- Modified Kirby-Bauer disc diffusion test is a standard method that can be used as a regular method to assess the susceptibility test to a bacterial isolate in the clinical laboratory.
- The method of disc diffusion was first introduced in 1966.
- It is standardized and widely examined.
Procedure of Modified Kirby Bauer method
- Make the inoculum from the plate of culture that was used for the initial one by placing a loop over the tops of every one of 3 to 5 colonies that have a similar appearance of the organism that is to be tested , and then transfer this growth to a tube filled with Saline. If the inoculum must be made from a purified culture, then suspend the confluent growth as well.
Note: Multiple similar colonies should be selected to reduce the chance of testing a non-representative colony. For example, you could pick one that is susceptible and not excluding the mutants that are resistant scattered in other colonies.
- Examine the tube in comparison to that of the 0.5 McFarland turbidity standard (approx cell density 1.5 108 CFU/ml) and then adjust the amount of test suspension to match that of that standard, by adding more of the bacteria and sterilized saline.
- Be sure to adjust the inoculum’s turbidity is vital in order to ensure that the lawn is almost or confluent.
- Innoculate the plates by placing an sterile swab in the inoculum. Eliminate any excess inoculum by pressing and turning the swab against the inside of the tube to the liquid level.
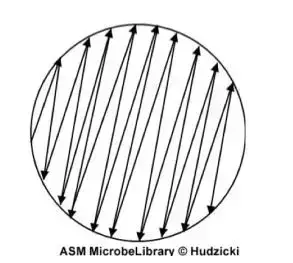
- The swab should be dragged across your medium’s surface 3 times, turning the plate at the 60 degrees after each time. Then, slide the swab along the edges of the agar surface. The swab will follow when it is traced across the plates (as illustrated in the image). Discard the swab in the appropriate container.
- Allow the inoculum to dry for several minutes (at at least 3 to 5 mins, but not longer than 15 mins) with room temperatures, keeping the lid shut.
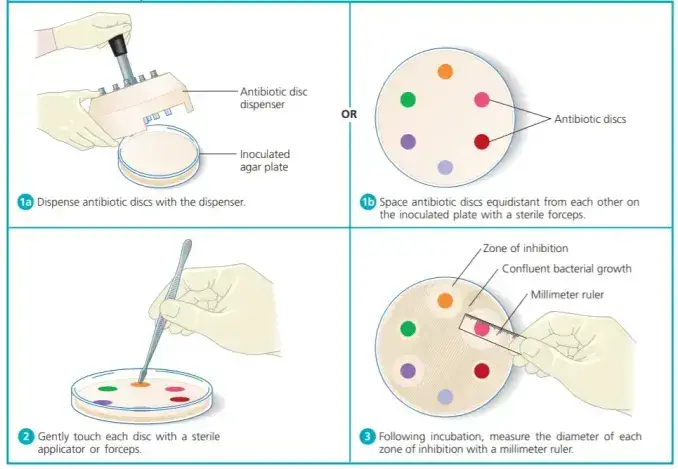
- Place the appropriate antimicrobial-impregnated disks on the surface of the agar (antimicrobial disks can be purchased from any reputable suppliers)
- Antimicrobial discs may be placed on the plates with inoculation by using forceps that are sterile. It is easy to use a template put the discs in a uniform manner or a needle-tip made of sterile material can be used for placing the discs with antibiotics onto the plates. Alternately using an anti-bacterial disc (as illustrated in image 2) could be used to place the discs on the plate that has been inoculated.
- Disks shouldn’t be placed more than 24 millimeters (center from center) in the Muller Hinton Agar Plate. Typically there should be no more than 12 disks are allowed on a 150-mm-wide plate or more than five disks on a 100mm plate. Do not place disks too close to an edge on the plate because the zones won’t be perfectly round and may be difficult to determine.
- Each disc needs to be gently pressed to ensure that it is in complete contact with the surface. They should not slide off after the plates are flipped during the incubation. Don’t push it into the plate.
- The plates must be put in an incubator set at 35 degrees Celsius within 30 minutes after preparation. Temperatures above 35 degC invalidate results for oxacillin/methicillin. Don’t incubate within an environment containing carbon dioxide. This can lower the pH of agar, which can cause mistakes due to an improper pH of the media.
- After the overnight incubation period for a few hours, the dimensions of every zone (including the disc’s diameter) must be measured and recorded in millimeters. The results are then interpretable in accordance with the susceptibility chart for antimicrobials.
Measurement of zone of inhibition
Measurements can be calculated
- Using a ruler on the underneath of the plate without lifting the lid.
- When the substance is transparent the area can be determined by the use of of calibrators.
- Templates can be used to evaluate the final outcome of susceptibility tests.
The limit of inhibition can be seen by the naked eye at the point where growth starts However, there are three exceptions:
- In the presence of sulfonamides or co-trimoxazo tiny growth can be observed in the inhibition zone. the growth shouldn’t be overlooked.
- When b-lactamase-producing staphylococci are tested against penicillin, zones of inhibition are produced with a heaped-up, clearly defined edge; these are readily recognizable when compared with the sensitive control, and regardless of the size of the zone of inhibition, they should be reported as resistant.
- Certain Proteus species could form a swarm of inhibition in the vicinity of certain antibiotics, however the zone of inhibition is generally clear and the tiny swarming growth should not be ignored.
Results of Modified Kirby Bauer method
Results are available at the end of 18 hours. After the incubation period, determine zones to millimeters (mm) with the ruler or caliper. Include dimensions of disks in your measurement.
Interpretation and reporting of results from antimicrobial susceptibility tests
- Based on the recently published CLSI guidelines, identify your susceptibility and resistance an organism to each of the drugs examined. Be aware that there are various charts for different species.
- In the case of each medication, write on the record sheet whether the zone size is sensitive (S) or intermediate (I) or resistance (R) according to the chart for interpretation. Sizes of zones are not provided to doctors.
Limitations of Modified Kirby Bauer method
If it is performed in accordance to standard protocols, disk diffusion yields results which can be used to identify the in vivo efficacy of the drug at issue however, it is not without limitations:
- Do not provide exact information on the concentration of inhibitory minimum (MIC).
- It is not able to provide accurate results for some antibiotics/organism combination, like penicillin G found in N. meningitidis as well as S. pneumoniae.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.