on the optical and dielectric properties, allowing for a deeper understanding of the interconversion processes and the factors influencing mutarotation.
Is Mutarotation A General Property Of Cyclic Sugars Bearing A Hemiacetal
Mutarotation is a general property exhibited by cyclic sugars bearing a hemiacetal group, as well as other chiral cyclic hemiacetals. This phenomenon was first discovered in 1846 by Augustin-Pierre Dubrunfaut, a French chemist known for his work on sugar production from beet sugar. While studying the optical rotation of glucose, Dubrunfaut observed that freshly dissolved glucose exhibited a rotational value twice that which was previously documented. He also investigated the mutarotation of lactose.
At the time of Dubrunfaut’s discovery, the structures of glucose and fructose had not yet been established. It wasn’t until 1895 when Tanret reported on the two anomers of glucose, which provided a comprehensive explanation for Dubrunfaut’s observations.
Regarding the interconversion of alpha-D-glucose to beta-D-glucose, the mechanism can be represented as follows:
- Protonation of the oxygen atom at the anomeric carbon (C1) of alpha-D-glucose.
- Intramolecular transfer of the proton from the anomeric carbon to the hydroxyl group at the C4 position, resulting in the formation of an open-chain aldehyde.
- Rotation of the open-chain aldehyde to a linear form, followed by reformation of the cyclic structure as beta-D-glucose.
- Deprotonation of the hydroxyl group at the C4 position to restore the hemiacetal structure.
The interconversion of alpha-D-glucose to beta-D-glucose involves the reversible breaking and formation of glycosidic bonds, allowing for the equilibrium between the two forms. This mechanism is an important aspect of the mutarotation phenomenon observed in cyclic sugars.
It is worth noting that drawing the mechanism for the interconversion of alpha-D-glucose to beta-D-glucose is a common examination question in the field of chemistry, as it requires an understanding of the structural dynamics and transformation processes involved in mutarotation.
Factors influence the rate of mutarotation
Several factors can influence the rate of mutarotation in a solution. These factors include:
- Temperature: The rate of mutarotation generally increases with an increase in temperature. Higher temperatures provide more thermal energy, leading to faster interconversion between the alpha and beta anomers.
- pH: The pH of the solution can affect the rate of mutarotation. Changes in pH can alter the concentration of protons, which can influence the stability and reactivity of the cyclic sugar, thereby affecting the rate of mutarotation.
- Concentration: The concentration of the sugar in the solution can impact the rate of mutarotation. Higher concentrations of the sugar can lead to increased collisions between molecules, promoting faster interconversion.
- Presence of Catalysts: Certain catalysts can accelerate the rate of mutarotation by facilitating the conversion between different anomers. For example, acids or bases can act as catalysts in the mutarotation of sugars.
- Solvent: The nature of the solvent can influence the rate of mutarotation. Different solvents can have varying effects on the stability and reactivity of the cyclic sugar, thereby affecting the rate of interconversion.
- Presence of Inhibitors: Some substances can inhibit or slow down the rate of mutarotation. These inhibitors may interfere with the interconversion process or stabilize one particular anomer, reducing the overall rate of mutarotation.
It’s important to note that the specific effects of these factors can vary depending on the particular sugar and experimental conditions.
Significance of mutarotation
Mutarotation holds significant importance in the study and understanding of sugars and their behavior. Here are some key significances of mutarotation:
- Identification and Characterization: Mutarotation provides a means to identify and characterize different sugar molecules. By measuring the rate and extent of mutarotation, scientists can determine the specific type of sugar present, its anomeric configuration (alpha or beta), and its optical activity.
- Determination of Purity and Composition: Mutarotation allows for the determination of the purity and composition of sugar samples. The equilibrium mixture of anomers obtained during mutarotation reflects the relative proportions of different forms present in a sample. This information is valuable in fields such as food science, pharmaceuticals, and biochemistry.
- Optical Activity and Chirality: Mutarotation is closely related to the optical activity of sugars. The different anomers of sugars exhibit distinct optical rotations due to their structural differences. By studying mutarotation, researchers can gain insights into the chirality and three-dimensional arrangement of sugar molecules.
- Kinetics and Reaction Mechanisms: The study of mutarotation provides insights into the kinetics and reaction mechanisms of sugar interconversions. By investigating the factors that influence the rate of mutarotation, scientists can better understand the underlying chemical processes involved in the conversion between alpha and beta anomers.
- Biochemical and Biological Significance: Mutarotation plays a crucial role in various biochemical and biological processes. It affects the behavior and properties of sugars in biological systems, such as their metabolism, transport, and interactions with enzymes and receptors. Understanding mutarotation is essential in fields like glycobiology and carbohydrate chemistry.
- Industrial Applications: Mutarotation has practical applications in industries such as food and beverage, pharmaceuticals, and biotechnology. It helps in the formulation and quality control of products containing sugars, as well as in the synthesis of specific sugar derivatives and analogs.
Overall, mutarotation is a fundamental phenomenon that contributes to our understanding of sugars, their structures, properties, and reactivity. It has implications in diverse fields and is a valuable tool for both research and practical applications.
FAQ
What is mutarotation?
Mutarotation refers to the spontaneous interconversion of different anomeric forms (alpha and beta) of a cyclic sugar in solution, resulting in a change in its specific rotation.
Which compounds exhibit mutarotation?
Mutarotation is commonly observed in cyclic sugars that possess a hemiacetal or hemiketal functional group, such as glucose, fructose, and lactose.
What is the significance of mutarotation?
Mutarotation is important because it allows for the formation of a dynamic equilibrium between different anomers, resulting in a mixture with specific optical properties. This property has implications in fields such as biochemistry, food science, and pharmaceuticals.
How is mutarotation measured?
Mutarotation can be measured using techniques such as polarimetry, where the change in optical rotation of a solution over time is monitored using a polarimeter.
What factors influence the rate of mutarotation?
The rate of mutarotation can be influenced by factors such as temperature, pH, concentration, and the presence of catalysts or inhibitors in the solution.
Can mutarotation occur in non-sugar compounds?
While mutarotation is commonly associated with sugars, it can also occur in other cyclic compounds that possess a hemiacetal or hemiketal group.
How does mutarotation relate to the equilibrium between alpha and beta anomers?
Mutarotation involves the dynamic interconversion between the alpha and beta anomers of a cyclic sugar, establishing an equilibrium state where the ratio of these forms remains constant.
Does mutarotation affect the taste or properties of sugars?
Mutarotation itself does not significantly alter the taste or properties of sugars. However, it can impact their chemical reactivity and interactions with other substances.
Can mutarotation be controlled or inhibited?
The rate of mutarotation can be influenced by various factors, but completely inhibiting mutarotation in a sugar solution is challenging due to the inherent nature of the equilibrium between different anomers.
Are there any practical applications of mutarotation?
Mutarotation has practical applications in fields such as carbohydrate chemistry, pharmaceutical formulation, and food science, where understanding the dynamic behavior of sugars is crucial for product development and quality control.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.