Millon’s Test is a classical biochemical test which is used for the detection of proteins containing the amino acid tyrosine. It is the process based on the presence of phenolic group in tyrosine residue of protein. In this test Millon’s reagent is used which is prepared by dissolving metallic mercury in concentrated nitric acid forming mercuric and mercurous nitrates. When the reagent is added to protein solution the protein is first denatured and a white or yellow precipitate is formed. On gentle heating this precipitate turns brick red or pink in colour which indicates a positive Millon’s test. This reaction is due to nitration of phenolic group of tyrosine followed by the formation of a mercury complex. The test is not specific only for proteins as other phenolic compounds also give positive result and due to toxic nature of mercury reagent its use is limited in routine laboratory analysis.
Objectives of Millon’s Test
- To detect proteins containing the amino acid tyrosine.
- To identify the presence of phenolic group in proteins or other compounds.
- To differentiate tyrosine from other amino acids which do not possess phenolic group.
- To detect tyrosine rich protein substances such as casein in milk and proteins present in meat.
Principle of Millon’s Test
Millon’s test is based on the principle of nitrification of phenolic group present in the amino acid tyrosine. It is the process in which Millon’s reagent containing mercuric and mercurous nitrates in concentrated nitric acid reacts with protein. In this reaction the nitric acid first nitrates the phenol group of tyrosine side chain and this nitrated compound then reacts with mercury ions. As a result a coloured complex is formed. Initially a white or yellow precipitate is produced due to denaturation of protein but on heating this precipitate turns brick red or reddish brown. This colour formation confirms the presence of tyrosine containing proteins and hence it is referred to as positive Millon’s test.
Requirements for Millon’s Test
- Reagents
- Millon’s reagent (a solution of mercuric and mercurous nitrates dissolved in concentrated nitric acid).
- Test sample such as protein solution (albumin, casein) or tyrosine solution.
- Distilled water for dilution and preparation of solutions.
- Laboratory Apparatus
- Safety Requirements
- Laboratory gloves, apron and safety goggles due to corrosive and toxic nature of reagent.
- Fume hood for performing the test safely and to avoid inhalation of mercury vapours.
Procedure of Millon’s Test
- Take about 2 mL of the given test solution (protein solution or tyrosine solution) in a clean and dry test tube.
- Add few drops or equal volume of Millon’s reagent to the test tube containing the sample.
- Mix the contents gently and observe the formation of white or yellow precipitate due to protein denaturation.
- Place the test tube in a water bath and heat gently for about 2 minutes or till boiling if colour does not appear initially.
- Observe the colour change of the precipitate or solution. The appearance of brick red or reddish brown colour indicates positive Millon’s test.
Result and Interpretation of Millon’s Test
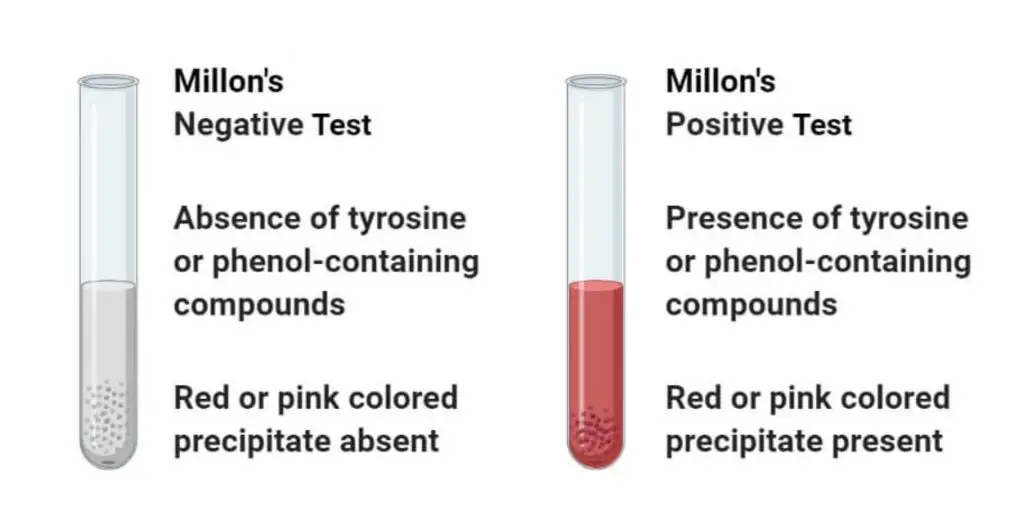
Positive result – A brick-red or reddish-brown colour is formed after gentle heating of the mixture. This indicates the presence of tyrosine in the protein. The colour may appear as precipitate or in solution.
Initial reaction – On addition of Millon’s reagent a white or yellow precipitate is formed immediately. This is due to denaturation of protein by mercury ions and not the specific tyrosine reaction.
Negative result – No red colour is developed even after heating. The solution remains colourless or the white precipitate does not change colour indicating absence of tyrosine residue.
False result / interferences – A red colour may be obtained with phenolic compounds like salicylic acid giving false positive result. Presence of chlorides may interfere with reaction and give false negative result.
Uses of Millon’s Test
- It is used for detection of proteins containing tyrosine amino acid.
- It is used to identify tyrosine due to presence of phenolic group in its structure.
- It is helpful in differentiation of tyrosine from other amino acids which do not give red colour reaction.
- It is used for detection of phenolic compounds like phenol salicylic acid and cresol.
- It is used in laboratory analysis of food proteins such as casein in milk and albumin in egg or meat.
- It is commonly used as a demonstration test in practical classes for study of protein reactions.
Advantages of Millon’s Test
- It is a highly sensitive test and can detect very small amount of protein containing tyrosine.
- It is specific for tyrosine due to presence of phenolic group in its side chain.
- It helps in differentiation of tyrosine from other amino acids.
- It is useful in laboratory identification of tyrosine rich proteins like casein and meat proteins.
- It can also be used for detection of phenolic compounds other than proteins.
Limitations of Millon’s Test
- It is not specific only for proteins and also gives positive result with phenolic compounds.
- Presence of chloride ions interferes with reaction and may give false negative result.
- Initial white or yellow precipitate formed may cause confusion during interpretation.
- The reagent contains mercury and nitric acid which are highly toxic and corrosive.
- It is a qualitative test and has been largely replaced by safer and more accurate methods.
- Aaron. (2023, June 5). Which protein assay is best for you? Azure Biosystems. https://azurebiosystems.com/blog/protein-assay-best/
- BYJU’S. (n.d.). Laboratory test of proteins. https://byjus.com/chemistry/laboratory-test-of-proteins/
- Fisher Scientific. (2024, February 9). Safety data sheet: Millon’s reagent. https://www.fishersci.com/store/msds?partNumber=AC458545000&productDescription=MILLON+S+REAGENT%2C+FOR+TH+500ML&vendorId=VN00032119&countryCode=US&language=en
- S D Fine-Chem Limited. (2018, June 16). Material safety data sheet: Millon’s reagent. https://sdfine.com/media/catalog/product/attachment/29085MSDS.pdf
- Sigma-Aldrich. (n.d.). Tools for protein quantitation. https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/protein-biology/protein-quantitation/tools-for-protein
- Subroto, E., Lembong, E., Filianty, F., Indiarto, R., Primalia, G., Putri, M. S. K. Z., Theodora, H. C., & Junar, S. (2020). The analysis techniques of amino acid and protein in food and agricultural products. International Journal of Scientific & Technology Research, 9(10), 29-36. https://www.ijstr.org/final-print/oct2020/The-Analysis-Techniques-Of-Amino-Acid-And-Protein-In-Food-And-Agricultural-Products.pdf
- Vedantu. (n.d.). Millon’s reagent: Preparation, uses & key reactions explained. https://www.vedantu.com/chemistry/millons-reagent
- Wikipedia. (2025). Millon’s reagent. https://en.wikipedia.org/wiki/Millon%27s_reagent
- Wisniak, J. (2020). Auguste-Nicolas-Eugène Millon: Millon reagent, chlorine derivatives, and blood. Revista CENIC Ciencias Químicas, 51(2), 386-401. https://www.redalyc.org/journal/1816/181676102012/
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.